Extracellular Vesicles, as Drug-Delivery Vehicles, Improve the Biological Activities of Astaxanthin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. EV Isolation
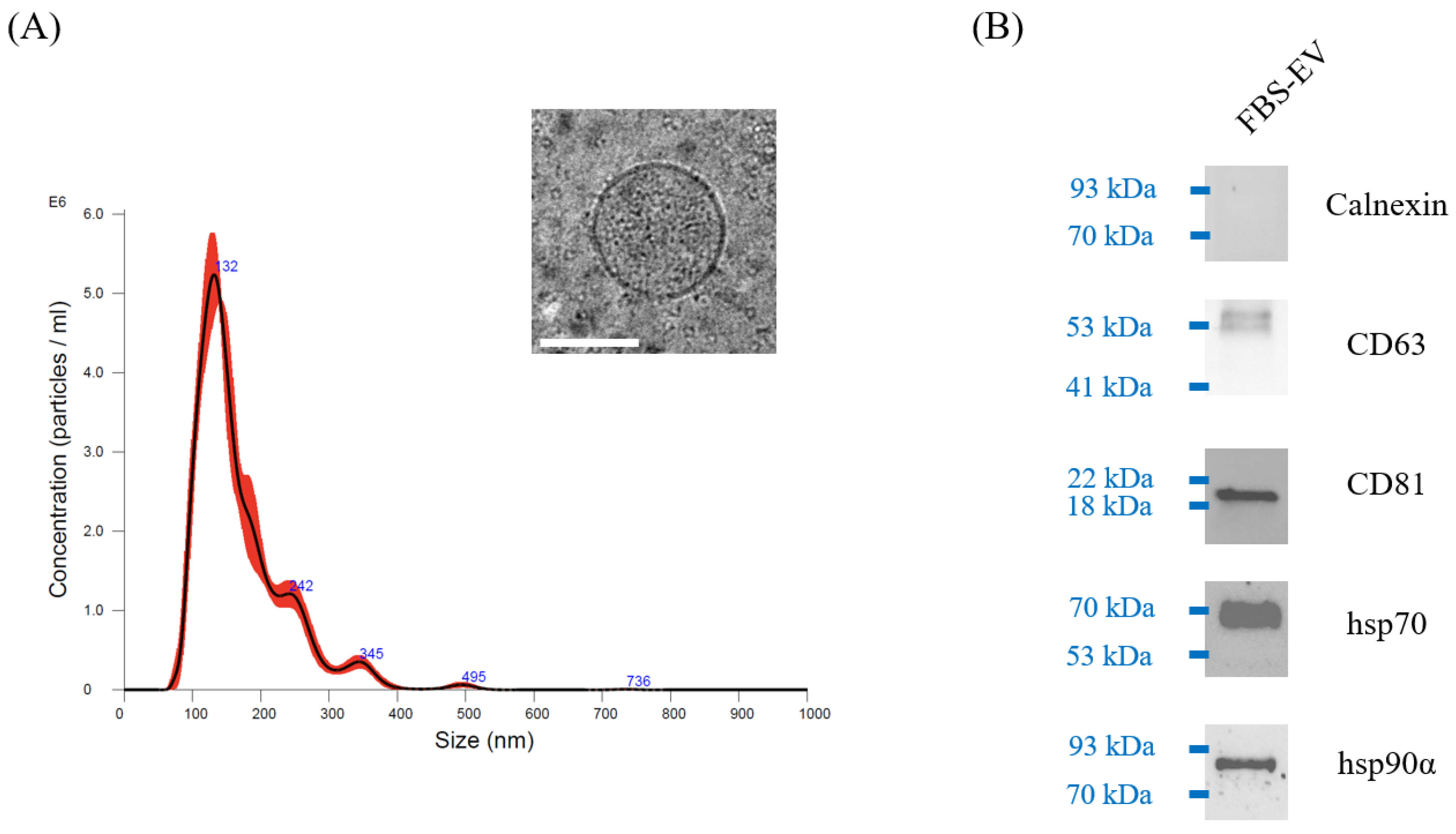
2.4. Nanoparticle Tracking Analysis (NTA)
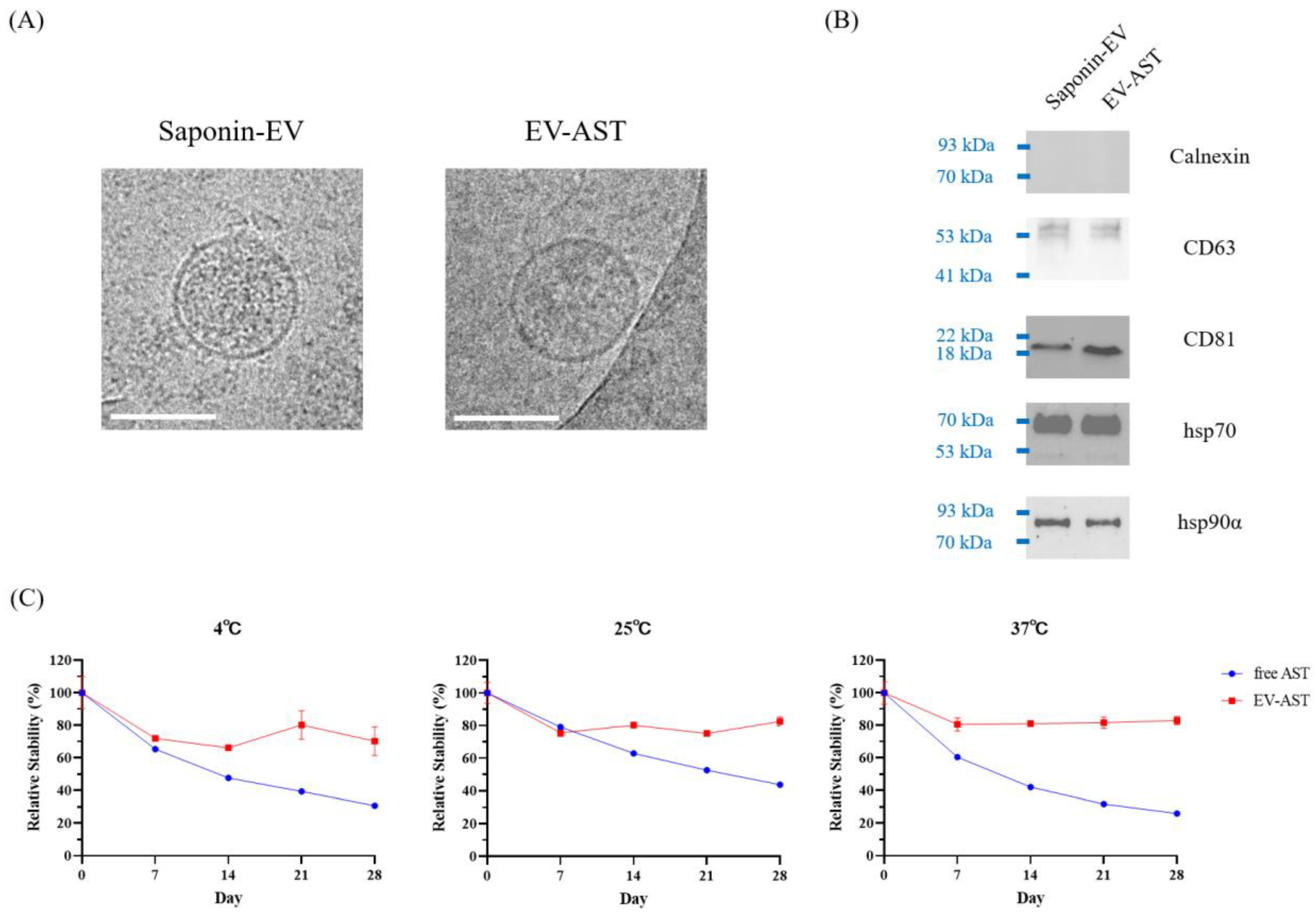
2.5. Western Blotting
2.6. Cryo-Transmission Electron Microscopy (Cryo-TEM)
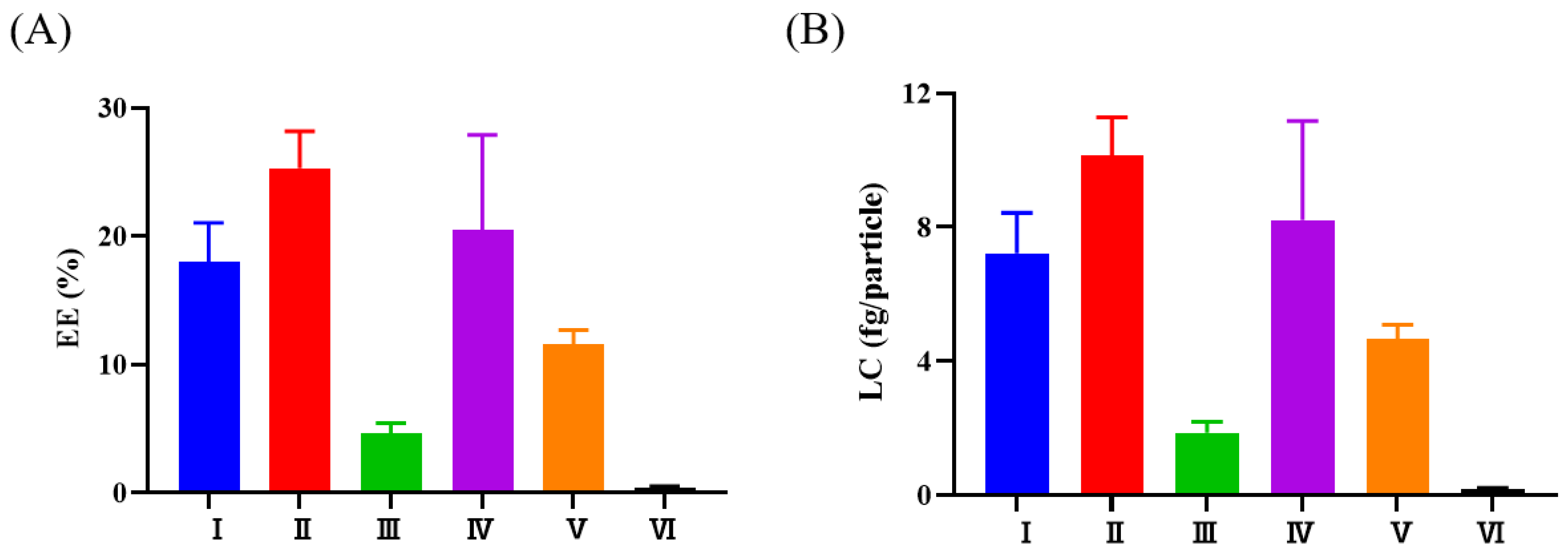
2.7. Drug-Loading Methods
2.8. Determination of AST Content in EV-AST
2.9. Thermal Stability of EV-ASTs
2.10. ABTS Radical-Scavenging Assay
2.11. Cell-Viability Assay
2.12. Cellular Antioxidant Activity (CAA) Assay
2.13. Anti-Inflammatory Assay
2.14. mRNA Analysis
2.15. Statistical Analysis
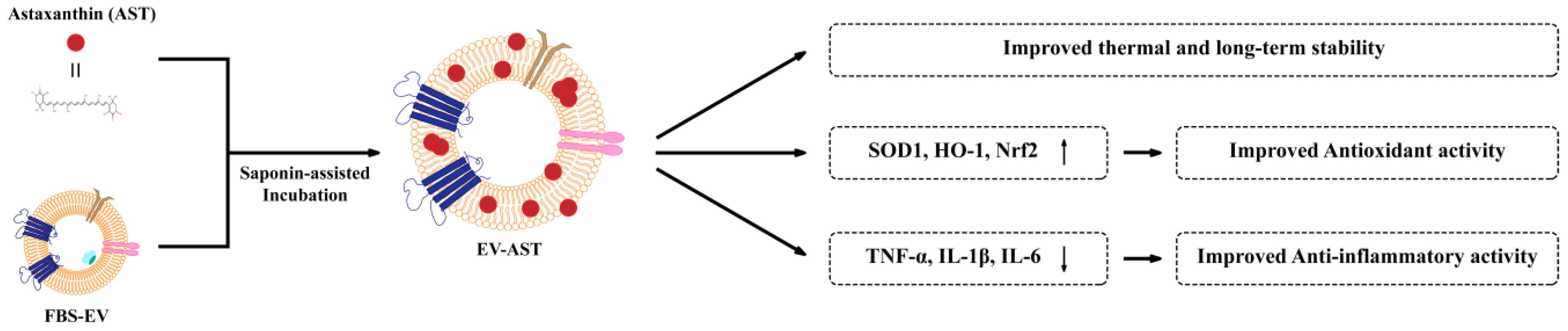
3. Results and Discussion
3.1. Characterization of FBS-EVs
3.2. Selection of the Methods Used to Load AST into FBS-EVs
3.3. Characterization of EV-ASTs
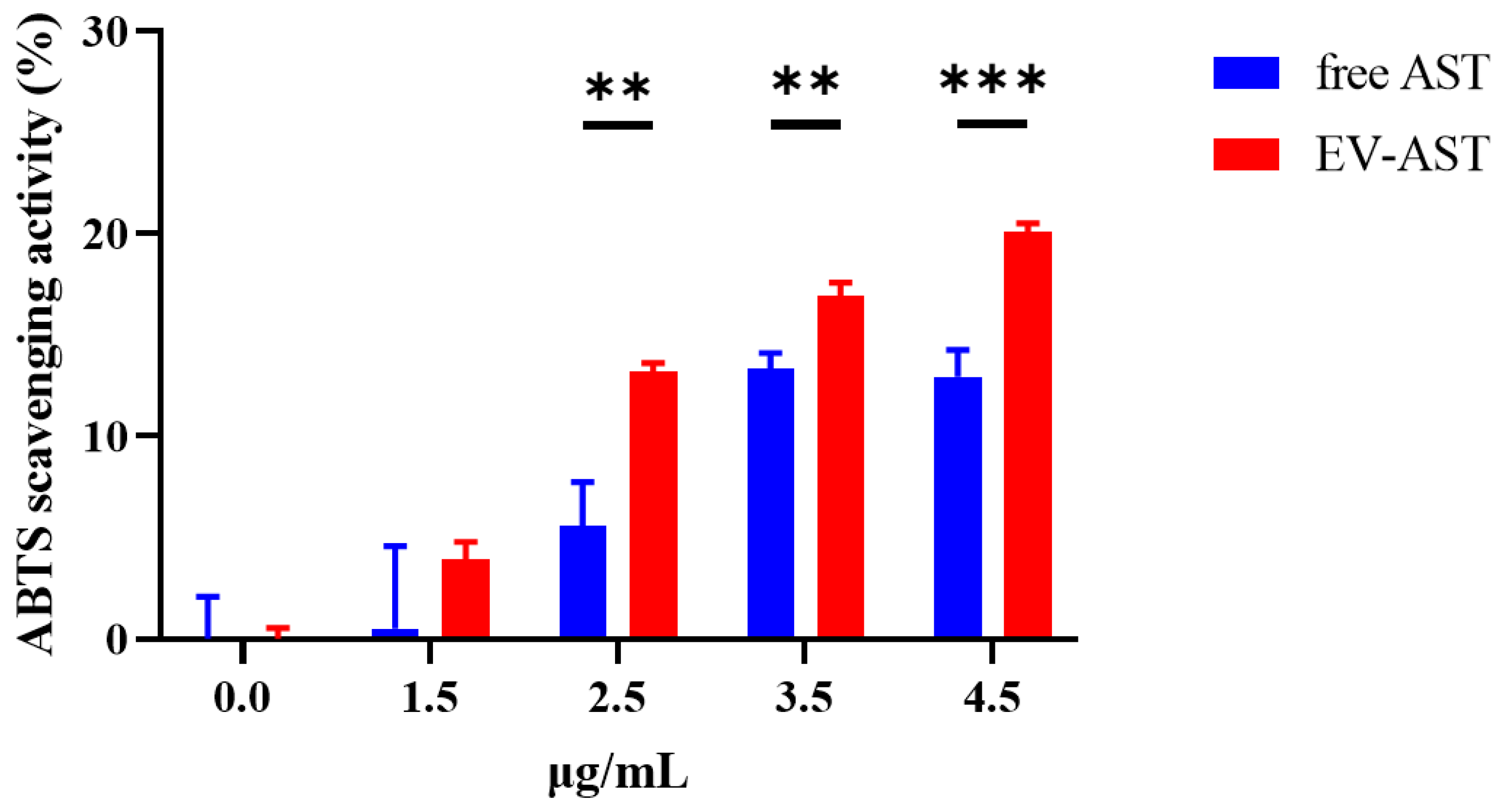
3.4. In Vitro Antioxidant Activity of Free AST and EV-ASTs
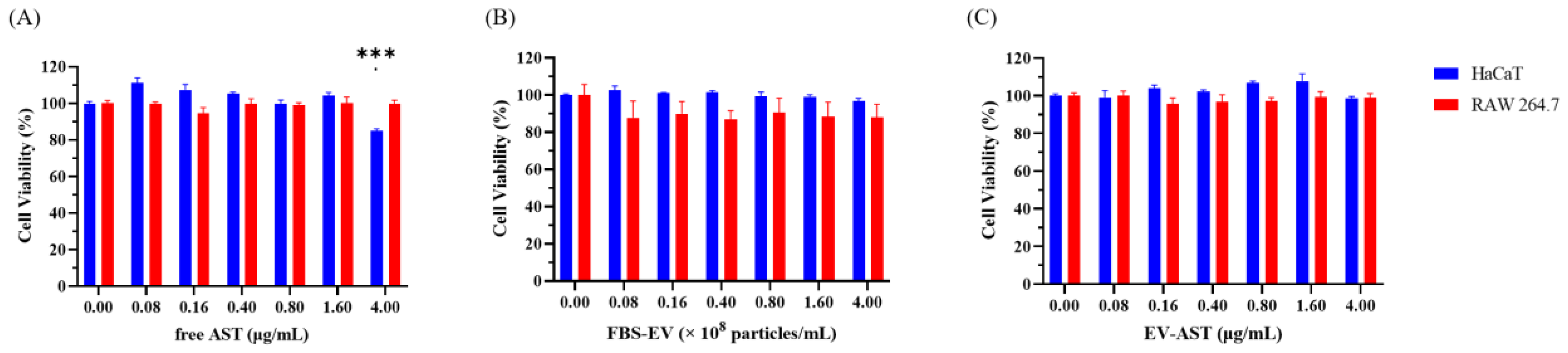
3.5. Evaluation of Cytotoxicity in HaCaT and RAW 264.7 Cells
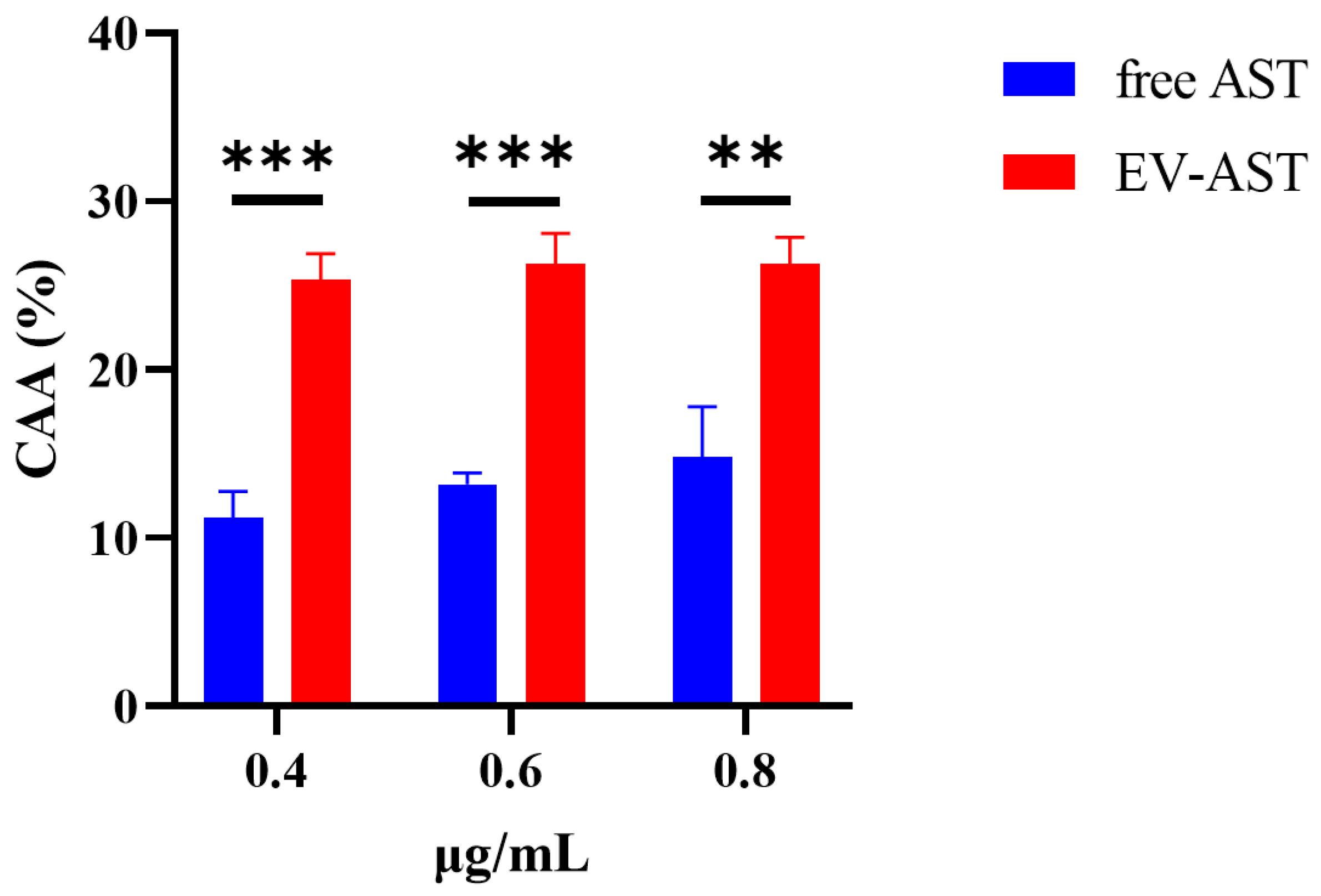
3.6. The CAA of Free AST and EV-ASTs
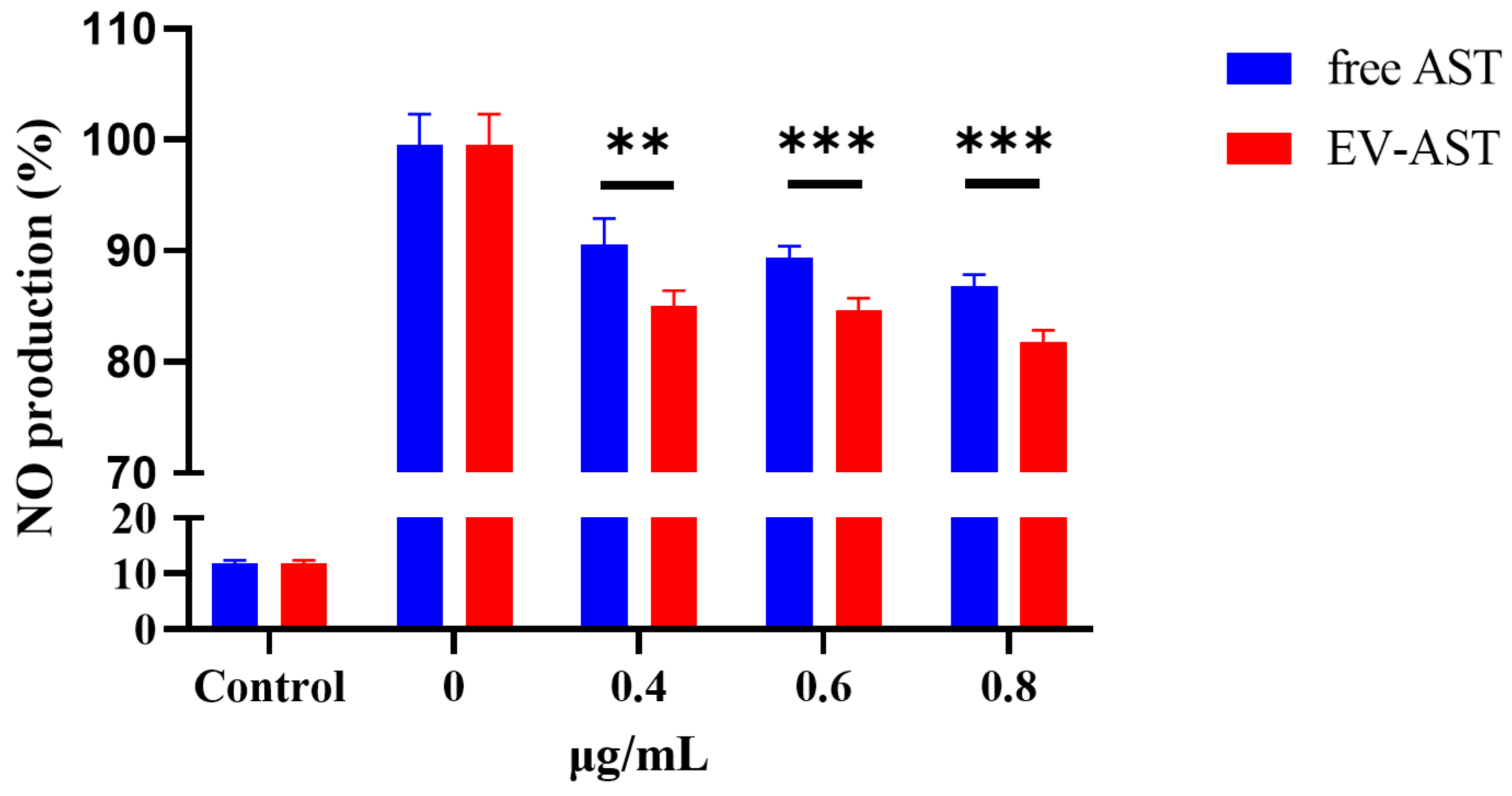
3.7. The Anti-Inflammatory Activity of Free AST and EV-AST
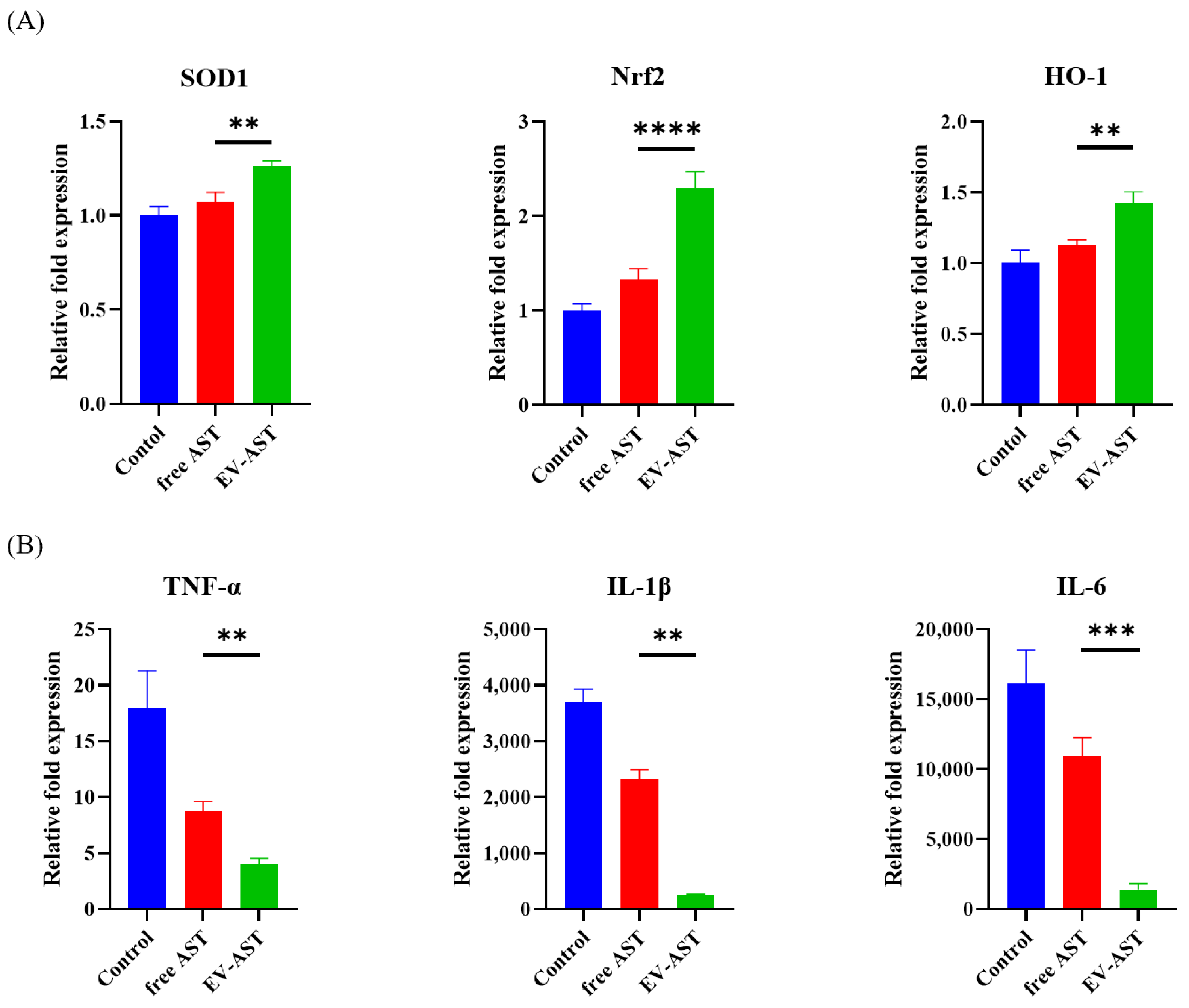
3.8. Assessment of Gene Expression Related to the Antioxidant and Anti-Inflammatory Activities of the EV-ASTs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Yamashita, E. Let Astaxanthin Be Thy Medicine. PharmaNutrition 2015, 3, 115–122. [Google Scholar] [CrossRef]
