Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity
Abstract
:1. Introduction
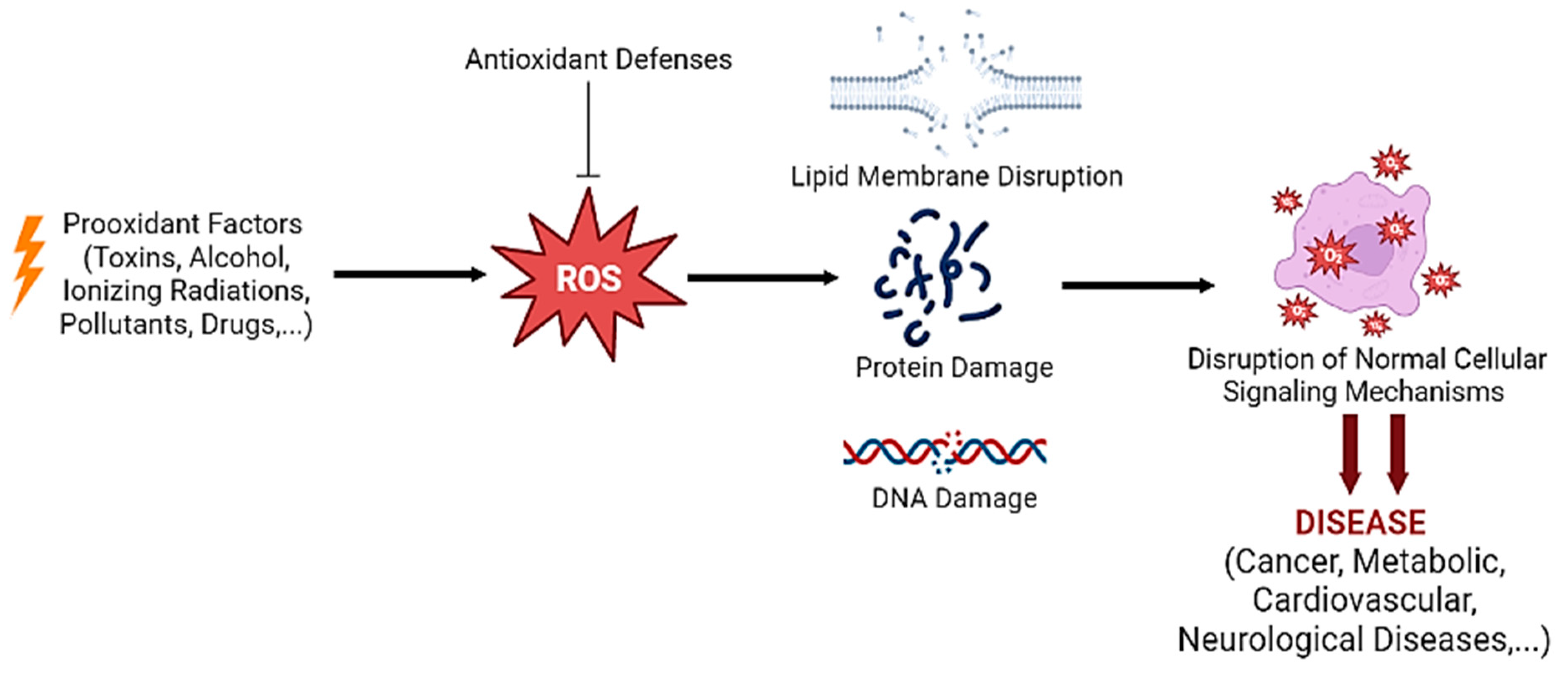
2. Oxidative Stress: An Overview
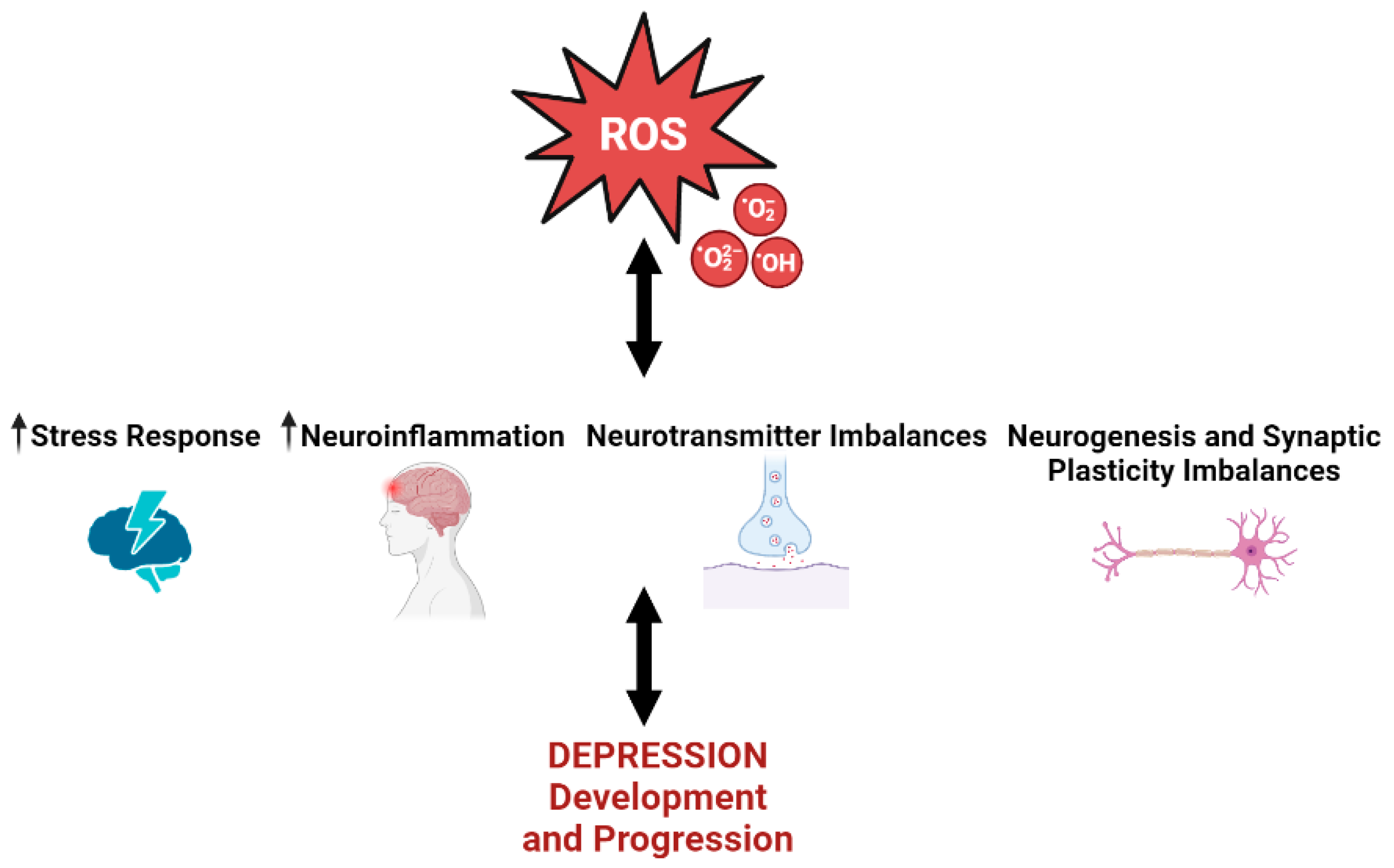
3. Oxidative Stress and Depression
3.1. Oxidative Stress and Depression’s Associated Stress Response
3.2. Oxidative Stress and Depression’s Associated Neuroinflammation
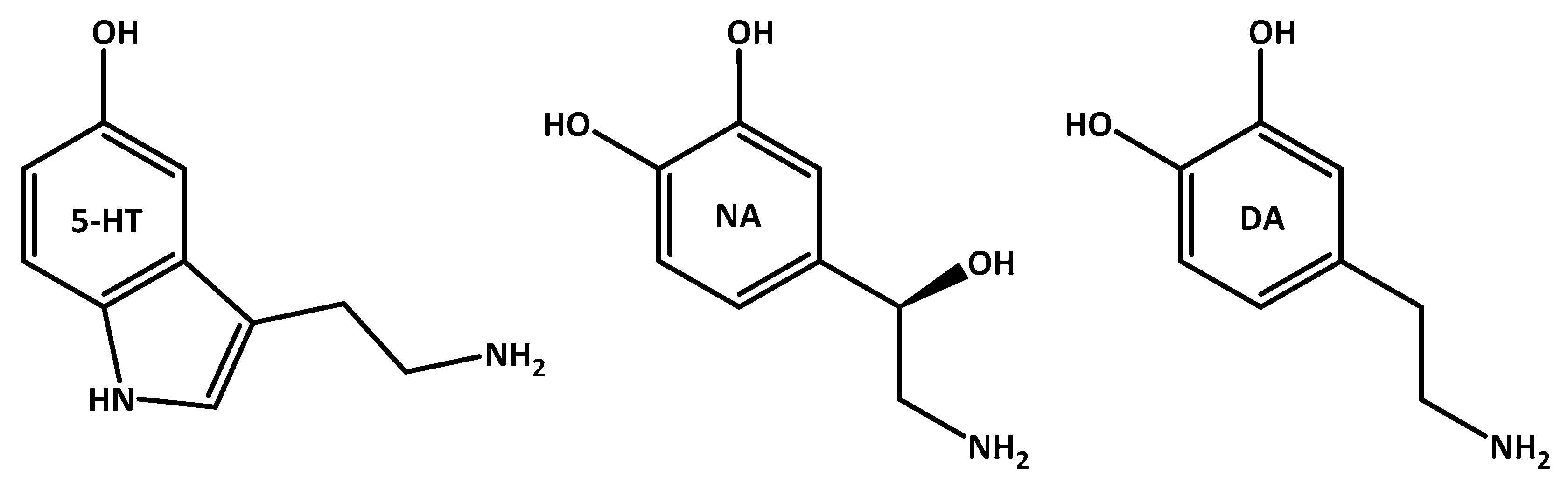
3.3. Oxidative Stress and Depression’s Associated Serotonin Imbalance
3.4. Oxidative Stress and Depression’s Associated Synaptic Plasticity and Neurogenesis Imbalance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 13 January 2023).
- Kennedy, S.H. Core symptoms of major depressive disorder: Relevance to diagnosis and treatment. Dialogues Clin. Neurosci. 2008, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, Z.Z.; Zhao, M.; Zhang, Y.; Chen, N.H. Role of non-coding RNA in the pathogenesis of depression. Gene 2020, 735, 144276. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Bajpai, A.; Verma, A.K.; Srivastava, M.; Srivastava, R. Oxidative Stress and Major Depression. J. Clin. Diagn. Res. 2014, 8, CC04–CC07. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- BioRender. Available online: https://biorender.com/ (accessed on 11 January 2023).
