Co-Targeting of BTK and TrxR as a Therapeutic Approach to the Treatment of Lymphoma
Abstract
:1. Introduction
2. Method and Materials
2.1. Cells and Reagents
2.2. Compounds Preparation
2.3. Cell Proliferation Assay
2.4. Isobologram Analysis
2.5. TrxR Activity Assay
2.6. Caspase-3 Activity Assay
2.7. Transient Transfections
2.8. Reverse Transcriptase-Quantitative PCR (RT-qPCR)
2.9. Western Blot Analysis
2.10. Bioinformatics
2.11. Human Protein Atlas
2.12. Statistical Analysis
3. Results
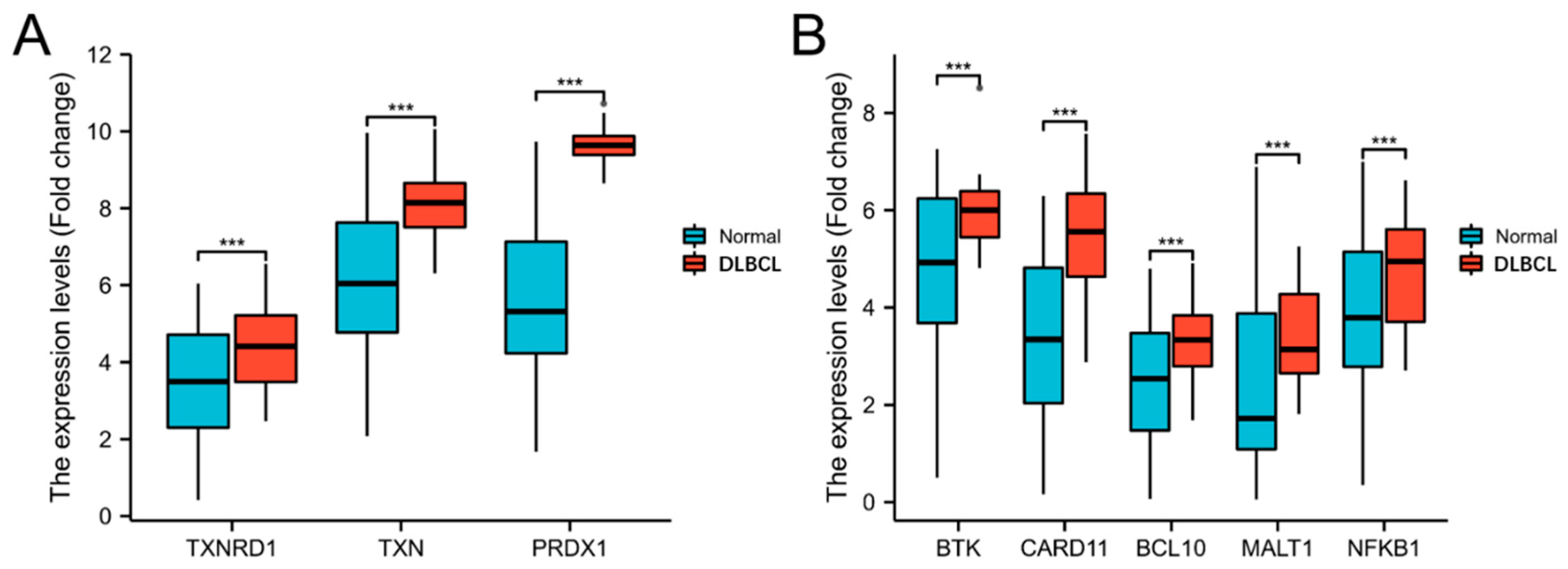
3.1. The Trx System and the BCR Signalling Pathway-Related Genes Are Overexpressed in DLBLC
3.2. The Gene Expression of TrxR Is Correlated with BCR Signalling Pathway-Related Gene Expression
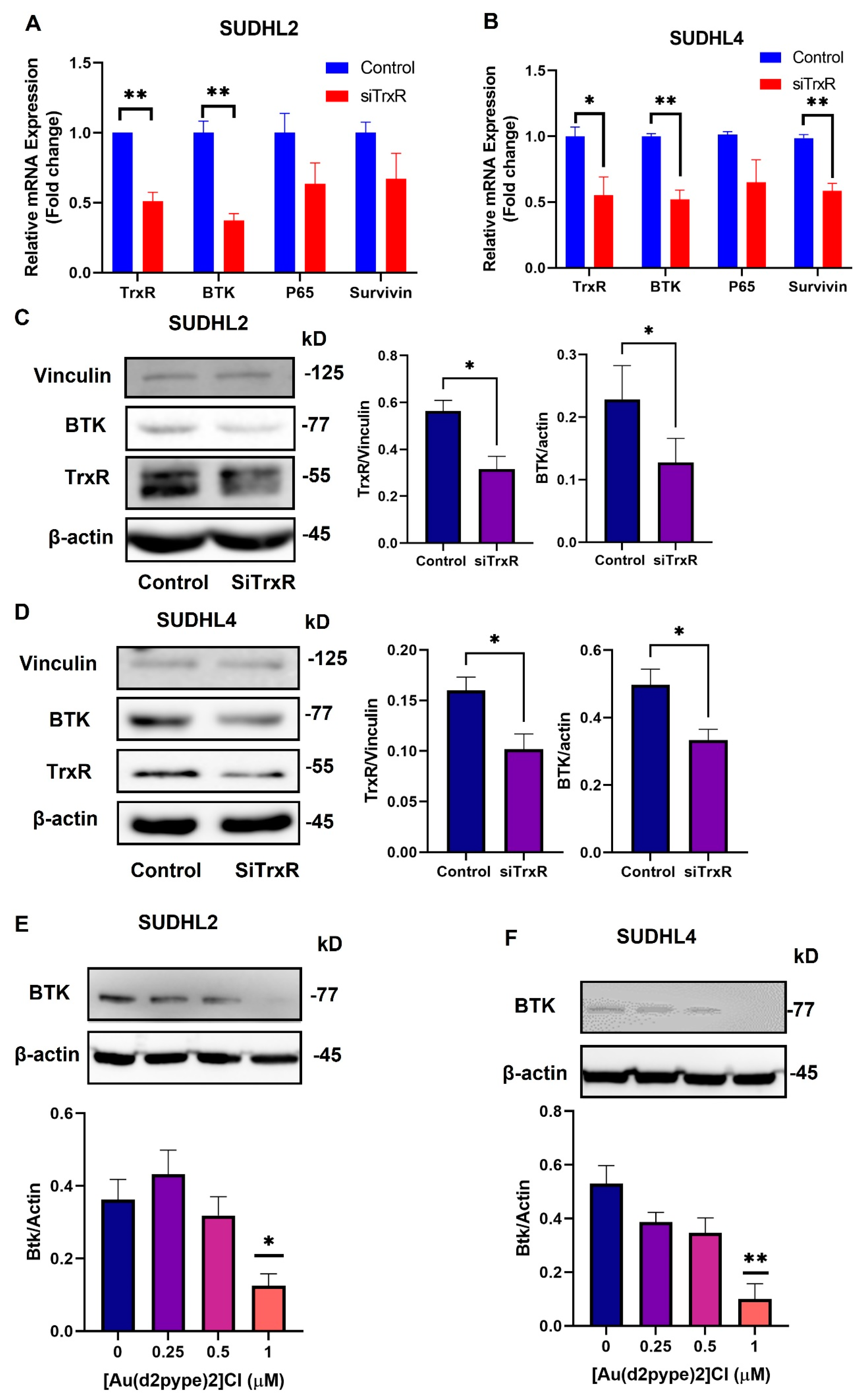
3.3. Knockdown of TrxR1 in Lymphoma Cells Decreased the Expression of BTK and Affected Its Downstream Signalling Pathway
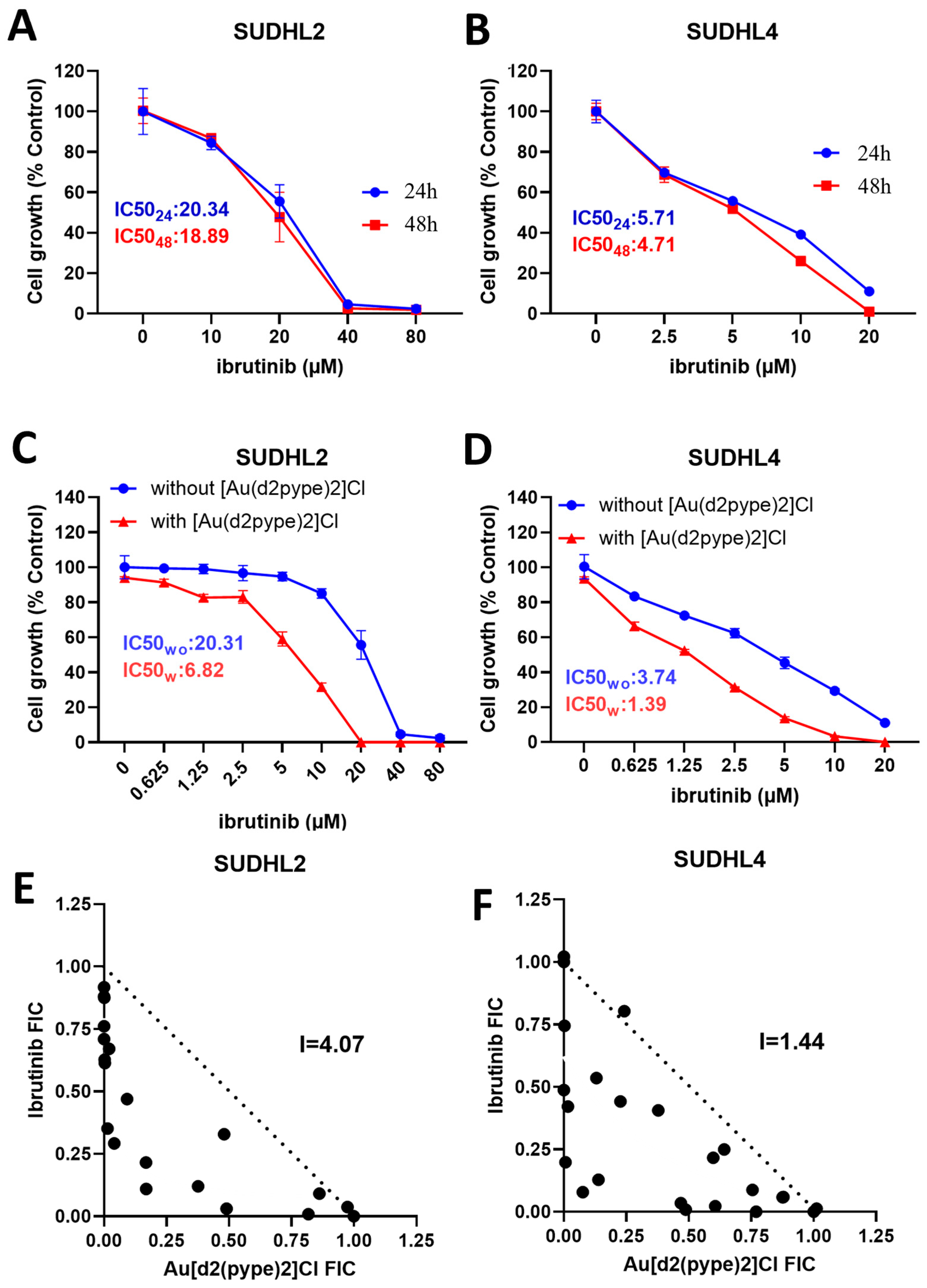
3.4. [Au(d2pype)2]Cl/Ibrutinib Cotreatment Synergistically Induced Cell Death in Lymphoma Cells
3.5. Ibrutinib/[Au(d2pype)2]Cl Cotreatment Induced Cell Death via Apoptosis and Ferroptosis in Lymphoma Cells
3.6. The [Au(d2pype)2]Cl/Ibrutinib Treatment Decreased the Total Expression of BTK in Non-Hodgkin’s Lymphoma Cell Lines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goparaju, K.; Caimi, P.F. Loncastuximab tesirine for treatment of relapsed or refractory diffuse large B cell lymphoma. Expert Opin. Biol. Ther. 2021, 21, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- He, M.Y.; Kridel, R. Treatment resistance in diffuse large B-cell lymphoma. Leukemia 2021, 35, 2151–2165. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Miyoshi, H.; Yamauchi, T.; Arakawa, F.; Kawano, R.; Muta, H.; Sugita, Y.; Akashi, K.; Ohshima, K. Composite lymphoma of peripheral T-cell lymphoma and Hodgkin lymphoma, mixed cellularity type; pathological and molecular analysis. Pathol. Int. 2017, 67, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.; Au-Yeung, R.; Chau, D.; Hwang, Y.-Y.; Loong, F.; Kwong, Y.-L. Epstein-Barr virus–positive diffuse large B-cell lymphoma after frontline brentuximab vedotin treatment of classical Hodgkin lymphoma. Ann. Hematol. 2022, 101, 1149–1152. [Google Scholar] [CrossRef]
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Blenk, S.; Engelmann, J.C.; Weniger, M.; Schultz, J.; Dittrich, M.; Rosenwald, A.; Müller-Hermelink, H.; Müller, T.; Dandekar, T. Germinal Center B Cell-Like (GCB) and Activated B Cell-Like (ABC) Type of Diffuse Large B Cell Lymphoma (DLBCL): Analysis of Molecular Predictors, Signatures, Cell Cycle State and Patient Survival. Cancer Inform. 2007, 3, 399–420. [Google Scholar] [CrossRef]
