Application of Plant Growth-Promoting Bacteria from Cape Verde to Increase Maize Tolerance to Salinity
Abstract
1. Introduction
2. Materials and Methods
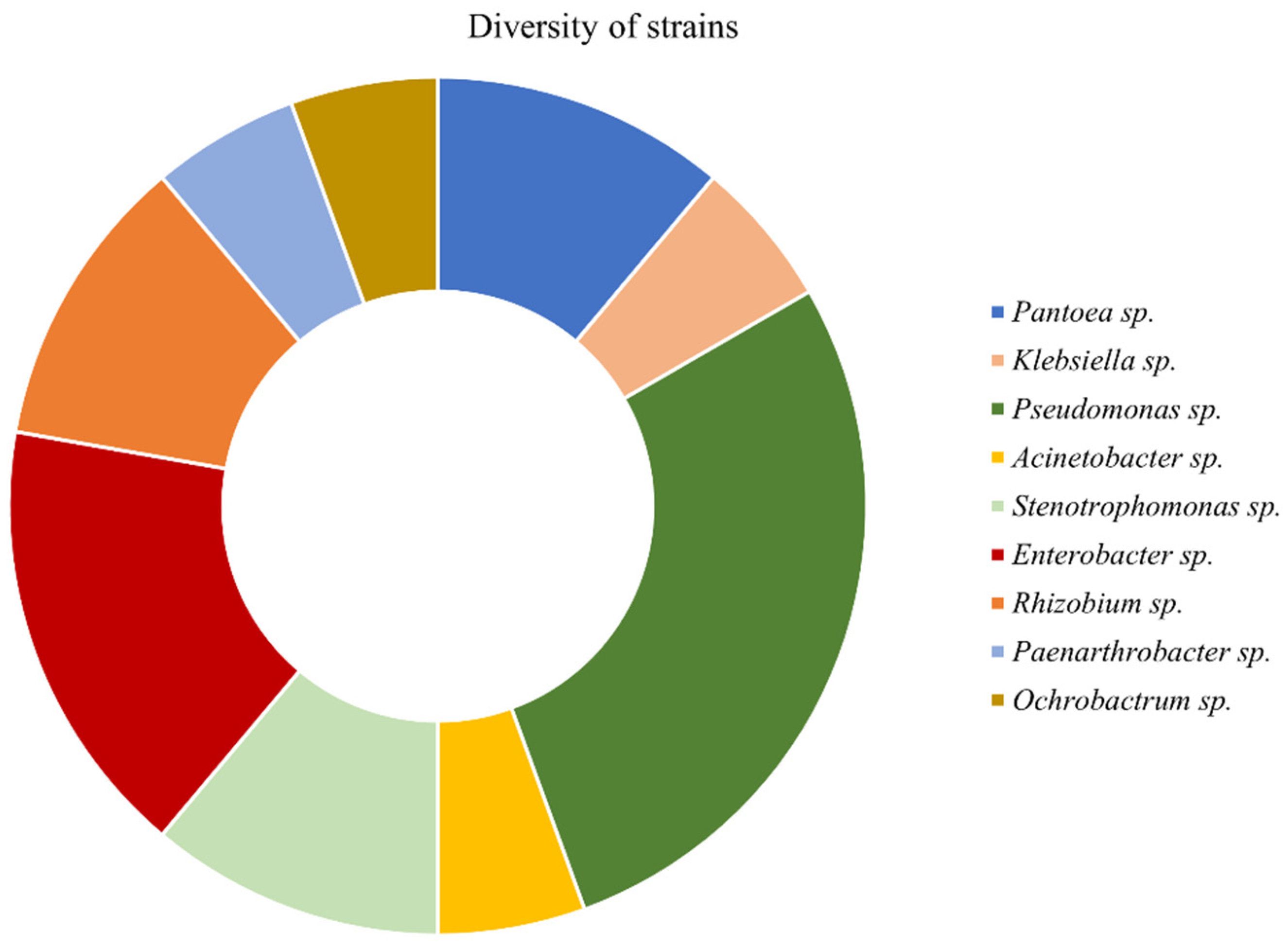
2.1. Bacterial Strains
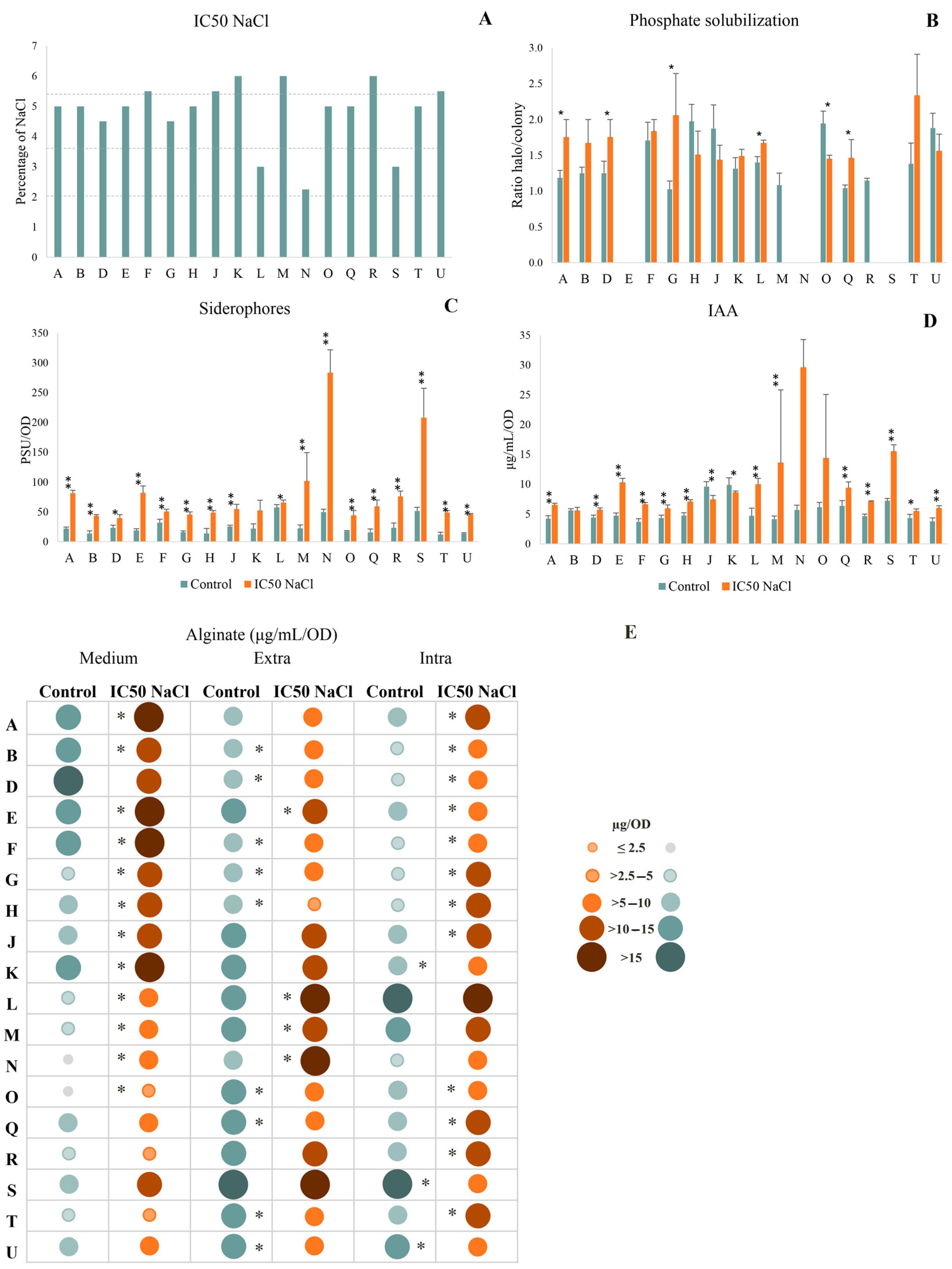
2.2. Bacterial Tolerance to Salinity
2.3. Plant Growth Promoting Abilities
2.3.1. Siderophore Production
2.3.2. Alginate Production
2.3.3. Indole Acetic Acid Production
2.3.4. Phosphate Solubilization
2.4. Plant Experiments
2.4.1. Zea mays Tolerance to NaCl
2.4.2. Greenhouse Experiment
2.4.3. Photosynthetic Pigments
2.5. Biochemical Analysis
2.5.1. Extraction
2.5.2. Lipid Peroxidation (LPO)
2.5.3. Superoxide Dismutase (SOD)
2.5.4. Catalase (CAT)
2.5.5. Glutathione S-Transferases (GST)
2.5.6. Protein
2.5.7. Protein Carbonylation
2.5.8. ETS
2.5.9. Soluble Sugars
2.5.10. Proline
2.6. Statistical Analysis
3. Results
3.1. Characterization of Strains and PGP Traits
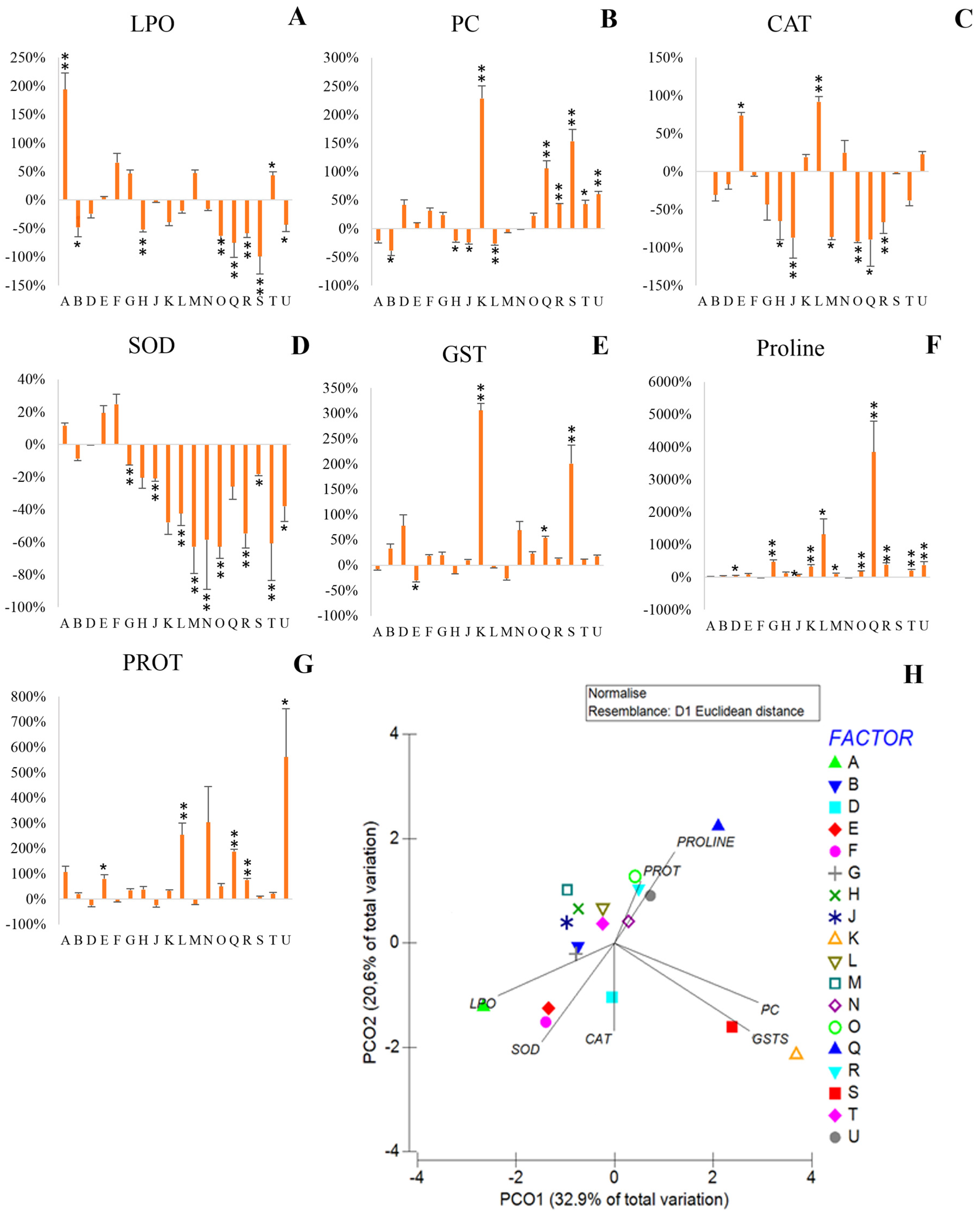
3.2. Biochemical Changes from Bacterial Exposure to NaCl
3.2.1. Cellular Damage
3.2.2. Antioxidant Response
3.2.3. Osmolyte Production-Proline
3.2.4. Protein Content
3.2.5. Multivariate Analysis
3.3. Response of Plants to Salinity
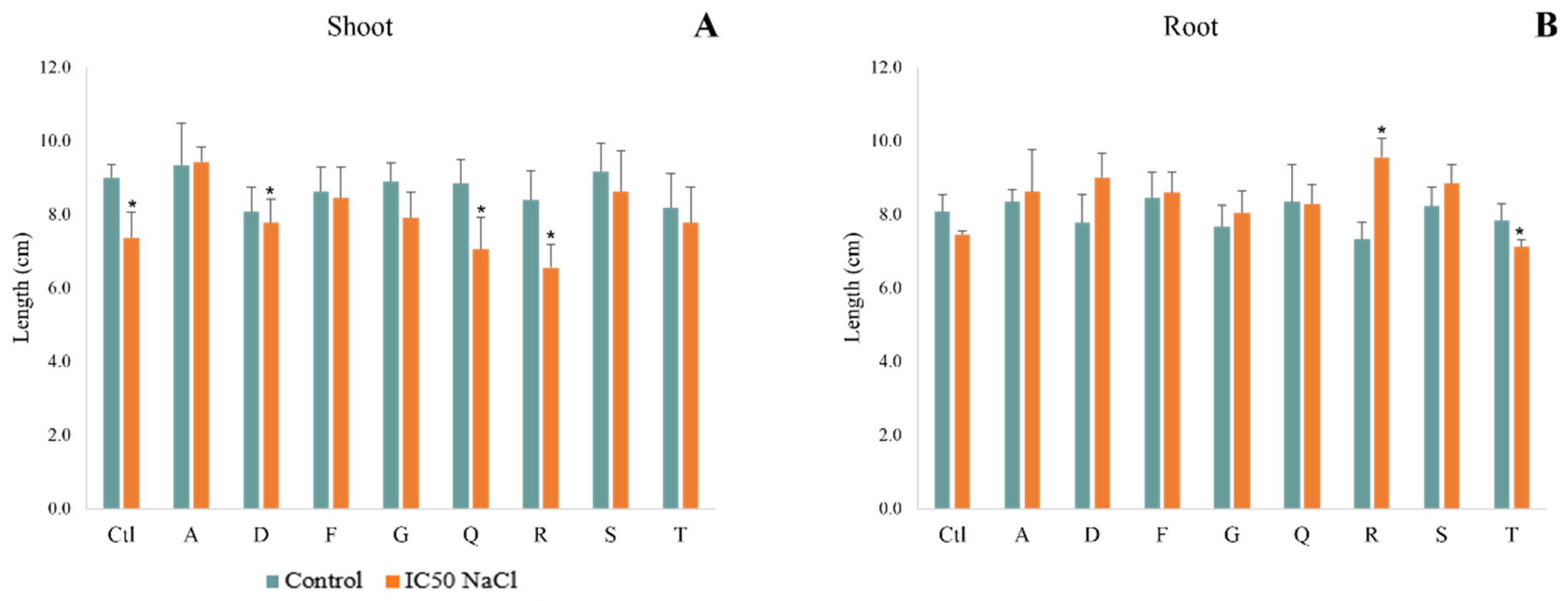
3.3.1. Biometric Parameters
3.3.2. Photosynthetic Pigments
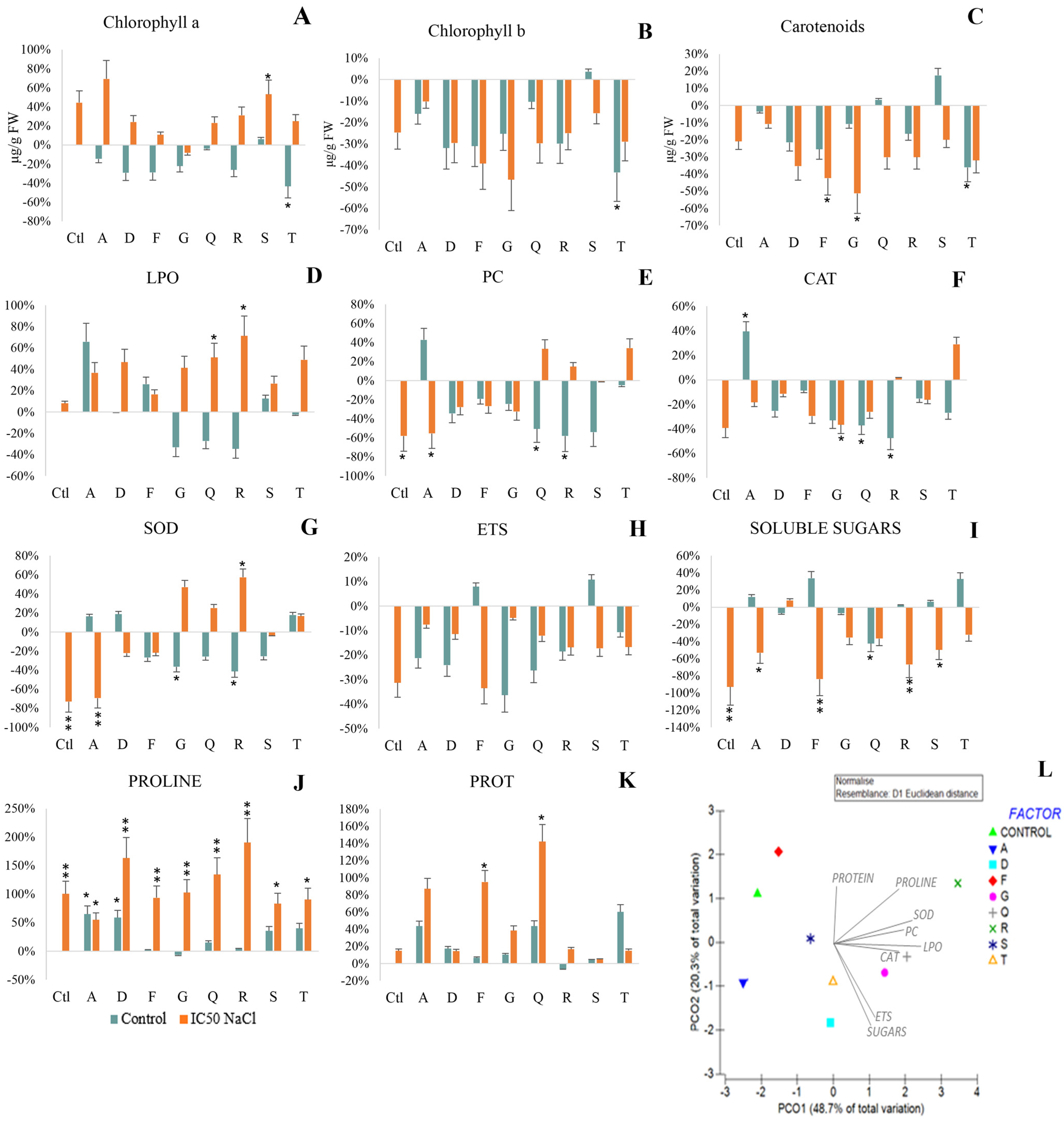
3.3.3. Cellular Damage
3.3.4. Antioxidant Response
3.3.5. Metabolism
3.3.6. Osmolyte Production
3.3.7. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar] [CrossRef]
- Akbari, M.; Alamdarlo, H.N.; Mosavi, S.H. The Effects of Climate Change and Groundwater Salinity on Farmers’ Income Risk. Ecol. Indic. 2020, 110, 105893. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate Change Impacts on Soil Salinity in Agricultural Areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil Salinity under Climate Change: Challenges for Sustainable Agriculture and Food Security. J. Environ. Manage. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Vengosh, A. Salinization and Saline Environments. In Treatise on Geochemistry; Lollar, B.S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2003; Volume 9, pp. 333–365. ISBN 9780080548074. [Google Scholar]
- George, E.; Horst, W.J.; Neumann, E. Adaptation of Plants to Adverse Chemical Soil Conditions. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 409–472. ISBN 9780123849052. [Google Scholar]