- Gonzalez-Hunt, C.P.; Wadhwa, M.; Sanders, L.H. DNA damage by oxidative stress: Measurement strategies for two genomes. Curr. Opin. Toxicol. 2018, 7, 87–94. [Google Scholar] [CrossRef]
- Zhu, D.; Shen, Z.; Liu, J.; Chen, J.; Liu, Y.; Hu, C.; Li, Z.; Li, Y. The ROS-mediated activation of STAT-3/VEGF signaling is involved in the 27-hydroxycholesterol-induced angiogenesis in human breast cancer cells. Toxicol. Lett. 2016, 264, 79–86. [Google Scholar] [CrossRef]
- Echizen, K.; Oshima, H.; Nakayama, M.; Oshima, M. The inflammatory microenvironment that promotes gastrointestinal cancer development and invasion. Adv. Biol. Regul. 2018, 68, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Reczek, C.R.; Birsoy, K.; Kong, H.; Martínez-Reyes, I.; Wang, T.; Gao, P.; Sabatini, D.M.; Chandel, N.S. A CRISPR screen identifies a pathway required for paraquat-induced cell death. Nat. Chem. Biol. 2017, 13, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Z.; Wang, L.; Wang, G.; Wang, Z.; Dong, X.B.; Wen, B.; Zhang, Z. The Pathogenesis of Diabetes Mellitus by Oxidative Stress and Inflammation: Its Inhibition by Berberine. Front. Pharmacol. 2018, 9, 782. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T.A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediat. Inflamm. 2010, 2010, 453892. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef] [PubMed]
- Dubois—Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Park. Dis. 2013, 3, 461. [Google Scholar] [CrossRef]
- Butterfield, D.A. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic. Res. 2002, 36, 1307–1313. [Google Scholar] [CrossRef]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/ RNS generation. J. Biomed. Sci. 2017, 24, 76. [Google Scholar] [CrossRef]
- Perier, C.; Bové, J.; Vila, M.; Przedborski, S. The rotenone model of Parkinson’s disease. Trends Neurosci. 2003, 26, 345–346. [Google Scholar] [CrossRef]
- Callio, J.; Oury, T.D.; Chu, C.T. Manganese Superoxide Dismutase Protects against 6-Hydroxydopamine Injury in Mouse Brains. J. Biol. Chem. 2005, 280, 18536. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Kaur, P.; Kaur, P.; Singh, H.; Batiha, G.E.S.; Verma, I. Exploring multifunctional antioxidants as potential agents for management of neurological disorders. Environ. Sci. Pollut. Res. Int. 2022, 29, 24458–24477. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Cardoso, A.; Vale, N. Highlighting Immune System and Stress in Major Depressive Disorder, Parkinson’s, and Alzheimer’s Diseases, with a Connection with Serotonin. Int. J. Mol. Sci. 2021, 22, 8525. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Antidepressants in Alzheimer’s Disease: A Focus on the Role of Mirtazapine. Pharmaceuticals 2021, 14, 930. [Google Scholar] [CrossRef]
- Moriarty, A.S.; Castleton, J.; Gilbody, S.; McMillan, D.; Ali, S.; Riley, R.D.; Chew-Graham, C.A. Predicting and preventing relapse of depression in primary care. Br. J. Gen. Pract. 2020, 70, 54–55. [Google Scholar] [CrossRef]
- Ferriani, L.O.; Silva, D.A.; Molina, M.d.C.B.; Mill, J.G.; Brunoni, A.R.; da Fonseca, M.d.J.M.; Moreno, A.B.; Benseñor, I.M.; de Aguiar, O.B.; Barreto, S.M.; et al. Associations of depression and intake of antioxidants and vitamin B complex: Results of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J. Affect. Disord. 2022, 297, 259–268. [Google Scholar] [CrossRef]
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef]
- Dutta, A.; Sarkar, P.; Shrivastava, S.; Chattopadhyay, A. Effect of Hypoxia on the Function of the Human Serotonin1AReceptor. ACS Chem. Neurosci. 2022, 13, 1456–1466. [Google Scholar] [CrossRef]
- Young, S.N. Elevated incidence of suicide in people living at altitude, smokers and patients with chronic obstructive pulmonary disease and asthma: Possible role of hypoxia causing decreased serotonin synthesis. J. Psychiatry Neurosci. 2013, 38, 423. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Niedermeier, M.; Hüfner, K.; van den Burg, E.; Kopp, M.; Stoop, R.; Burtscher, M.; Gatterer, H.; Millet, G.P. The interplay of hypoxic and mental stress: Implications for anxiety and depressive disorders. Neurosci. Biobehav. Rev. 2022, 138, 104718. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Rocha, R.; Correia, A.S.; Mota, B.; Madeira, M.D.; Vale, N.; Cardoso, A. Repurposed Edaravone, Metformin, and Perampanel as a Potential Treatment for Hypoxia–Ischemia Encephalopathy: An In Vitro Study. Biomedicines 2022, 10, 3043. [Google Scholar] [CrossRef]
- Hinds, J.A.; Sanchez, E.R. The Role of the Hypothalamus–Pituitary–Adrenal (HPA) Axis in Test-Induced Anxiety: Assessments, Physiological Responses, and Molecular Details. Stress 2022, 2, 146–155. [Google Scholar] [CrossRef]
- Raff, H. CORT, Cort, B, Corticosterone, and now Cortistatin: Enough Already! Endocrinology 2016, 157, 3307–3308. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Lewis, M.; Licinio, J. Translational research in endocrinology and neuroimmunology applied to depression. Biomed. Chem. Curr. Trends Dev. 2015, 119–131. [Google Scholar] [CrossRef]