- Yamashita, E. Astaxanthin as a Medical Food. Funct. Foods Health Dis. 2013, 3, 254. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Johnson, E.A.; An, G.-H. Astaxanthin from Microbial Sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Cis Astaxanthin and Especially 9-Cis Astaxanthin Exhibits a Higher Antioxidant Activity In Vitro Compared to the All-Trans Isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef]
- White, D.A.; Ørnsrud, R.; Davies, S.J. Determination of Carotenoid and Vitamin A Concentrations in Everted Salmonid Intestine Following Exposure to Solutions of Carotenoid in Vitro. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 136, 683–692. [Google Scholar] [CrossRef]
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a Potential Neuroprotective Agent for Neurological Diseases. Mar. Drugs 2015, 13, 5750–5766. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin Decreased Oxidative Stress and Inflammation and Enhanced Immune Response in Humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Zhang, X.; Wu, Q.; Li, W.; Zhang, Q.-R.; Wang, C.-X.; Zhou, X.-M.; Li, H.; Shi, J.-X.; Zhou, M.-L. Astaxanthin Alleviates Early Brain Injury Following Subarachnoid Hemorrhage in Rats: Possible Involvement of Akt/Bad Signaling. Mar. Drugs 2014, 12, 4291–4310. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic Benefits of Astaxanthin on Humans Subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Miyachi, M.; Matsuno, T.; Asano, K.; Mataga, I. Anti-Inflammatory Effects of Astaxanthin in the Human Gingival Keratinocyte Line NDUSD-1. J. Clin. Biochem. Nutr. 2015, 56, 171–178. [Google Scholar] [CrossRef]