- de Souza Oliveira Filho, J.; Junior, C.R.P.; Pereira, M.G.; Valladares, G.S.; Camara, R. Sodification and Solodization Processes: Pedogenesis or Natural Soil Degradation? J. S. Am. Earth Sci. 2020, 104, 102909. [Google Scholar] [CrossRef]
- Ghassemi, F.; Jakeman, A.J.; Nix, H.A. Salinisation of Land and Water Resources: Human Causes, Extent, Management and Case Studies; CAB International: Wallingford, UK, 1995; ISBN 0851989. [Google Scholar]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of Salinity Stress on Plants and Its Tolerance Strategies: A Review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, M.R.; Chaabani, S.; Roula, R.; Muscolo, A. Bio-Priming Mitigates Detrimental Effects of Salinity on Maize Improving Antioxidant Defense and Preserving Photosynthetic Efficiency. Plant Physiol. Biochem. 2018, 132, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Xue, C.; Xiong, Y.; Meng, X.; Li, B.; Shen, R.; Lan, P. Proteomic Dissection of the Similar and Different Responses of Wheat to Drought, Salinity and Submergence during Seed Germination. J. Proteom. 2020, 220, 103756. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 17. [Google Scholar] [CrossRef]
- Bernstein, N. Plants and Salt: Plant Response and Adaptations to Salinity. In Model Ecosystems in Extreme Environments; Seckbach, J., Rampelotto, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 101–112. ISBN 9780128127421. [Google Scholar]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and Concepts in Quantifying Resistance to Drought, Salt and Freezing, Abiotic Stresses That Affect Plant Water Status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Sharma, S.; Verslues, P.E. Mechanisms Independent of Abscisic Acid (ABA) or Proline Feedback Have a Predominant Role in Transcriptional Regulation of Proline Metabolism during Low Water Potential and Stress Recovery. Plant Cell Environ. 2010, 33, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global Plant-Responding Mechanisms to Salt Stress: Physiological and Molecular Levels and Implications in Biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Sumithra, K.; Jutur, P.P.; Carmel, B.D.; Reddy, A.R. Salinity-Induced Changes in Two Cultivars of Vigna radiata: Responses of Antioxidative and Proline Metabolism. Plant Growth Regul. 2006, 50, 11–22. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A New Perspective of Phytohormones in Salinity Tolerance: Regulation of Proline Metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol 2017, 33, 197. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant Growth Promoting Bacteria in Agriculture: Two Sides of a Coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Abdelaal, K.; Alkahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The Role of Plant Growth-Promoting Bacteria in Alleviating the Adverse Effects of Drought on Plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Badri, D.V.; Weir, T.L.; van der Lelie, D.; Vivanco, J.M. Rhizosphere Chemical Dialogues: Plant–Microbe Interactions. Curr. Opin. Biotechnol. 2009, 20, 642–650. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced Systemic Resistance (ISR) in Plants: Mechanism of Action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef]
- Cardoso, P.; Freitas, R.; Figueira, E. Salt Tolerance of Rhizobial Populations from Contrasting Environmental Conditions: Understanding the Implications of Climate Change. Ecotoxicology 2015, 24, 143–152. [Google Scholar] [CrossRef]
- Cruz, C.; Cardoso, P.; Santos, J.; Matos, D.; Figueira, E. Bioprospecting Soil Bacteria from Arid Zones to Increase Plant Tolerance to Drought: Growth and Biochemical Status of Maize Inoculated with Plant Growth-Promoting Bacteria Isolated from Sal Island, Cape Verde. Plants 2022, 11, 2912. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified Microplate Method for Rapid and Efficient Estimation of Siderophore Produced by Bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.S.; O’Sullivan, E.; D’Aoust, L.N.; Omer, A.; Bonner-Weir, S.; Fisher, R.J.; Weir, G.C.; Colton, C.K. Quantitative Assessment of Islets of Langerhans Encapsulated in Alginate. Tissue Eng. Part C Methods 2011, 17, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.A.; Weber, R.P. Colorimetric Estimation od Indole Acetic Acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef]
- Lopes, T.; Cardoso, P.; Matos, D.; Rocha, R.; Pires, A.; Marques, P.; Figueira, E. Graphene Oxide Influence in Soil Bacteria Is Dose Dependent and Changes at Osmotic Stress: Growth Variation, Oxidative Damage, Antioxidant Response, and Plant Growth Promotion Traits of a Rhizobium Strain. Nanotoxicology 2022, 16, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Figueira, E.; Matos, D.; Cardoso, P.; Pires, A.; Fernandes, C.; Tauler, R.; Bedia, C. A Biochemical and Lipidomic Approach to Perceive Halimione portulacoides (L.) Response to Mercury: An Environmental Perspective. Mar. Pollut. Bull. 2023, 186, 114393. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Johansson, L.H.; Håkan Borg, L.A. A Spectrophotometric Method for Determination of Catalase Activity in Small Tissue Samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- William, H.H.; Michael, J.P.; William, B.J. Glutathione S-Transferases: The First Enzymatic StepP in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Robinson, H.W.; Hogden, C.G.; Co-investigator, N. The Biuret Reaction in the Determination of Serum Proteins. I. A Study of the Conditions Necessary for the Production of a Stable Colour Which Bears a Quantitative Relationship to the Protein Concentration. J. Biol. Chem. 1940, 135, 707–725. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-Dinitrophenylhydrazine Spectrophotometric Assay for Quantification of Carbonyls in Oxidized Proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Udechukwu, M.C.; Yiridoe, C.; Gibson, A.; Gong, M. Antioxidant Mechanism of Potato Protein Hydrolysates against in Vitro Oxidation of Reduced Glutathione. J. Funct. Foods 2016, 20, 195–203. [Google Scholar] [CrossRef]
- King, F.D.; Packard, T.T. Respiration and the Activity of the Respiratory Electron Transport System in Marine Zooplankton1. Limnol. Oceanogr. 1975, 20, 849–854. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Anderson, M.; Gorley, R.N.; Clarke, K. PERMANOVA+ for Primer: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Fukami, J.; de la Osa, C.; Ollero, F.J.; Megías, M.; Hungria, M. Co-Inoculation of Maize with Azospirillum brasilense and Rhizobium tropici as a Strategy to Mitigate Salinity Stress. Funct. Plant Biol. 2017, 45, 328–339. [Google Scholar] [CrossRef]
- Li, H.Q.; Jiang, X.W. Inoculation with Plant Growth-Promoting Bacteria (PGPB) Improves Salt Tolerance of Maize Seedling. Russ. J. Plant Physiol. 2017, 64, 235–241. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of Inoculation with Plant Growth-Promoting Bacteria (PGPB) on Amelioration of Saline Stress in Maize (Zea Mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining Halophytes for Plant Growth-Promoting Halotolerant Bacteria to Enhance the Salinity Tolerance of Non-Halophytic Crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Glick, B.R. Halotolerant Plant Growth–Promoting Bacteria: Prospects for Alleviating Salinity Stress in Plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Nabti, E.; Schmid, M.; Hartmann, A. Application of Halotolerant Bacteria to Restore Plant Growth under Salt Stress. In Halophiles: Biodiversity and Sustainable Exploitation; Maheshwari, D.K., Saraf, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 235–259. ISBN 9783319145952. [Google Scholar]