- Hemmerle, A.M.; Herman, J.P.; Seroogy, K.B. Stress, depression and Parkinson’s disease. Exp. Neurol. 2012, 233, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. J. Neurosci. 1985, 5, 1222–1227. [Google Scholar] [CrossRef]
- Cryan, J.F.; Leonard, B.E. (Eds.) The Concept of Depression as a Dysfunction of the Immune System. In Depression: From Psychopathology to Pharmacotherapy; Karger Publishers: Basel, Switzerland, 2010; Volume 27, pp. 53–71. [Google Scholar] [CrossRef]
- Belleau, E.L.; Treadway, M.T.; Pizzagalli, D.A. The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol. Psychiatry 2019, 85, 443–453. [Google Scholar] [CrossRef]
- Song, Y.; Miyaki, K.; Suzuki, T.; Sasaki, Y.; Tsutsumi, A.; Kawakami, N.; Shimazu, A.; Takahashi, M.; Inoue, A.; Kan, C.; et al. Altered DNA methylation status of human brain derived neurotrophis factor gene could be useful as biomarker of depression. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2014, 165, 357–364. [Google Scholar] [CrossRef]
- Trifunovic, S.; Stevanovic, I.; Milosevic, A.; Ristic, N.; Janjic, M.; Bjelobaba, I.; Savic, D.; Bozic, I.; Jakovljevic, M.; Tesovic, K.; et al. The Function of the Hypothalamic–Pituitary–Adrenal Axis During Experimental Autoimmune Encephalomyelitis: Involvement of Oxidative Stress Mediators. Front. Neurosci. 2021, 15, 649485. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Hunter, R.; Wei, Y.; Blumenthal, R.; Falke, C.; Khairova, R.; Zhou, R.; Yuan, P.; Machado-Vieira, R.; et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc. Natl. Acad. Sci. USA 2009, 106, 3543–3548. [Google Scholar] [CrossRef]
- Spiers, J.G.; Chen, H.J.C.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takahashi, T.; Sumitani, K.; Takatsu, H.; Urano, S. Glucocorticoid Generates ROS to Induce Oxidative Injury in the Hippocampus, Leading to Impairment of Cognitive Function of Rats. J. Clin. Biochem. Nutr. 2010, 47, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Aquino, G.A.; Sousa, C.N.S.; Medeiros, I.S.; Almeida, J.C.; Cysne Filho, F.M.S.; Santos Júnior, M.A.; Vasconcelos, S.M.M. Behavioral alterations, brain oxidative stress, and elevated levels of corticosterone associated with a pressure injury model in male mice. J. Basic Clin. Physiol. Pharmacol. 2021, 33, 789–801. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; Rikel, L.; da Silva, E.B.; Simão da Silva, K.A.B.; Zeni, A.L.B. Cholecalciferol counteracts depressive-like behavior and oxidative stress induced by repeated corticosterone treatment in mice. Eur. J. Pharmacol. 2018, 833, 451–461. [Google Scholar] [CrossRef]
- Zeni, A.L.B.; Camargo, A.; Dalmagro, A.P. Lutein prevents corticosterone-induced depressive-like behavior in mice with the involvement of antioxidant and neuroprotective activities. Pharmacol. Biochem. Behav. 2019, 179, 63–72. [Google Scholar] [CrossRef]
- Xiao, Q.; Xiong, Z.; Yu, C.; Zhou, J.; Shen, Q.; Wang, L.; Xie, X.; Fu, Z. Antidepressant activity of crocin-I is associated with amelioration of neuroinflammation and attenuates oxidative damage induced by corticosterone in mice. Physiol. Behav. 2019, 212, 112699. [Google Scholar] [CrossRef]
- Song, L.; Wu, X.; Wang, J.; Guan, Y.; Zhang, Y.; Gong, M.; Wang, Y.; Li, B. Antidepressant effect of catalpol on corticosterone-induced depressive-like behavior involves the inhibition of HPA axis hyperactivity, central inflammation and oxidative damage probably via dual regulation of NF-κB and Nrf2. Brain Res. Bull. 2021, 177, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Shuster, A.L.; Rocha, F.E.; Wayszceyk, S.; de Lima, D.D.; Barauna, S.C.; Lopes, B.G.; Alberton, M.D.; Magro, D.D.D. Protective effect of Myrcia pubipetala Miq. against the alterations in oxidative stress parameters in an animal model of depression induced by corticosterone. Brain Res. 2021, 1774, 147725. [Google Scholar] [CrossRef]
- Ozyurek, P.; Cevik, C.; Kilic, I.; Aslan, A. Effects of Day and Night Shifts on Stress, Anxiety, Quality of Life, and Oxidative Stress Parameters in Nurses. Florence Nightingale J. Nurs. 2021, 29, 81–92. [Google Scholar] [CrossRef]
- Samad, N.; Rafeeque, M.; Imran, I. Free-L-Cysteine improves corticosterone-induced behavioral deficits, oxidative stress and neurotransmission in rats. Metab. Brain Dis. 2022. [Google Scholar] [CrossRef]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective effects of Hericium erinaceus (Bull.: Fr.) Pers. against high-dose corticosterone-induced oxidative stress in PC-12 cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, R.; She, Y.; Liu, X.; Zhao, H.; Li, C.; Jia, Y. A new perspective on depression and neuroinflammation: Non-coding RNA. J. Psychiatr. Res. 2022, 148, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Lamers, F.; Milaneschi, Y.; Smit, J.H.; Schoevers, R.A.; Wittenberg, G.; Penninx, B.W.J.H. Longitudinal Association Between Depression and Inflammatory Markers: Results From the Netherlands Study of Depression and Anxiety. Biol. Psychiatry 2019, 85, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.N.; Rizavi, H.S.; Zhang, H.; Bhaumik, R.; Ren, X. Abnormal protein and mRNA expression of inflammatory cytokines in the prefrontal cortex of depressed individuals who died by suicide. J. Psychiatry Neurosci. 2018, 43, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.; Shea, D.T.; McKim, D.B.; Reader, B.F.; Sheridan, J.F. Imipramine attenuates neuroinflammatory signaling and reverses stress-induced social avoidance. Brain. Behav. Immun. 2015, 46, 212–220. [Google Scholar] [CrossRef]