- Park, S.-A.; Ahn, J.-B.; Choi, S.-H.; Lee, J.-S.; Lee, H.G. The Effects of Particle Size on the Physicochemical Properties of Optimized Astaxanthin-Rich Xanthophyllomyces Dendrorhous-Loaded Microparticles. LWT-Food Sci. Technol. 2014, 55, 638–644. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of Astaxanthin-Loaded Core–Shell Nanoparticles Consisting of Chitosan Oligosaccharides and Poly(Lactic-Co-Glycolic Acid): Enhancement of Water Solubility, Stability, and Bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef]
- Galarza, J.I.; Arredondo Vega, B.O.; Villón, J.; Henríquez, V. Deesterification of Astaxanthin and Intermediate Esters from Haematococcus Pluvialis Subjected to Stress. Biotechnol. Rep. 2019, 23, e00351. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, J.; Zhong, B.; Huang, J.; Jiang, L.; Jiang, Y.; Yuan, J.; Sun, J.; Dai, L.; Yang, C.; et al. Exosomes as Potential Sources of Biomarkers in Colorectal Cancer. Cancer Lett. 2020, 476, 13–22. [Google Scholar] [CrossRef]
- Kim, S.; Han, J.; Park, J.S.; Kim, J.H.; Lee, E.S.; Cha, B.S.; Park, K.S. DNA Barcode-Based Detection of Exosomal MicroRNAs Using Nucleic Acid Lateral Flow Assays for the Diagnosis of Colorectal Cancer. Talanta 2022, 242, 123306. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Kwon, W.Y.; Park, K.S. Exosomes for Non-Invasive Cancer Monitoring. Biotechnol. J. 2019, 14, 1800430. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Kwon, W.Y.; Park, K.S. A Simple Approach for Rapid and Cost-Effective Quantification of Extracellular Vesicles Using a Fluorescence Polarization Technique. J. Biol. Eng. 2019, 13, 31. [Google Scholar] [CrossRef]
- Cha, B.S.; Park, K.S.; Park, J.S. Signature MRNA Markers in Extracellular Vesicles for the Accurate Diagnosis of Colorectal Cancer. J. Biol. Eng. 2020, 14, 4. [Google Scholar] [CrossRef]