- Shilev, S. Plant-Growth-Promoting Bacteria Mitigating Soil Salinity Stress in Plants. Appl. Sci. 2020, 10, 7326. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.M.; Kim, K.M.; Lee, I.J. Halotolerant Bacteria Mitigate the Effects of Salinity Stress on Soybean Growth by Regulating Secondary Metabolites and Molecular Responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.; Rai, S.; Ilyas, N.; Sayyed, R.Z.; Al-Turki, A.I.; El Enshasy, H.A.; Simarmata, T. Halotolerant Rhizobacteria for Salinity-Stress Mitigation: Diversity, Mechanisms and Molecular Approaches. Sustainability 2022, 14, 490. [Google Scholar] [CrossRef]
- Tank, N.; Saraf, M. Salinity-Resistant Plant Growth Promoting Rhizobacteria Ameliorates Sodium Chloride Stress on Tomato Plants. J. Plant Interact. 2010, 5, 51–58. [Google Scholar] [CrossRef]
- Albdaiwi, R.N.; Khyami-Horani, H.; Ayad, J.Y.; Alananbeh, K.M.; Al-Sayaydeh, R. Isolation and Characterization of Halotolerant Plant Growth Promoting Rhizobacteria from Durum Wheat (Triticum turgidum subsp. durum) Cultivated in Saline Areas of the Dead Sea Region. Oxid. Med. Cell. Longev. 2020, 10, 1639. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of High Salinity Stress Damage by Plant Growth-Promoting Bacterial Endophytes That Contain ACC Deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alzahrani, S.M.; Ali, H.M.; Alayafi, A.A.; Ahmad, M. Serratia liquefaciens KM4 Improves Salt Stress Tolerance in Maize by Regulating Redox Potential, Ion Homeostasis, Leaf Gas Exchange and Stress-Related Gene Expression. Int. J. Mol. Sci. 2018, 19, 3310. [Google Scholar] [CrossRef]
- Fatima, T.; Mishra, I.; Verma, R.; Arora, N.K. Mechanisms of Halotolerant Plant Growth Promoting Alcaligenes sp. Involved in Salt Tolerance and Enhancement of the Growth of Rice under Salinity Stress. 3 Biotech 2020, 10, 361. [Google Scholar] [CrossRef]
- Ahemad, M.; Zaidi, A.; Khan, M.S.; Oves, M. Biological Importance of Phosphorus and Phosphate Solubilizing Microbes—An Overview. In Phosphate Solubilizing Microbes for Crop Improvement; Khan, M., Zaidi, A., Eds.; Nova Science Publishers: Happauge, NY, USA, 2009; pp. 1–14. ISBN 9781617285615. [Google Scholar]