- Gorlova, A.; Svirin, E.; Pavlov, D.; Cespuglio, R.; Proshin, A.; Schroeter, C.A.; Lesch, K.-P.; Strekalova, T. Understanding the Role of Oxidative Stress, Neuroinflammation and Abnormal Myelination in Excessive Aggression Associated with Depression: Recent Input from Mechanistic Studies. Int. J. Mol. Sci. 2023, 24, 915. [Google Scholar] [CrossRef]
- Sánchez-Ramón, S.; Liu, L.; Reale, M.; Rivas-Arancibia, S.; Solleiro-Villavicencio, H. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4 + T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 1, 114. [Google Scholar] [CrossRef]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef]
- Liu, H.; Lin Liu, L.; Chen, J.; Wen Chen, Y.; Chai, Y.; Shan Liu, Q.; Cheng, Y. Muscone with Attenuation of Neuroinflammation and Oxidative Stress Exerts Antidepressant-Like Effect in Mouse Model of Chronic Restraint Stress. Oxidative Med. Cell. Longev. 2022, 2022, 3322535. [Google Scholar] [CrossRef]
- Ren, Y.; Sun-Waterhouse, D.; Ouyang, F.; Tan, X.; Li, D.; Xu, L.; Li, B.; Wang, Y.; Li, F. Apple phenolic extracts ameliorate lead-induced cognitive impairment and depression- and anxiety-like behavior in mice by abating oxidative stress, inflammation and apoptosis via the miR-22-3p/SIRT1 axis. Food Funct. 2022, 13, 2647–2661. [Google Scholar] [CrossRef]
- Zhuo, R.; Cheng, X.; Luo, L.; Yang, L.; Zhao, Y.; Zhou, Y.; Peng, L.; Jin, X.; Cui, L.; Liu, F.; et al. Cinnamic Acid Improved Lipopolysaccharide-Induced Depressive-Like Behaviors by Inhibiting Neuroinflammation and Oxidative Stress in Mice. Pharmacology 2022, 107, 281–289. [Google Scholar] [CrossRef]
- Sarandol, A.; Sarandol, E.; Eker, S.S.; Erdinc, S.; Vatansever, E.; Kirli, S. Major depressive disorder is accompanied with oxidative stress: Short-term antidepressant treatment does not alter oxidative–antioxidative systems. Hum. Psychopharmacol. Clin. Exp. 2007, 22, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar] [CrossRef]
- Moradi Vastegani, S.; Hajipour, S.; Sarkaki, A.; Basir, Z.; Parisa Navabi, S.; Farbood, Y.; Khoshnam, S.E. Curcumin mitigates lipopolysaccharide-induced anxiety/depression-like behaviors, blood–brain barrier dysfunction and brain edema by decreasing cerebral oxidative stress in male rats. Neurosci. Lett. 2022, 782, 136697. [Google Scholar] [CrossRef] [PubMed]
- Kheiry, M.; Dianat, M.; Badavi, M.; Mard, S.A.; Bayati, V. p-Coumaric Acid Attenuates Lipopolysaccharide-Induced Lung Inflammation in Rats by Scavenging ROS Production: An In Vivo and In Vitro Study. Inflammation 2019, 42, 1939–1950. [Google Scholar] [CrossRef]
- Yu, X.D.; Zhang, D.; Xiao, C.L.; Zhou, Y.; Li, X.; Wang, L.; He, Z.; Reilly, J.; Xiao, Z.Y.; Shu, X. P-Coumaric Acid Reverses Depression-Like Behavior and Memory Deficit Via Inhibiting AGE-RAGE-Mediated Neuroinflammation. Cells 2022, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sahu, K.; Kapil, L.; Singh, C.; Singh, A. Quercetin ameliorates lipopolysaccharide-induced neuroinflammation and oxidative stress in adult zebrafish. Mol. Biol. Rep. 2022, 49, 3247–3258. [Google Scholar] [CrossRef]
- Tayab, M.A.; Islam, M.N.; Chowdhury, K.A.A.; Tasnim, F.M. Targeting neuroinflammation by polyphenols: A promising therapeutic approach against inflammation-associated depression. Biomed. Pharmacother. 2022, 147, 112668. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behl, T.; Rana, T.; Alotaibi, G.H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A.A.H.; et al. Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed. Pharmacother. 2022, 146, 112545. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.K.d.S.; Turones, L.C.; Campos, H.M.; Nazareth, A.M.; Thomaz, D.V.; Gil, E.d.S.; Ghedini, P.C.; Rocha, F.F.d.; Menegatti, R.; Fajemiroye, J.O.; et al. LQFM212, a piperazine derivative, exhibits potential antioxidant effect as well as ameliorates LPS-induced behavioral, inflammatory and oxidative changes. Life Sci. 2023, 312, 121199. [Google Scholar] [CrossRef]
- Hursitoglu, O.; Kurutas, E.B.; Strawbridge, R.; Oner, E.; Gungor, M.; Tuman, T.C.; Uygur, O.F. Serum NOX1 and Raftlin as new potential biomarkers of Major Depressive Disorder: A study in treatment-naive first episode patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 121, 110670. [Google Scholar] [CrossRef]
- Baghaei Naeini, F.; Hassanpour, S.; Asghari, A. Resveratrol exerts anxiolytic-like effects through anti-inflammatory and antioxidant activities in rats exposed to chronic social isolation. Behav. Brain Res. 2023, 438, 114201. [Google Scholar] [CrossRef] [PubMed]