- Bagheri Hashkavayi, A.; Cha, B.S.; Lee, E.S.; Kim, S.; Park, K.S. Advances in Exosome Analysis Methods with an Emphasis on Electrochemistry. Anal. Chem. 2020, 92, 12733–12740. [Google Scholar] [CrossRef]
- Lee, E.S.; Cha, B.S.; Kim, S.; Park, K.S. Synthesis of Exosome-Based Fluorescent Gold Nanoclusters for Cellular Imaging Applications. Int. J. Mol. Sci. 2021, 22, 4433. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Cha, B.S.; Lee, E.S.; Park, K.S. Dual Rolling Circle Amplification-Enabled Ultrasensitive Multiplex Detection of Exosome Biomarkers Using Electrochemical Aptasensors. Anal. Chim. Acta 2022, 1205, 339762. [Google Scholar] [CrossRef]
- Fujita, D.; Arai, T.; Komori, H.; Shirasaki, Y.; Wakayama, T.; Nakanishi, T.; Tamai, I. Apple-Derived Nanoparticles Modulate Expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 Cells. Mol. Pharm. 2018, 15, 5772–5780. [Google Scholar] [CrossRef]
- Patel, M.M.; Patel, B.M. Crossing the Blood–Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef]
- Hood, J.L.; Wickline, S.A. A Systematic Approach to Exosome-Based Translational Nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 458–467. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv. Mater. 2019, 31, 1802896. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome Engineering: Current Progress in Cargo Loading and Targeted Delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Ho, E.A. Challenges in the Development and Establishment of Exosome-Based Drug Delivery Systems. J. Control. Release 2021, 329, 894–906. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.v.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Eitan, E.; Zhang, S.; Witwer, K.W.; Mattson, M.P. Extracellular Vesicle–Depleted Fetal Bovine and Human Sera Have Reduced Capacity to Support Cell Growth. J. Extracell. Vesicles 2015, 4, 26373. [Google Scholar] [CrossRef]