- Painschab, M.S.; Kohler, R.; Kimani, S.; Mhango, W.; Kaimila, B.; Zuze, T.; Mithi, V.; Kasonkanji, E.; Mumba, N.; Nyasosela, R.; et al. Comparison of best supportive care, CHOP, or R-CHOP for treatment of diffuse large B-cell lymphoma in Malawi: A cost-effectiveness analysis. Lancet Glob. Health 2021, 9, e1305–e1313. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Seto, A.G.; Beatty, X.; Hermreck, M.; Gilles, M.-E.; Stroopinsky, D.; Pinter-Brown, L.C.; Pestano, L.; Marchese, C.; Avigan, D.; et al. Cobomarsen, an Oligonucleotide Inhibitor of miR-155, Slows DLBCL Tumor Cell Growth In Vitro and In Vivo. Clin. Cancer Res. 2021, 27, 1139–1149. [Google Scholar] [CrossRef]
- Hara, T.; Yoshikawa, T.; Goto, H.; Sawada, M.; Yamada, T.; Fukuno, K.; Kasahara, S.; Shibata, Y.; Matsumoto, T.; Mabuchi, R.; et al. R-THP-COP versus R-CHOP in patients younger than 70 years with untreated diffuse large B cell lymphoma: A randomized, open-label, noninferiority phase 3 trial. Hematol. Oncol. 2018, 36, 638–644. [Google Scholar] [CrossRef]
- Küppers, R. Mechanisms of B-cell lymphoma pathogenesis. Nat. Rev. Cancer 2005, 5, 251–262. [Google Scholar] [CrossRef]
- Tanaka, S.; Baba, Y. Baba, Y. B Cell Receptor Signaling. In B Cells in Immunity and Tolerance; Wang, J.-Y., Ed.; Springer: Singapore, 2020; Volume 1254, pp. 23–36. [Google Scholar] [CrossRef]
- Xue, C.; Wang, X.; Zhang, L.; Qu, Q.; Zhang, Q.; Jiang, Y. Ibrutinib in B-cell lymphoma: Single fighter might be enough? Cancer Cell Int. 2020, 20, 467. [Google Scholar] [CrossRef] [PubMed]
- Efremov, D.G.; Turkalj, S.; Laurenti, L. Mechanisms of B Cell Receptor Activation and Responses to B Cell Receptor Inhibitors in B Cell Malignancies. Cancers 2020, 12, 1396. [Google Scholar] [CrossRef]
- Xu, W.; Berning, P.; Lenz, G. Targeting B-cell receptor and PI3K signaling in diffuse large B-cell lymphoma. Blood 2021, 138, 1110–1119. [Google Scholar] [CrossRef]
- Pontoriero, M.; Fiume, G.; Vecchio, E.; de Laurentiis, A.; Albano, F.; Iaccino, E.; Mimmi, S.; Pisano, A.; Agosti, V.; Giovannone, E.; et al. Activation of NF-κB in B cell receptor signaling through Bruton’s tyrosine kinase-dependent phosphorylation of IκB-α. J. Mol. Med. 2019, 97, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Rai, K.; Shukla, V.; Chaturvedi, N.K.; Bociek, R.G.; Pirruccello, S.J.; Band, H.; Lu, R.; Joshi, S.S. Sprouty 2: A novel attenuator of B-cell receptor and MAPK-Erk signaling in CLL. Blood 2016, 127, 2310–2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, P.G.; Laurenti, L.; Gobessi, S.; Sica, S.; Leone, G.; Efremov, D. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood 2008, 111, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.J.; Yu, L.; Bäckesjö, C.-M.; Vargas, L.; Faryal, R.; Aints, A.; Christensson, B.; Berglöf, A.; Vihinen, M.; Nore, B.F.; et al. Bruton’s tyrosine kinase (Btk): Function, regulation, and transformation with special emphasis on the PH domain. Immunol. Rev. 2009, 228, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Cameron, F.; Sanford, M. Ibrutinib: First Global Approval. Drugs 2014, 74, 263–271. [Google Scholar] [CrossRef]
- Dubovsky, J.A.; Beckwith, K.A.; Woyach, J.A.; Jaglowski, S.M.; Hessler, J.; Chang, B.Y.; Larkin, K.; Stefanovski, M.R.; Frissora, F.W.; Smith, L.L.; et al. Ibrutinib Is an Irreversible Molecular Inhibitor of Interleukin-2 Inducible Kinase: Expanding Therapeutic Potential and Modulating a Th1 Selective Pressure in CD4 T-Cells. Blood 2012, 120, 775. [Google Scholar] [CrossRef]
- McMullen, J.R.; Boey, E.J.H.; Ooi, J.; Seymour, J.F.; Keating, M.J.; Tam, C.S. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood 2014, 124, 3829–3830. [Google Scholar] [CrossRef] [Green Version]
- Paydas, S. Management of adverse effects/toxicity of ibrutinib. Crit. Rev. Oncol. Hematol. 2019, 136, 56–63. [Google Scholar] [CrossRef]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzawa, A. Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate. Arch. Biochem. Biophys. 2017, 617, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Muri, J.; Thut, H.; Feng, Q.; Kopf, M. Thioredoxin-1 distinctly promotes NF-κB target DNA binding and NLRP3 inflammasome activation independently of Txnip. eLife 2020, 9, e53627. [Google Scholar] [CrossRef]
- Wang, S.; Di Trapani, G.; Tonissen, K.F. Expanding the armory for treating lymphoma: Targeting redox cellular status through thioredoxin reductase inhibition. Pharmacol. Res. 2022, 177, 106134. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Sanda, T.; Asamitsu, K. NF-κB Signaling and Carcinogenesis. Curr. Pharm. Des. 2007, 13, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Stafford, W.C.; Peng, X.; Olofsson, M.H.; Zhang, X.; Luci, D.K.; Lu, L.; Cheng, Q.; Trésaugues, L.; Dexheimer, T.S.; Coussens, N.P.; et al. Irreversible inhibition of cytosolic thioredoxin reductase 1 as a mechanistic basis for anticancer therapy. Sci. Transl. Med. 2018, 10, eaaf7444. [Google Scholar] [CrossRef] [Green Version]
- Sze, J.H.; Raninga, P.V.; Nakamura, K.; Casey, M.; Khanna, K.K.; Berners-Price, S.J.; Di Trapani, G.; Tonissen, K.F. Anticancer activity of a Gold(I) phosphine thioredoxin reductase inhibitor in multiple myeloma. Redox Biol. 2020, 28, 101310. [Google Scholar] [CrossRef]