- Birmann, P.T.; Casaril, A.M.; Zugno, G.P.; Acosta, G.G.; Severo Sabedra Sousa, F.; Collares, T.; Seixas, F.K.; Jacob, R.G.; Brüning, C.A.; Savegnago, L.; et al. Flower essential oil of Tagetes minuta mitigates oxidative stress and restores BDNF-Akt/ERK2 signaling attenuating inflammation- and stress-induced depressive-like behavior in mice. Brain Res. 2022, 1784, 147845. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, X.; Qian, P.; Wu, H. Indoor air pollution from solid fuels use, inflammation, depression and cognitive function in middle-aged and older Chinese adults. J. Affect. Disord. 2022, 319, 370–376. [Google Scholar] [CrossRef]
- Yao, Y.; Man, L.; Du, J.; Wu, D.; Yang, L.; Peng, F.; Han, L.; Zhao, T.; Zhou, W. Astilbin ameliorates depressive-like behavior caused by postnatal immune activation through Menin-regulated astrocyte inflammation. J. Affect. Disord. 2022, 301, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry 2022, 28, 564–578. [Google Scholar] [CrossRef]
- Poletti, S.; Paolini, M.; Mazza, M.G.; Palladini, M.; Furlan, R.; Querini, P.R.; Benedetti, F. Lower levels of glutathione in the anterior cingulate cortex associate with depressive symptoms and white matter hyperintensities in COVID-19 survivors. Eur. Neuropsychopharmacol. 2022, 61, 71–77. [Google Scholar] [CrossRef]
- Manosso, L.M.; Camargo, A.; Dafre, A.L.; Rodrigues, A.L.S. Vitamin E for the management of major depressive disorder: Possible role of the anti-inflammatory and antioxidant systems. Nutr. Neurosci. 2022, 25, 1310–1324. [Google Scholar] [CrossRef]
- Filatova, E.V.; Shadrina, M.I.; Slominsky, P.A. Major Depression: One Brain, One Disease, One Set of Intertwined Processes. Cells 2021, 10, 1283. [Google Scholar] [CrossRef]
- Ogata, N.; de Souza Dantas, L.M.; Crowell-Davis, S.L. Selective Serotonin Reuptake Inhibitors. Vet. Psychopharmacol. 2022, 103–128. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022. [Google Scholar] [CrossRef]
- Müller, A.; Leichert, L.I. Redox proteomics. In Oxidative Stress and Redox Regulation; Springer: Dordrecht, The Netherlands, 2013; pp. 157–186. [Google Scholar] [CrossRef]
- Ribaudo, G.; Bortoli, M.; Witt, C.E.; Parke, B.; Mena, S.; Oselladore, E.; Zagotto, G.; Hashemi, P.; Orian, L. ROS-Scavenging Selenofluoxetine Derivatives Inhibit in Vivo Serotonin Reuptake. ACS Omega 2022, 7, 8314–8322. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Cardoso, A.; Vale, N. Significant Differences in the Reversal of Cellular Stress Induced by Hydrogen Peroxide and Corticosterone by the Application of Mirtazapine or L-Tryptophan. Int. J. Transl. Med. 2022, 2, 482–505. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Pourtau, L.; Gaudout, D.; Moras, B.; Vignault, A.; Monchaux De Oliveira, C.; Gabaston, J.; Vaysse, C.; Bertrand, K.; et al. Circulating Human Serum Metabolites Derived from the Intake of a Saffron Extract (Safr’InsideTM) Protect Neurons from Oxidative Stress: Consideration for Depressive Disorders. Nutrients 2022, 14, 1511. [Google Scholar]
- Jorgensen, A.; Köhler-Forsberg, K.; Henriksen, T.; Weimann, A.; Brandslund, I.; Ellervik, C.; Poulsen, H.E.; Knudsen, G.M.; Frokjaer, V.G.; Jorgensen, M.B. Systemic DNA and RNA damage from oxidation after serotonergic treatment of unipolar depression. Transl. Psychiatry 2022, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Omachi, T.; Matsuyama, N.; Hasegawa, Y. Nacre extract from pearl oyster suppresses LPS-induced depression and anxiety. J. Funct. Foods 2023, 100, 105373. [Google Scholar] [CrossRef]
- Yu, H.; Shao, S.; Xu, J.; Guo, H.; Zhong, Z.; Xu, J. Persimmon leaf extract alleviates chronic social defeat stress-induced depressive-like behaviors by preventing dendritic spine loss via inhibition of serotonin reuptake in mice. Chin. Med. 2022, 17, 65. [Google Scholar] [CrossRef]
- Vašíček, O.; Lojek, A.; Číž, M. Serotonin and its metabolites reduce oxidative stress in murine RAW264.7 macrophages and prevent inflammation. J. Physiol. Biochem. 2020, 76, 49–60. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, S.; Yang, X.; Li, W.; Si, J.; Yang, X. The antidepressant potential of lactobacillus casei in the postpartum depression rat model mediated by the microbiota-gut-brain axis. Neurosci. Lett. 2022, 774, 136474. [Google Scholar] [CrossRef]
- Citri, A.; Malenka, R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y. Involvement of Brain-Derived Neurotrophic Factor in Late-Life Depression. Am. J. Geriatr. Psychiatry 2013, 21, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.M.; Underwood, M.D.; Huang, Y.Y.; Hsiung, S.C.; Liu, Y.; Simpson, N.R.; Bakalian, M.J.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; et al. Association of BDNF Val66Met Polymorphism and Brain BDNF Levels with Major Depression and Suicide. Int. J. Neuropsychopharmacol. 2018, 21, 528–538. [Google Scholar] [CrossRef]
- Chen, B.; Dowlatshahi, D.; MacQueen, G.M.; Wang, J.F.; Young, L.T. Increased hippocampal bdnf immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef]
- Kumar, A.; Pareek, V.; Faiq, M.A.; Ghosh, S.K.; Kumari, C. ADULT NEUROGENESIS IN HUMANS: A Review of Basic Concepts, History, Current Research, and Clinical Implications. Innov. Clin. Neurosci. 2019, 16, 30. [Google Scholar] [PubMed]