- Lehrich, B.; Liang, Y.; Khosravi, P.; Federoff, H.; Fiandaca, M. Fetal Bovine Serum-Derived Extracellular Vesicles Persist within Vesicle-Depleted Culture Media. Int. J. Mol. Sci. 2018, 19, 3538. [Google Scholar] [CrossRef]
- Ochieng, J.; Pratap, S.; Khatua, A.K.; Sakwe, A.M. Anchorage-Independent Growth of Breast Carcinoma Cells Is Mediated by Serum Exosomes. Exp. Cell Res. 2009, 315, 1875–1888. [Google Scholar] [CrossRef]
- Dong, M.; Wu, S.; Xu, H.; Yu, X.; Wang, L.; Bai, H.; Niu, W. FBS-Derived Exosomes as a Natural Nano-Scale Carrier for Icariin Promote Osteoblast Proliferation. Front. Bioeng. Biotechnol. 2021, 9, 615920. [Google Scholar] [CrossRef]
- Cha, B.S.; Lee, E.S.; Kim, S.; Kim, J.M.; Hwang, S.H.; Oh, S.S.; Park, K.S. Simple Colorimetric Detection of Organophosphorus Pesticides Using Naturally Occurring Extracellular Vesicles. Microchem. J. 2020, 158, 105130. [Google Scholar] [CrossRef]
- Emelyanov, A.; Shtam, T.; Kamyshinsky, R.; Garaeva, L.; Verlov, N.; Miliukhina, I.; Kudrevatykh, A.; Gavrilov, G.; Zabrodskaya, Y.; Pchelina, S.; et al. Cryo-Electron Microscopy of Extracellular Vesicles from Cerebrospinal Fluid. PLoS ONE 2020, 15, e0227949. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.-W.; Chan, L.-C.; Wei, Y.; Hsu, J.-M.; Xia, W.; Cha, J.-H.; Hou, J.; Hsu, J.L.; Sun, L.; et al. Exosomal PD-L1 Harbors Active Defense Function to Suppress T Cell Killing of Breast Cancer Cells and Promote Tumor Growth. Cell Res. 2018, 28, 862–864. [Google Scholar] [CrossRef]