- Arnér, E.S. Perspectives of TrxR1-based cancer therapies. In Oxidative Stress; Sies, H., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 639–667. [Google Scholar] [CrossRef]
- Zhang, X.; Selvaraju, K.; Saei, A.A.; D’Arcy, P.; Zubarev, R.A.; Arnér, E.S.; Linder, S. Repurposing of auranofin: Thioredoxin reductase remains a primary target of the drug. Biochimie 2019, 162, 46–54. [Google Scholar] [CrossRef]
- Saei, A.A.; Gullberg, H.; Sabatier, P.; Beusch, C.M.; Johansson, K.; Lundgren, B.; Arvidsson, P.I.; Arnér, E.S.; Zubarev, R.A. Comprehensive chemical proteomics for target deconvolution of the redox active drug auranofin. Redox Biol. 2020, 32, 101491. [Google Scholar] [CrossRef]
- Berners-Price, S.J.; Filipovska, A. Gold compounds as therapeutic agents for human diseases. Metallomics 2011, 3, 863–873. [Google Scholar] [CrossRef]
- Rackham, O.; Shearwood, A.-M.J.; Thyer, R.; McNamara, E.; Davies, S.M.; Callus, B.A.; Miranda-Vizuete, A.; Berners-Price, S.J.; Cheng, Q.; Arnér, E.S.; et al. Substrate and inhibitor specificities differ between human cytosolic and mitochondrial thioredoxin reductases: Implications for development of specific inhibitors. Free Radic. Biol. Med. 2011, 50, 689–699. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Y.; Woods, K.; Di Trapani, G.; Tonissen, K.F. Investigating the Thioredoxin and Glutathione Systems’ Response in Lymphoma Cells after Treatment with [Au(d2pype)2]CL. Antioxidants 2021, 10, 104. [Google Scholar] [CrossRef]
- Clapper, E.; Wang, S.; Raninga, P.V.; Di Trapani, G.; Tonissen, K.F. Cross-talk between Bcr-abl and the Thioredoxin System in Chronic Myeloid Leukaemia: Implications for CML Treatment. Antioxidants 2020, 9, 207. [Google Scholar] [CrossRef]
- Berners-Price, S.J.; Bowen, R.J.; Hambley, T.W.; Healy, P.C. NMR and structural studies of gold(I) chloride adducts with bidentate 2-, 3- and 4-pyridyl phosphines. J. Chem. Soc. Dalton Trans. 1999, 8, 1337–1346. [Google Scholar] [CrossRef]
- Hall, M.J.; Middleton, R.F.; Westmacott, D. The fractional inhibitory concentration (FIC) index as a measure of synergy. J. Antimicrob. Chemother. 1983, 11, 427–433. [Google Scholar] [CrossRef]
- Nair, H.B.; Sung, B.; Yadav, V.R.; Kannappan, R.; Chaturvedi, M.M.; Aggarwal, B.B. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem. Pharmacol. 2010, 80, 1833–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafleur, M.A.; Drew, A.F.; de Sousa, E.L.; Blick, T.; Bills, M.; Walker, E.C.; Williams, E.D.; Waltham, M.; Thompson, E.W. Upregulation of matrix metalloproteinases (MMPs) in breast cancer xenografts: A major induction of stromal MMP-13. Int. J. Cancer 2005, 114, 544–554. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Matute, J.; Fernandez-Garcia, C.