- Dey, G.; Banerjee, P.; Sharma, R.K.; Maity, J.P.; Etesami, H.; Shaw, A.K.; Huang, Y.H.; Huang, H.B.; Chen, C.Y. Management of Phosphorus in Salinity-Stressed Agriculture for Sustainable Crop Production by Salt-Tolerant Phosphate-Solubilizing Bacteria—A Review. Agronomy 2021, 11, 1552. [Google Scholar] [CrossRef]
- Sá, C.; Cardoso, P.; Figueira, E. Alginate as a Feature of Osmotolerance Differentiation among Soil Bacteria Isolated from Wild Legumes Growing in Portugal. Sci. Total Environ. 2019, 681, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Van De Mortel, M.; Nielsen, L.; De Guzman, G.N.; Li, X.; Halverson, L.J. Alginate Production by Pseudomonas putida Creates a Hydrated Microenvironment and Contributes to Biofilm Architecture and Stress Tolerance under Water-Limiting Conditions. J. Bacteriol. 2007, 189, 8290–8299. [Google Scholar] [CrossRef]
- Freeman, B.C.; Chen, C.; Yu, X.; Nielsen, L.; Peterson, K.; Beattie, G.A. Physiological and Transcriptional Responses to Osmotic Stress of Two Pseudomonas syringae Strains That Differ in Epiphytic Fitness and Osmotolerance. J. Bacteriol. 2013, 195, 4742–4752. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.H.A.; Alkhalifah, D.H.M.; Al Yousef, S.A.; Beemster, G.T.S.; Mousa, A.S.M.; Hozzein, W.N.; AbdElgawad, H. Salinity Stress Enhances the Antioxidant Capacity of Bacillus and Planococcus Species Isolated From Saline Lake Environment. Front. Microbiol. 2020, 11, 561816. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Prášil, I.T.; Vítámvás, P. Protein Contribution to Plant Salinity Response and Tolerance Acquisition. Int. J. Mol. Sci 2013, 14, 6757–6789. [Google Scholar] [CrossRef]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R. Abbas Shoukat, Maqsood Ul Hussan, Muhammad Ishtiaq Sarwar. A Review: Impact of Salinity on Plant Growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar] [CrossRef]
- Azeem, M.; Haider, M.Z.; Javed, S.; Saleem, M.H.; Alatawi, A. Drought Stress Amelioration in Maize (Zea mays L.) by Inoculation of Bacillus spp. Strains under Sterile Soil Conditions. Agriculture 2022, 12, 50. [Google Scholar] [CrossRef]
- Gupta, A.; Rai, S.; Bano, A.; Khanam, A.; Sharma, S.; Pathak, N. Comparative Evaluation of Different Salt-Tolerant Plant Growth-Promoting Bacterial Isolates in Mitigating the Induced Adverse Effect of Salinity in Pisum sativum. Biointerface Res. Appl. Chem. 2021, 11, 13141–13154. [Google Scholar] [CrossRef]
- Hmaeid, N.; Wali, M.; Metoui-Ben Mahmoud, O.; Pueyo, J.J.; Ghnaya, T.; Abdelly, C. Efficient Rhizobacteria Promote Growth and Alleviate NaCl-Induced Stress in the Plant Species Sulla Carnosa. Appl. Soil Ecol. 2019, 133, 104–113. [Google Scholar] [CrossRef]