- Ban, J.-J.; Lee, M.; Im, W.; Kim, M. Low PH Increases the Yield of Exosome Isolation. Biochem. Biophys. Res. Commun. 2015, 461, 76–79. [Google Scholar] [CrossRef]
- Tang, X.; Chang, C.; Guo, J.; Lincoln, V.; Liang, C.; Chen, M.; Woodley, D.T.; Li, W. Tumour-Secreted Hsp90α on External Surface of Exosomes Mediates Tumour-Stromal Cell Communication via Autocrine and Paracrine Mechanisms. Sci. Rep. 2019, 9, 15108. [Google Scholar] [CrossRef] [Green Version]
- Asea, A.; Jean-Pierre, C.; Kaur, P.; Rao, P.; Linhares, I.M.; Skupski, D.; Witkin, S.S. Heat Shock Protein-Containing Exosomes in Mid-Trimester Amniotic Fluids. J. Reprod. Immunol. 2008, 79, 12–17. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, D.; Ma, X.; Wang, J.; Hou, W.; Zhang, W. Exosomes as Drug Carriers for Cancer Therapy and Challenges Regarding Exosome Uptake. Biomed. Pharmacother. 2020, 128, 110237. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-Encapsulated Paclitaxel to Overcome MDR in Cancer Cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Bagheri, E.; Abnous, K.; Farzad, S.A.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Targeted Doxorubicin-Loaded Mesenchymal Stem Cells-Derived Exosomes as a Versatile Platform for Fighting against Colorectal Cancer. Life Sci. 2020, 261, 118369. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Barua, H.T.; Zhang, Y.; Midge, S.B.; Nguyen, T.N.G.; Lee, B.-J.; Duan, W. Development of a Nanoamorphous Exosomal Delivery System as an Effective Biological Platform for Improved Encapsulation of Hydrophobic Drugs. Int. J. Pharm. 2019, 566, 697–707. [Google Scholar] [CrossRef]