-E.; Blanco-Colio, L.M.; Burillo, E.; Fortuño, A.; Martinez-Pinna, R.; Llamas-Granda, P.; Beloqui, O.; Egido, J.; Zalba, G.; et al. Thioredoxin-1/peroxiredoxin-1 as sensors of oxidative stress mediated by NADPH oxidase activity in atherosclerosis. Free Radic. Biol. Med. 2015, 86, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, G.W.; Kwon, S.H. The HDAC6-selective inhibitor is effective against non-Hodgkin lymphoma and synergizes with ibrutinib in follicular lymphoma. Mol. Carcinog. 2019, 58, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-P.; Ezell, S.A.; Schweighofer, K.J.; Cheung, L.W.; Hsieh, S.; Apatira, M.; Sirisawad, M.; Eckert, K.; Hsu, S.J.; Chen, C.-T.; et al. Combination of Ibrutinib and ABT-199 in Diffuse Large B-Cell Lymphoma and Follicular Lymphoma. Mol. Cancer Ther. 2017, 16, 1246–1256. [Google Scholar] [CrossRef] [Green Version]
- Ding, N.; Li, X.; Shi, Y.; Ping, L.; Wu, L.; Fu, K.; Feng, L.; Zheng, X.; Song, Y.; Pan, Z.; et al. Irreversible dual inhibitory mode: The novel Btk inhibitor PLS-123 demonstrates promising anti-tumor activity in human B-cell lymphoma. Oncotarget 2015, 6, 15122–15136. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Yang, X.; Wu, Q.; An, P.; Jin, X.; Liu, W.; Huang, X.; Li, Y.; Yan, S.; et al. Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal Transduct. Target. Ther. 2020, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Boullosa, L.F.; Van Loenhout, J.; Flieswasser, T.; De Waele, J.; Hermans, C.; Lambrechts, H.; Cuypers, B.; Laukens, K.; Bartholomeus, E.; Siozopoulou, V.; et al. Auranofin reveals therapeutic anticancer potential by triggering distinct molecular cell death mechanisms and innate immunity in mutant p53 non-small cell lung cancer. Redox Biol. 2021, 42, 101949. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [Green Version]
- Vandromme, M.; Gauthier-Rouvière, C.; Lamb, N.; Fernandez, A. Regulation of transcription factor localization: Fine-tuning of gene expression. Trends Biochem. Sci. 1996, 21, 59–64. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, J.; Lei, Y.; Zhou, S.; Wei, Y.; Huang, C. Targeting Metabolic–Redox Circuits for Cancer Therapy. Trends Biochem. Sci. 2019, 44, 401–414. [Google Scholar] [CrossRef]