- Aloe, L.; Alleva, E.; Fiore, M. Stress and nerve growth factor: Findings in animal models and humans. Pharmacol. Biochem. Behav. 2002, 73, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.; Zhao, X.; Van Praag, H.; Wodtke, K.; Gage, F.H.; Christie, B.R. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male sprague-dawley rats in vivo. Neuroscience 2004, 124, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Lee, H.; Young, A.H.; Aarsland, D.; Thuret, S. Adult Hippocampal Neurogenesis in Major Depressive Disorder and Alzheimer’s Disease. Trends Mol. Med. 2020, 26, 803–818. [Google Scholar] [CrossRef]
- Boldrini, M.; Santiago, A.N.; Hen, R.; Dwork, A.J.; Rosoklija, G.B.; Tamir, H.; Arango, V.; John Mann, J. Hippocampal Granule Neuron Number and Dentate Gyrus Volume in Antidepressant-Treated and Untreated Major Depression. Neuropsychopharmacology 2013, 38, 1068–1077. [Google Scholar] [CrossRef]
- Boldrini, M.; Hen, R.; Underwood, M.D.; Rosoklija, G.B.; Dwork, A.J.; Mann, J.J.; Arango, V. Hippocampal Angiogenesis and Progenitor Cell Proliferation Are Increased with Antidepressant Use in Major Depression. Biol. Psychiatry 2012, 72, 562–571. [Google Scholar] [CrossRef]
- Boldrini, M.; Underwood, M.D.; Hen, R.; Rosoklija, G.B.; Dwork, A.J.; John Mann, J.; Arango, V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 2009, 34, 2376–2389. [Google Scholar] [CrossRef]
- Ferreira, A.; Castro, J.P.; Andrade, J.P.; Dulce Madeira, M.; Cardoso, A. Cafeteria-diet effects on cognitive functions, anxiety, fear response and neurogenesis in the juvenile rat. Neurobiol. Learn. Mem. 2018, 155, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Łuszczki, E.; Michońska, I.; Dereń, K. The Mediterranean Diet and the Western Diet in Adolescent Depression-Current Reports. Nutrients 2022, 14, 4390. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; Moreira, J.B.; Costa, M.; Rodrigues, R.S.; Sebastião, A.M.; Xapelli, S.; Solá, S. The Mitochondrial Antioxidant Sirtuin3 Cooperates with Lipid Metabolism to Safeguard Neurogenesis in Aging and Depression. Cells 2022, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-T.; Yin, H.; Hu, C.; Zeng, J.; Zhang, S.; Chen, S.; Zheng, W.; Li, M.; Jin, L.; Liu, Y.; et al. Tilapia Skin Peptides Ameliorate Cyclophosphamide-Induced Anxiety- and Depression-Like Behavior via Improving Oxidative Stress, Neuroinflammation, Neuron Apoptosis, and Neurogenesis in Mice. Front. Nutr. 2022, 9, 882175. [Google Scholar] [CrossRef]
- Suwannakot, K.; Sritawan, N.; Naewla, S.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin Attenuates Methotrexate-Induced Reduction of Antioxidant Activity Related to Decreases of Neurogenesis in Adult Rat Hippocampus and Prefrontal Cortex. Oxid. Med. Cell Longev. 2022, 2022, 1596362. [Google Scholar] [CrossRef]
- de Sousa, C.N.S.; Medeiros, I.d.S.; Vasconcelos, G.S.; de Aquino, G.A.; Cysne Filho, F.M.S.; de Almeida Cysne, J.C.; Macêdo, D.S.; Vasconcelos, S.M.M. Involvement of oxidative pathways and BDNF in the antidepressant effect of carvedilol in a depression model induced by chronic unpredictable stress. Psychopharmacology 2022, 239, 297–311. [Google Scholar] [CrossRef]
- Ryu, D.; Jee, H.-J.; Kim, S.-Y.; Hwang, S.-H.; Pil, G.-B.; Jung, Y.-S. Luteolin-7-O-Glucuronide Improves Depression-like and Stress Coping Behaviors in Sleep Deprivation Stress Model by Activation of the BDNF Signaling. Nutrients 2022, 14, 3314. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Qi, K.; Xu, W.; Wei, Y. Rosmarinic acid relieves LPS-induced sickness and depressive-like behaviors in mice by activating the BDNF/Nrf2 signaling and autophagy pathway. Behav. Brain Res. 2022, 433, 114006. [Google Scholar] [CrossRef]
- Abbas, F.; Eladl, M.A.; El-Sherbiny, M.; Abozied, N.; Nabil, A.; Mahmoud, S.M.; Mokhtar, H.I.; Zaitone, S.A.; Ibrahim, D. Celastrol and thymoquinone alleviate aluminum chloride-induced neurotoxicity: Behavioral psychomotor performance, neurotransmitter level, oxidative-inflammatory markers, and BDNF expression in rat brain. Biomed. Pharmacother. 2022, 151, 113072. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Li, M.; Zou, C.; Wang, K.; Zhang, W.; Huang, X.; Wang, Y. Walnut polyphenols and the active metabolite urolithin A improve oxidative damage in SH-SY5Y cells by up-regulating PKA/CREB/BDNF signaling. Food Funct. 2023. [Google Scholar] [CrossRef]
| Compound | Strain | Doses | Route of Administration | Main Findings |
|---|---|---|---|---|
| Cholecalciferol [57] | Male Swiss mice | 2.5, 7.5, 25 μg/kg | Oral | Counteracted depressive-like behavior and oxidative stress |
| Lutein [58] | Male Swiss mice | 0.1, 1, and 10 mg/kg | Oral | Counteracted depressive-like behavior and oxidative stress |
| Crocin-I [59] | C57BL/6 J mice | 20 and 40 mg/kg | Oral | Counteracted neuroinflammation and oxidative damage |
| Catalpol [60] | Kunming mice | 20 mg/kg | Intragastric | Inhibited the HPA axis hyperactivity, central inflammation, and oxidative damage |
| Myrcia pubipetala Miq. [61] | Male Swiss mice | 50, 100, or 150 mg/Kg | Oral | Antioxidant effects |
| L-Cysteine [63] | Male albino rats | 150 mg/kg/mL | Oral | Reduced corticosterone levels and increased antioxidant defenses |