- Hood, J.L. Post Isolation Modification of Exosomes for Nanomedicine Applications. Nanomedicine 2016, 11, 1745–1756. [Google Scholar] [CrossRef]
- Shany, S.; Bernheimer, A.W.; Grushoff, P.S.; Kim, K.-S. Evidence for Membrane Cholesterol as the Common Binding Site for Cereolysin, Streptolysin O and Saponin. Mol. Cell Biochem. 1974, 3, 179–186. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Tu, C.; Chen, X.; Yang, B.; Huo, Y.; Li, Y.; Chen, A.-Z.; Lan, P.; Zhang, Y.S.; et al. High-Throughput Single-Cell Analysis of Exosome Mediated Dual Drug Delivery, In Vivo Fate and Synergistic Tumor Therapy. Nanoscale 2020, 12, 13742–13756. [Google Scholar] [CrossRef]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. MiRNA in Plasma Exosome Is Stable under Different Storage Conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Walker, R.B.; Everette, J.D. Comparative Reaction Rates of Various Antioxidants with ABTS Radical Cation. J. Agric. Food Chem. 2009, 57, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Furger, C. Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts. Antioxidants 2021, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering Exosomes as Refined Biological Nanoplatforms for Drug Delivery. Acta Pharm. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Diniyah, N.; Alam, M.B.; Choi, H.J.; Lee, S.H. Lablab Purpureus Protects Hacat Cells from Oxidative Stress-Induced Cell Death through Nrf2-Mediated Heme Oxygenase-1 Expression via the Activation of P38 and Erk1/2. Int. J. Mol. Sci. 2020, 21, 8583. [Google Scholar] [CrossRef]