- Chen, A.C.-H.; Arany, P.; Huang, Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.; Blackwell, T.S.; et al. Low-Level Laser Therapy Activates NF-κB via Generation of Reactive Oxygen Species in Mouse Embryonic Fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, V.; Hay, N. Molecular Pathways: Reactive Oxygen Species Homeostasis in Cancer Cells and Implications for Cancer Therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purohit, V.; Simeone, D.M.; Lyssiotis, C.A. Metabolic Regulation of Redox Balance in Cancer. Cancers 2019, 11, 955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, W.H.; Young, R.M.; Schmitz, R.; Yang, Y.; Pittaluga, S.; Wright, G.; Lih, C.-J.; Williams, P.M.; Shaffer, A.L.; Gerecitano, J.; et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015, 21, 922–926. [Google Scholar] [CrossRef]
- Hamadani, M.; Balasubramanian, S.; Hari, P.N. Ibrutinib in Refractory Classic Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 1381–1382. [Google Scholar] [CrossRef]
- Jin, J.; Wang, L.; Tao, Z.; Zhang, J.; Lv, F.; Cao, J.; Hu, X. PDGFD induces ibrutinib resistance of diffuse large B-cell lymphoma through activation of EGFR. Mol. Med. Rep. 2020, 21, 2209–2219. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, C.; Kopp, N.; Layer, J.V.; Redd, R.A.; Tschuri, S.; Haebe, S.; Van Bodegom, D.; Bird, L.; Christie, A.L.; Christodoulou, A.; et al. HSP90 inhibition overcomes ibrutinib resistance in mantle cell lymphoma. Blood 2016, 128, 2517–2526. [Google Scholar] [CrossRef]
- Chiron, D.; Di Liberto, M.; Martin, P.; Huang, X.; Sharman, J.; Blecua, P.; Mathew, S.; Vijay, P.; Eng, K.; Ali, S.; et al. Cell-Cycle Reprogramming for PI3K Inhibition Overrides a Relapse-Specific C481S BTK Mutation Revealed by Longitudinal Functional Genomics in Mantle Cell Lymphoma. Cancer Discov. 2014, 4, 1022–1035. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Barta, S.K. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019, 94, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Piechotta, V.; Jakob, T.; Langer, P.; Monsef, I.; Scheid, C.; Estcourt, L.J.; Ocheni, S.; Theurich, S.; Kuhr, K.; Scheckel, B.; et al. Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: A network meta-analysis. Cochrane Database Syst. Rev. 2019, 11, CD013487. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, H.; Cao, M.; Wang, L.; Wu, S.; Fang, B. Auranofin Enhances Ibrutinib’s Anticancer Activity in EGFR-Mutant Lung Adenocarcinoma. Mol. Cancer Ther. 2018, 17, 2156–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, M.-K.; Shin, S.; Ye, D.-J.; An, H.-G.; Kwon, T.-U.; Baek, H.-S.; Kwon, Y.-J.; Chun, Y.-J. Combined treatment with auranofin and trametinib induces synergistic apoptosis in breast cancer cells. J. Toxicol. Environ. Health Part A 2021, 84, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.R. Enhanced targeting of mitochondrial peroxide defense by the combined use of thiosemicarbazones and inhibitors of thioredoxin reductase. Free Radic. Biol. Med. 2016, 91, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Tsubata, T. Involvement of Reactive Oxygen Species (ROS) in BCR Signaling as a Second Messenger. B Cells Immun. Toler. 2020, 1254, 37–46. [Google Scholar] [CrossRef]