- Khan, M.A.; Hamayun, M.; Asaf, S.; Khan, M.; Yun, B.W.; Kang, S.M.; Lee, I.J. Rhizospheric Bacillus spp. Rescues Plant Growth Under Salinity Stress via Regulating Gene Expression, Endogenous Hormones, and Antioxidant System of Oryza sativa L. Front. Plant Sci. 2021, 12, 665590. [Google Scholar] [CrossRef] [PubMed]
- Germ, M.; Kreft, I.; Stibilj, V.; Urbanc-Berčič, O. Combined Effects of Selenium and Drought on Photosynthesis and Mitochondrial Respiration in Potato. Plant Physiol. Biochem. 2007, 45, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Pastore, D.; Trono, D.; Laus, M.N.; Fonzo, N.D.; Flagella, Z. Possible Plant Mitochondria Involvement in Cell Adaptation to Drought Stress A Case Study: Durum Wheat Mitochondria. J. Exp. Bot. 2007, 58, 195–210. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum Lipoferum FK1 Confers Improved Salt Tolerance in Chickpea (Cicer arietinum L.) by Modulating Osmolytes, Antioxidant Machinery and Stress-Related Genes Expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-Tolerant Plant Growth-Promoting Bacillus pumilus Strain JPVS11 to Enhance Plant Growth Attributes of Rice and Improve Soil Health under Salinity Stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef] [PubMed]
- Zilaie, M.N.; Arani, A.M.; Etessami, H.; Dinarvand, M.; Dolati, A. Halotolerant Plant Growth-Promoting Rhizobacteria-Mediated Alleviation of Salinity and Dust Stress and Improvement of Forage Yield in the Desert Halophyte Seidlitzia rosmarinus. Environ. Exp. Bot. 2022, 201, 104952. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S.; Sengupta, D.N. Amelioration of Salinity Stress by Exogenously Applied Spermidine or Spermine in Three Varieties of Indica Rice Differing in Their Level of Salt Tolerance. J. Plant Physiol. 2011, 168, 317–328. [Google Scholar] [CrossRef]
- Baena, G.; Feria, A.B.; Echevarría, C.; Monreal, J.A.; García-Mauriño, S. Salinity Promotes Opposite Patterns of Carbonylation and Nitrosylation of C4 Phosphoenolpyruvate Carboxylase in Sorghum Leaves. Planta 2017, 246, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Kaur, N. Sugar Signalling and Gene Expression in Relation to Carbohydrate Metabolism under Abiotic Stresses in Plants. J. Bioc. 2005, 30, 761–776. [Google Scholar] [CrossRef]