| Compound | Strain | Doses | Route of Administration | Main Findings |
|---|---|---|---|---|
| Muscone [72] | C57BL/6 mice | 10 mg/kg | Intragastric | Ameliorated depression-like behavior by regulating inflammatory and oxidative stress markers |
| Apple phenolic extracts [73] | Kunming mice | 200 ppm | Intragastric | Regulation of oxidative stress, neuroinflammation, and apoptosis |
| Cinnamic acid [74] | C57BL/6J mice | 50, 100 and 200 mg/kg | Intragastric | Improved depressive-like behavior by inhibiting neuroinflammation and oxidative stress |
| Curcumin [77] | Wistar rats | 50 mg/kg | Intragastric | Attenuated anxiety/depression-like behaviors, decreasing oxidative stress and neuroinflammation |
| p-Coumaric acid [78,79] | Sprague Dawley rats [78]; male Institute of Cancer Research (ICR) mice [79] | 25, 50, and 100 mg/kg [78]; 75 mg/kg [79] | Intraperitoneal injection [78,79] | Protective role against inflammation and oxidative stress [78,79] |
| Quercetin [80] | Zebrafish | 50 and 100 mg/kg | Intraperitoneal injection | Alleviated oxidative stress and neuroinflammation |
| LQFM212 [84] | Albino Swiss mice | 54 μmol/kg (18.8 mg/kg) | Oral | Antioxidant effects and ameliorated behavioral, inflammatory, and oxidative changes |
| Resveratrol [86] | Wistar rats | 20, 40, or 80 mg/kg | Intraperitoneal injection | Antioxidant, anti-inflammatory, and antidepressant effects |
| Flower essential oil of Tagetes minuta [87] | Adult Swiss mice | 10 and 50 mg/kg | Intragastric | Attenuated depressive-like behavior by reducing oxidative stress and inflammation and control of BDNF-related pathways |
| Astilbin [89] | C57/BL6 mice | 2, 4, 6, and 8 mg/kg | Intraperitoneal injection | Ameliorated depressive-like behavior by regulating astrocyte-mediated neuroinflammation |
| Compound | Strain | Doses | Route of Administration | Main Findings |
|---|---|---|---|---|
| Saffron extract [99] | Healthy volunteers | 300 mg | Oral | Protected human neurons from oxidative stress, stimulating the production of dopamine, 5-HT, and BDNF |
| Escitalopram [100] | Patients with unipolar depression | 10–20 mg | Oral | Reduced DNA and RNA damage from oxidation |
| Duloxetine [100] | Patients with unipolar depression | 30–120 mg | Oral | Reduced DNA and RNA damage from oxidation |
| Nacre extract [101] | ICR mice | 50 and 100 mg/kg | Intraperitoneal injection | Suppressed depression and anxiety behavior, attenuating the high levels of oxidative stress |
| Persimmon leaf extract [102] | Single-housed CD-1 mice and C57BL/6 mice | 30.0–60.0 mg/kg | Intragastric | Prevented dendritic spine loss, alleviating the depressive-like behavior |
| Lactobacillus casei [104] | Sprague Dawley rats | 8 × 108 CFU/kg/day | Intragastric | Improved depression-like behaviors, increased expression of monoamines, and decreased levels of oxidative stress |
| Compound | Strain | Doses | Route of Administration | Main Findings |
|---|---|---|---|---|
| Tilapia skin peptides [120] | C57BL/6 mice | 250, 500, and 1000 mg/kg | Intraperitoneal injection | Improved depression-like behavior by regulating oxidative stress and neurogenesis |
| Flower essential oil of Tagetes minuta [87] | Adult Swiss mice | 10 and 50 mg/kg | Intragastric | Attenuated depressive-like behavior by reducing oxidative stress and control of BDNF-related pathways |
| Melatonin [121] | Sprague Dawley rats | 8 mg/kg | Intraperitoneal injection | Increased antioxidant markers and increased neurogenesis |
| Carvedilol [122] | Adult Swiss mice | 5 and 10 mg/kg | Oral | Increased glutathione and BDNF concentrations, and decreased oxidative stress, presenting antidepressant-like effects |
| Luteolin-7-O-glucuronide [123] | C57BL/6 mice | 0.3, 1, and 3 mg/kg | Oral | Antioxidant properties and improved depression-like behavior, activating BDNF signaling |
| Rosmarinic acid [124] | Specific pathogen-free (SPF) C57BL/6 mice | 80 mg/kg | Intraperitoneal injection | Reversed depressive behaviors by promoting the expression of BDNF, increased expression of antioxidant enzymes, reduced inflammation |
| Celastrol [125] | Albino Wistar rats | 1 mg/kg | Intraperitoneal injection | Alleviated depressive and anxiety behaviors, increased BDNF expression, and downregulated the oxido-inflammatory markers |
| Thymoquinone [125] | Albino Wistar rats | 10 mg/kg | Intraperitoneal injection | Alleviated depressive and anxiety behaviors, increased BDNF expression, and downregulated the oxido-inflammatory markers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. https://doi.org/10.3390/antiox12020470
Correia AS, Cardoso A, Vale N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants. 2023; 12(2):470. https://doi.org/10.3390/antiox12020470
Chicago/Turabian StyleCorreia, Ana Salomé, Armando Cardoso, and Nuno Vale. 2023. "Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity" Antioxidants 12, no. 2: 470. https://doi.org/10.3390/antiox12020470
APA StyleCorreia, A. S., Cardoso, A., & Vale, N. (2023). Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants, 12(2), 470. https://doi.org/10.3390/antiox12020470







