Encapsulation of Folic Acid and α-Tocopherol in Lysozyme Particles and Their Bioaccessibility in the Presence of DNA
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Encapsulation Efficiency of Folic Acid and α-Tocopherol
2.4. Particle Characterization
2.5. Antioxidant Activity
2.6. In Vitro Release
2.7. Bioaccessibility
2.8. Statistical Analysis
3. Results and Discussion
3.1. Encapsulation of Folic Acid and α-Tocopherol by Lysozyme
3.2. Antioxidant Activity of Lysozyme and Folic Acid/α-Tocopherol
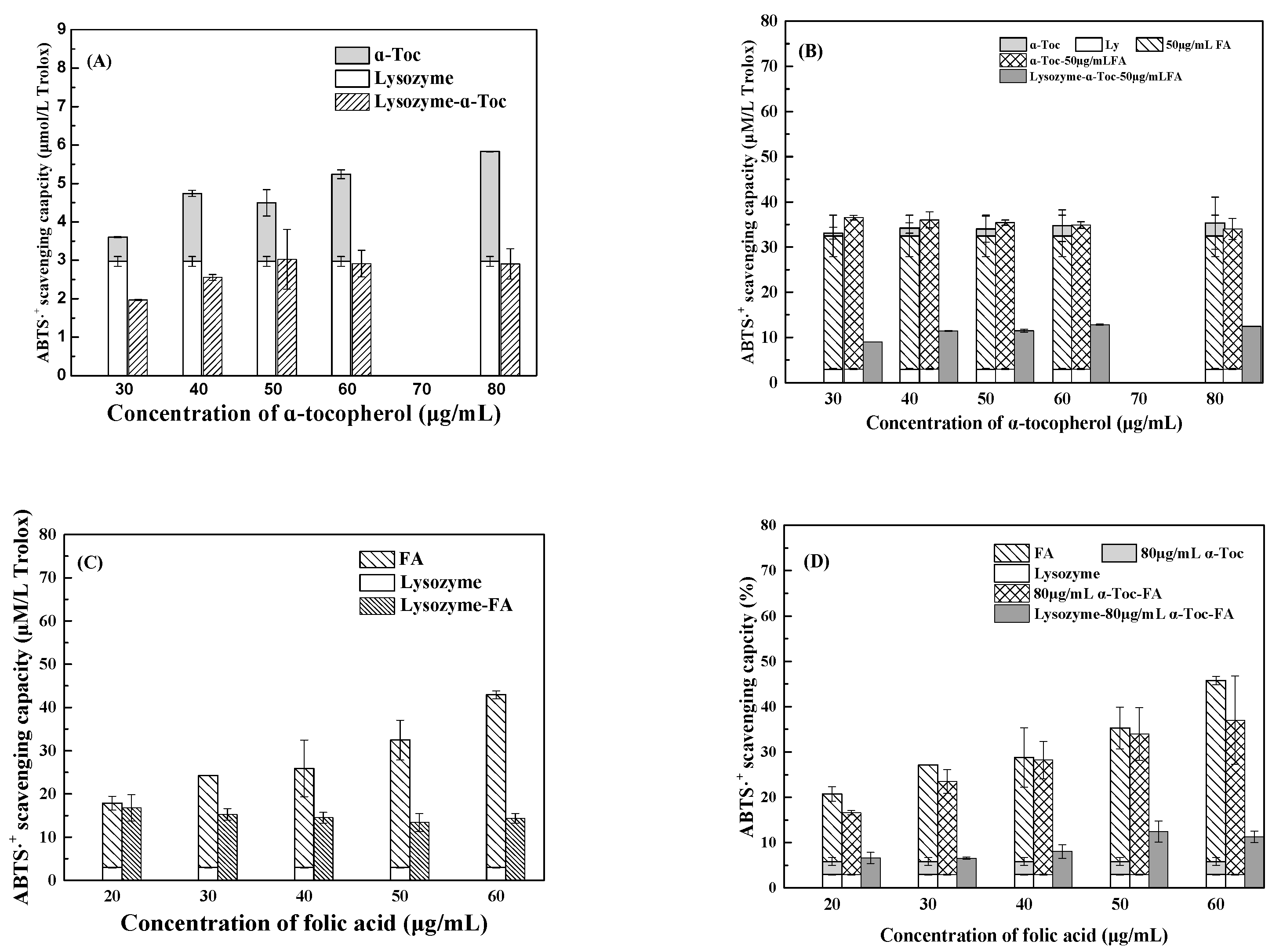
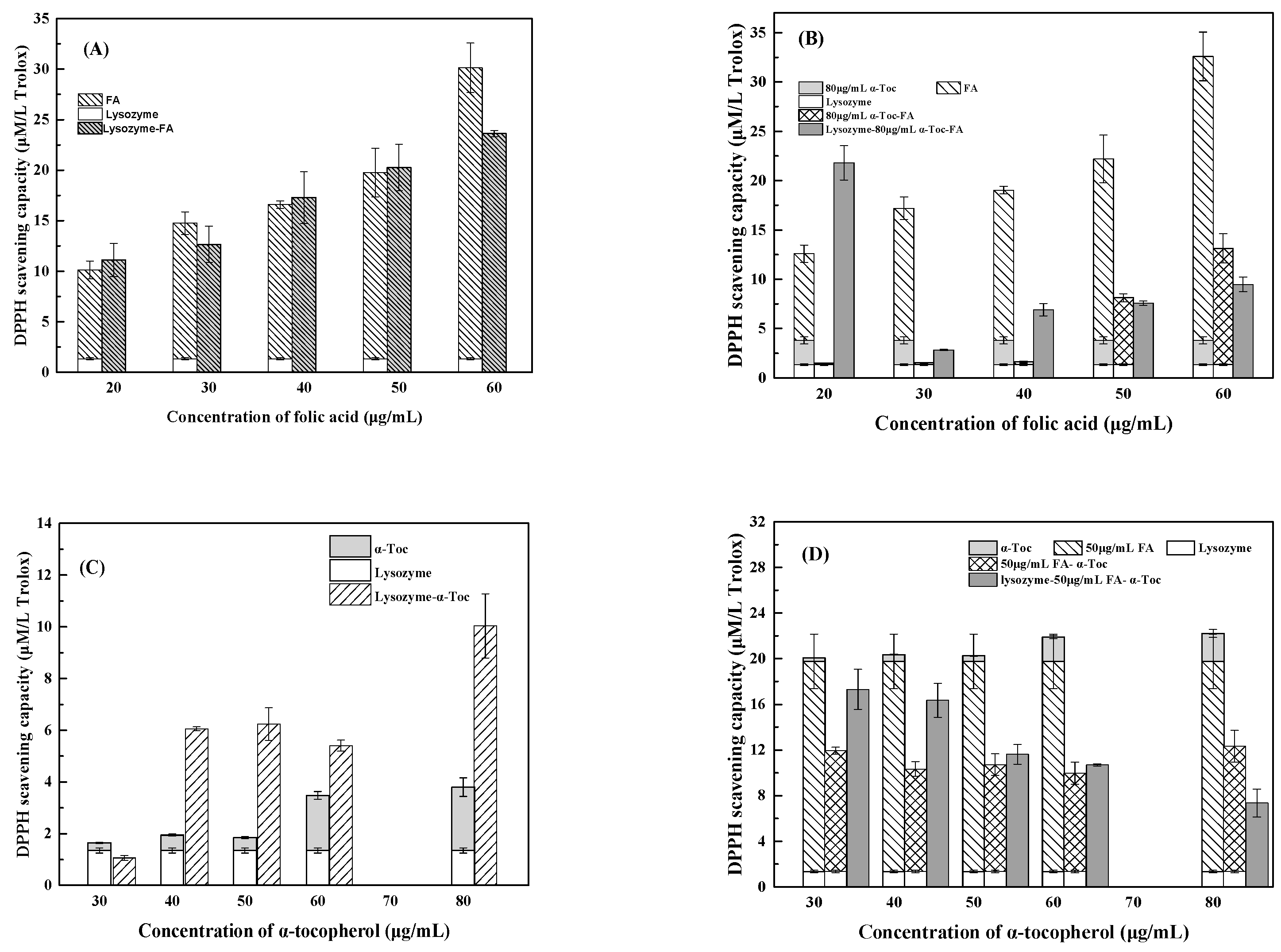
3.2.1. ABTS Assay
3.2.2. DPPH Assay
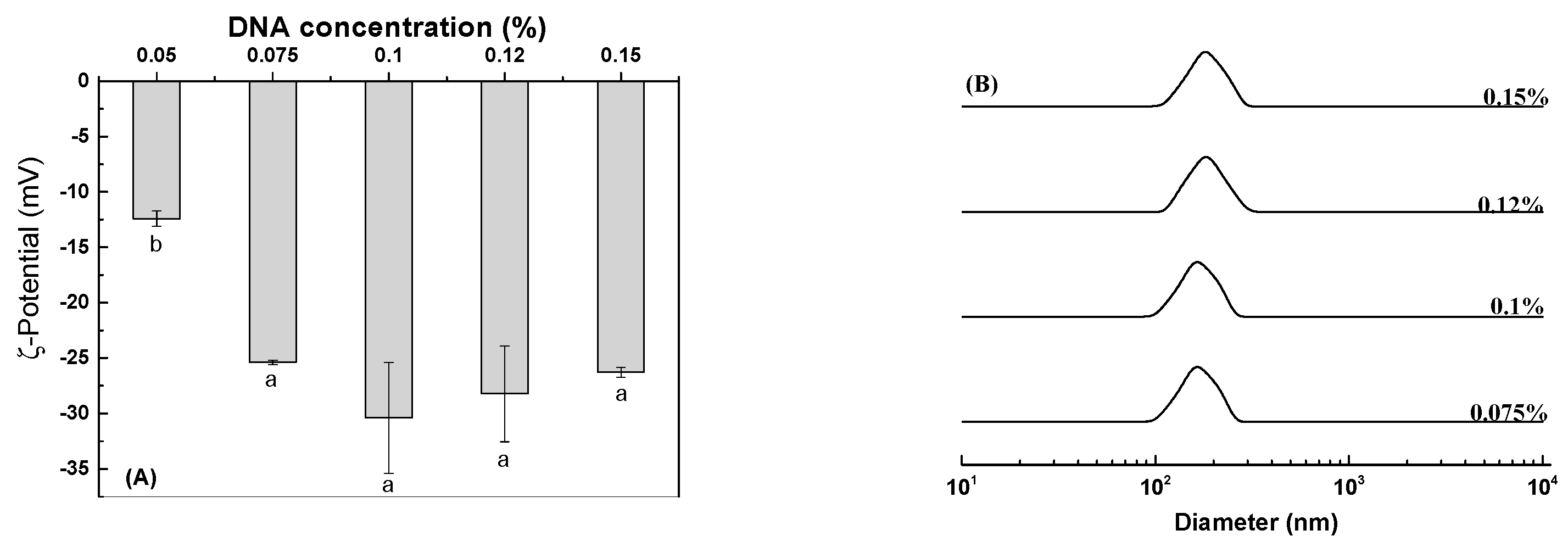
3.3. Lysozyme-DNA Complex Particles
3.4. In Vitro Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aditya, N.P.; Aditya, S.; Yang, H.; Won, H.; Ook, S.