- Cancer Council Australia. Non-Hodgkin Lymphoma. 2023. Available online: https://www.cancer.org.au/cancer-information/types-of-cancer/non-hodgkin-lymphoma (accessed on 3 February 2023).
- Kotla, S.; Singh, N.K.; Rao, G.N. ROS via BTK-p300-STAT1-PPARγ signaling activation mediates cholesterol crystals-induced CD36 expression and foam cell formation. Redox Biol. 2017, 11, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Schmitz, R. Molecular Subgroups of Diffuse Large B Cell Lymphoma: Biology and Implications for Clinical Practice. Curr. Oncol. Rep. 2022, 24, 13–21. [Google Scholar] [CrossRef]
- Mai, Y.; Yu, J.J.; Bartholdy, B.; Xu-Monette, Z.Y.; Knapp, E.E.; Yuan, F.; Chen, H.; Ding, B.B.; Yao, Z.; Das, B.; et al. An oxidative stress-based mechanism of doxorubicin cytotoxicity suggests new therapeutic strategies in ABC-DLBCL. Blood 2016, 128, 2797–2807. [Google Scholar] [CrossRef] [Green Version]
- Frick, M.; Dörken, B.; Lenz, G. The molecular biology of diffuse large B-cell lymphoma. Ther. Adv. Hematol. 2011, 2, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susanibar-Adaniya, S.; Barta, S.K. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. Am. J. Hematol. 2021, 96, 617–629. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, H.; Zhang, L.; Wu, M.; Zhang, F.; Yang, D.; Shen, J.; Chen, J. Glycyrrhetinic acid induces oxidative/nitrative stress and drives ferroptosis through activating NADPH oxidases and iNOS, and depriving glutathione in triple-negative breast cancer cells. Free Radic. Biol. Med. 2021, 173, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Angeli, J.P.F.; Shah, R.; Pratt, D.A.; Conrad, M. Ferroptosis Inhibition: Mechanisms and Opportunities. Trends Pharmacol. Sci. 2017, 38, 489–498. [Google Scholar] [CrossRef]
- Das, K.C.; Muniyappa, H.; Kundumani-Sridharan, V.; Subramani, J. Thioredoxin Decreases Anthracycline Cardiotoxicity, But Sensitizes Cancer Cell Apoptosis. Cardiovasc. Toxicol. 2021, 21, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Naveenkumar, S.K.; Hemshekhar, M.; Jagadish, S.; Manikanta, K.; Vishalakshi, G.J.; Kemparaju, K.; Girish, K.S. Melatonin restores neutrophil functions and prevents apoptosis amid dysfunctional glutathione redox system. J. Pineal Res. 2020, 69, e12676. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [Green Version]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef] [Green Version]
- Ramadass, V.; Vaiyapuri, T.; Tergaonkar, V. Small Molecule NF-κB Pathway Inhibitors in Clinic. Int. J. Mol. Sci. 2020, 21, 5164. [Google Scholar] [CrossRef]