- Dubey, R.S.; Singh, A.K. Salinity Induces Accumulation of Soluble Sugars and Alters the Activity of Sugar Metabolising Enzymes in Rice Plants. Biol. Plant. 1999, 42, 233–239. [Google Scholar] [CrossRef]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Plant Growth-Promoting Rhizobacteria Ameliorates Salinity Stress in Pea (Pisum sativum). J. Plant Growth Regul. 2022, 41, 647–656. [Google Scholar] [CrossRef]
- Doğan, M. Antioxidative and Proline Potentials as a Protective Mechanism in Soybean Plants under Salinity Stress. African J. Biotechnol. 2011, 10, 5972–5978. [Google Scholar] [CrossRef]
- Heuer, B. Role of Proline in Plant Response to Drought and Salinity. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 213–238. ISBN 9781439813997. [Google Scholar]
- Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; El Enshasy, H.; Hanapi, S.Z.; Ilyas, N.; Elgorban, A.M.; Bahkali, A.H.; Marraiki, N. Inoculation of Klebsiella variicola Alleviated Salt Stress and Improved Growth and Nutrients in Wheat and Maize. Agronomy 2021, 11, 927. [Google Scholar] [CrossRef]
- Prittesh, P.; Avnika, P.; Kinjal, P.; Jinal, H.N.; Sakthivel, K.; Amaresan, N. Amelioration Effect of Salt-Tolerant Plant Growth-Promoting Bacteria on Growth and Physiological Properties of Rice (Oryza ativa) under Salt-Stressed Conditions. Arch. Microbiol. 2020, 202, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Maurya, S.K.; Singh, D.P. Salinity Tolerance in Free Living Plant Growth Promoting Rhizobacteria. Indian J. Sci. Res 2012, 3, 73–78. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, C.; Cardoso, P.; Santos, J.; Matos, D.; Sá, C.; Figueira, E. Application of Plant Growth-Promoting Bacteria from Cape Verde to Increase Maize Tolerance to Salinity. Antioxidants 2023, 12, 488. https://doi.org/10.3390/antiox12020488
Cruz C, Cardoso P, Santos J, Matos D, Sá C, Figueira E. Application of Plant Growth-Promoting Bacteria from Cape Verde to Increase Maize Tolerance to Salinity. Antioxidants. 2023; 12(2):488. https://doi.org/10.3390/antiox12020488
Chicago/Turabian StyleCruz, Catarina, Paulo Cardoso, Jacinta Santos, Diana Matos, Carina Sá, and Etelvina Figueira. 2023. "Application of Plant Growth-Promoting Bacteria from Cape Verde to Increase Maize Tolerance to Salinity" Antioxidants 12, no. 2: 488. https://doi.org/10.3390/antiox12020488
APA StyleCruz, C., Cardoso, P., Santos, J., Matos, D., Sá, C., & Figueira, E. (2023). Application of Plant Growth-Promoting Bacteria from Cape Verde to Increase Maize Tolerance to Salinity. Antioxidants, 12(2), 488. https://doi.org/10.3390/antiox12020488