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Khan, M.A.; Cheng, H.; Liang, L. Co-encapsulation of α-tocopherol and resveratrol within zein nanoparticles: Impact on antioxidant activity and stability. J. Food Eng. 2019, 247, 9–18. [Google Scholar] [CrossRef]
- Yin, X.; Fu, X.; Cheng, H.; Liang, L. α-Tocopherol and naringenin in whey protein isolate particles: Partition, antioxidant activity, stability and bioaccessibility. Food Hydrocoll. 2020, 106, 105895. [Google Scholar] [CrossRef]
- Diarrassouba, F.; Remondetto, G.; Garrait, G.; Alvarez, P.; Beyssac, E.; Subirade, M. Self-assembly of β-lactoglobulin and egg white lysozyme as a potential carrier for nutraceuticals. Food Chem. 2015, 173, 203–209. [Google Scholar] [CrossRef]
- Huang, W.; Wang, L.; Wei, Y.; Cao, M.; Xie, H.; Wu, D. Fabrication of lysozyme/κ-carrageenan complex nanoparticles as a novel carrier to enhance the stability and in vitro release of curcumin. Int. J. Biol. Macromol. 2020, 146, 444–452. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, J.; Huang, L.; Jing, J.; Wang, N.; Wang, L. Curcumin encapsulation and protection based on lysozyme nanoparticles. Food Sci. Nutr. 2019, 7, 2702–2707. [Google Scholar] [CrossRef]
- Ren, D.; Qi, J.; Xie, A.; Jia, M.; Yang, X.; Xiao, H. Encapsulation in lysozyme/A. Sphaerocephala Krasch polysaccharide nanoparticles increases stability and bioefficacy of curcumin. J. Funct. Foods 2017, 38, 100–109. [Google Scholar] [CrossRef]
- Ji, C.-Y. Preparation and Characterization of DNA-Bioactive Compound Complexes and DNA-Bioactive Compound-Zein Composite Particles. Master’s Thesis, Jiangnan University, Wuxi, China, 2021. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx&dbname=CMFD202201&filename=1021757116.nh (accessed on 16 December 2021).