- Raninga, P.V.; Di Trapani, G.; Vuckovic, S.; Tonissen, K.F. TrxR1 inhibition overcomes both hypoxia-induced and acquired bortezomib resistance in multiple myeloma through NF-κβ inhibition. Cell Cycle 2016, 15, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Kelleher, Z.T.; Sha, Y.; Foster, M.W.; Foster, W.M.; Forrester, M.T.; Marshall, H.E. Thioredoxin-mediated Denitrosylation Regulates Cytokine-induced Nuclear Factor κB (NF-κB) Activation. J. Biol. Chem. 2014, 289, 3066–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butturini, E.; De Prati, A.C.; Mariotto, S. Redox Regulation of STAT1 and STAT3 Signaling. Int. J. Mol. Sci. 2020, 21, 7034. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Mohamed, A.J.; Simonson, O.E.; Vargas, L.; Blomberg, K.E.M.; Björkstrand, B.; Arteaga, H.J.; Nore, B.F.; Smith, C.I.E. Proteasome-dependent autoregulation of Bruton tyrosine kinase (Btk) promoter via NF-κB. Blood 2008, 111, 4617–4626. [Google Scholar] [CrossRef] [PubMed]



| Protein Name | Gene Name | * Accession Number | Forward | Reverse |
|---|---|---|---|---|
| L32 | RPL32 | NC_000003.12 | 5′CAGGGTTCGTAGAAGATTCAAGGG3′ | 5′CTTGGAGGAAAACATTGTGAGCGATC3′ |
| TrxR1 | TXNRD1 | NC_000012.12 | 5′GGAATCCACCCTGTCTCTGC3′ | 5′ACGAGCCAGTGGTTTGCAGT3′ |
| BTK | BTK | NC_000023.11 | 5′CTGAAGAACTAAGGAAGCGGTGGATT3′ | 5′ACTTGTGGAGACTGGTGCTGCT3′ |
| P65 | RelA | NC_000011.10 | 5′ATATGAGACCTTCAAGAGCATCA3′ | 5′ATAGTTGATGGTGCTCAGGGATGA3′ |
| Survivin | Survivin | NC_000011.10 | 5′ACCACCGCATCTCTACATTCAAGAACT3′ | 5′TCCCAGCCTTCCAGCTCCTT3′ |
| Normal Lymph Node | NHL | |
|---|---|---|
| TrxR (CAB015834) | Low | High |
| BTK (HPA001198) | Medium | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Clapper, E.; Tonissen, K.F.; Di Trapani, G. Co-Targeting of BTK and TrxR as a Therapeutic Approach to the Treatment of Lymphoma. Antioxidants 2023, 12, 529. https://doi.org/10.3390/antiox12020529
Wang S, Clapper E, Tonissen KF, Di Trapani G. Co-Targeting of BTK and TrxR as a Therapeutic Approach to the Treatment of Lymphoma. Antioxidants. 2023; 12(2):529. https://doi.org/10.3390/antiox12020529
Chicago/Turabian StyleWang, Sicong, Erin Clapper, Kathryn F. Tonissen, and Giovanna Di Trapani. 2023. "Co-Targeting of BTK and TrxR as a Therapeutic Approach to the Treatment of Lymphoma" Antioxidants 12, no. 2: 529. https://doi.org/10.3390/antiox12020529
APA StyleWang, S., Clapper, E., Tonissen, K. F., & Di Trapani, G. (2023). Co-Targeting of BTK and TrxR as a Therapeutic Approach to the Treatment of Lymphoma. Antioxidants, 12(2), 529. https://doi.org/10.3390/antiox12020529