- Han, M.; Lee, D.; Lee, S.H.; Kim, T.H. Oxidative Stress and Antioxidant Pathway in Allergic Rhinitis. Antioxidants 2021, 10, 1266. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Jin, C.; Wu, S.; Lu, X.; Hu, X.; Sun, Y.; Cai, Y. The Effect of Nuclear Factor Erythroid 2-Related Factor/Antioxidant Response Element Signalling Pathway in the Lanthanum Chloride-Induced Impairment of Learning and Memory in Rats. J. Neurochem. 2017, 140, 463–475. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, J.M.; Kim, S.; Yoon, M.J.; Park, K.S. Chemical Transformation of Astaxanthin from Haematococcus Pluvialis Improves Its Antioxidative and Anti-Inflammatory Activities. ACS Omega 2020, 5, 19120–19130. [Google Scholar] [CrossRef]
- Dong, J.; Li, J.; Cui, L.; Wang, Y.; Lin, J.; Qu, Y.; Wang, H. Cortisol Modulates Inflammatory Responses in LPS-Stimulated RAW264.7 Cells via the NF-ΚB and MAPK Pathways. BMC Vet. Res. 2018, 14, 30. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.J.; Cha, B.S.; Kim, D.; Lee, E.S.; Kim, S.; Han, J.; Shin, J.; Kim, S.; Park, K.S. Extracellular Vesicles, as Drug-Delivery Vehicles, Improve the Biological Activities of Astaxanthin. Antioxidants 2023, 12, 473. https://doi.org/10.3390/antiox12020473
Jang YJ, Cha BS, Kim D, Lee ES, Kim S, Han J, Shin J, Kim S, Park KS. Extracellular Vesicles, as Drug-Delivery Vehicles, Improve the Biological Activities of Astaxanthin. Antioxidants. 2023; 12(2):473. https://doi.org/10.3390/antiox12020473
Chicago/Turabian StyleJang, Young Jun, Byung Seok Cha, Doyeon Kim, Eun Sung Lee, Seokjoon Kim, Jinjoo Han, Jiye Shin, Seokhwan Kim, and Ki Soo Park. 2023. "Extracellular Vesicles, as Drug-Delivery Vehicles, Improve the Biological Activities of Astaxanthin" Antioxidants 12, no. 2: 473. https://doi.org/10.3390/antiox12020473