- Zhang, R.; Wang, Y.; Yang, G. DNA–Lysozyme Nanoarchitectonics: Quantitative Investigation on Charge Inversion and Compaction. Polymers 2022, 14, 1377. [Google Scholar] [CrossRef]
- Naderi, N.; House, J.D. Recent developments in folate nutrition. Adv. Food Nutr. Res. 2018, 83, 195–213. [Google Scholar]
- Sun, C.I.Q. Nutrition and Food Hygiene, 7th ed.; People’s Medical Publishing House: Beijing, China, 2012. [Google Scholar]
- Chapeau, A.L.; Tavares, G.M.; Hamon, P.; Croguennec, T.; Poncelet, D.; Bouhallab, S. Spontaneous co-assembly of lactoferrin and β-lactoglobulin as a promising biocarrier for vitamin B9. Food Hydrocoll. 2016, 57, 280–290. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. The progress and application of vitamin E encapsulation—A review. Food Hydrocoll. 2021, 121, 106998. [Google Scholar] [CrossRef]
- Li, X.R.; Jia, J.J.; Yan, Y.H.; Ni, T.J. Comparative studies on interactions of l-ascorbic acid, α-tocopherol, procyanidin B3, β-carotene, and astaxanthin with lysozyme using fluorescence spectroscopy and molecular modeling methods. J. Food Biochem. 2017, 41, e12338. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Subirade, M.; Zhou, P.; Liang, L. A study of multi-ligand beta-lactoglobulin complex formation. Food Chem. 2014, 165, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Fang, Z.; Liu, T.; Gao, Y.; Liang, L. A study on β-lactoglobulin-triligand-pectin complex particle: Formation, characterization and protection. Food Hydrocoll. 2018, 84, 93–103. [Google Scholar] [CrossRef]
- Dong, H.; Yin, X.; Cheng, H.; Choijilsuren, N.; Chen, X.; Liang, L. Antioxidant activity and stability of α-tocopherol, resveratrol and epigallocatechin-3-gallate in mixture and complexation with bovine serum albumin. Int. J. Food Sci. Technol. 2021, 56, 1788–1800. [Google Scholar] [CrossRef]
- Khan, M.A.; Chen, L.; Liang, L. Improvement in storage stability and resveratrol retention by fabrication of hollow zein-chitosan composite particles. Food Hydrocoll. 2021, 113, 106477. [Google Scholar] [CrossRef]
- Feng, W.; Yue, C.; Ni, Y.; Liang, L. Preparation and characterization of emulsion-filled gel beads for the encapsulation and protection of resveratrol and α-tocopherol. Food Res. Int. 2018, 108, 161–171. [Google Scholar] [CrossRef]
- Bao, H.; Ni, Y.; Dong, H.; Liang, L. α-Tocopherol and resveratrol in emulsion-filled whey protein gels: Co-encapsulation and in vitro digestion. Int. Dairy J. 2020, 104, 104649. [Google Scholar] [CrossRef]
- Mohammed, A.S.Y.; Dyab, A.K.; Taha, F.; Abd El-Mageed, A.I. Encapsulation of folic acid (vitamin B9) into sporopollenin microcapsules: Physico-chemical characterisation, in vitro controlled release and photoprotection study. Mater. Sci. Eng. C 2021, 128, 112271. [Google Scholar] [CrossRef]
- Kurmaz, S.V.; Fadeeva, N.V.; Skripets, J.A.; Komendant, R.I.; Ignatiev, V.M.; Emel’yanova, N.S.; Soldatova, Y.V.; Faingold, I.I.; Poletaeva, D.A.; Kotelnikova, R.A. New water-soluble forms of α-tocopherol: Preparation and study of antioxidant activity in vitro. Mendeleev Commun. 2022, 32, 117–119. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, X.; Yin, Y.; Xia, J.; Mei, Y. Preparation and evaluation of a chitosan-coated antioxidant liposome containing vitamin C and folic acid. J. Microencapsul. 2018, 35, 272–280. [Google Scholar] [CrossRef]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Chang, M.; Lu, M.; Liu, R.; Jin, Q.; Wang, X. Effects of interaction between α-tocopherol, oryzanol, and phytosterol on the antiradical activity against DPPH radical. LWT 2019, 112, 108206. [Google Scholar] [CrossRef]
- Lundberg, D.; Carnerup, A.M.; Schillén, K.; Miguel, M.D.G.; Lindman, B. Phase behavior and coassembly of DNA and lysozyme in dilute aqueous mixtures: A model investigation of DNA-protein interactions. Langmuir 2010, 26, 2986–2988. [Google Scholar] [CrossRef]
- Yang, T.; Wei, G.; Li, Z. Electrostatic assembly of protein lysozyme on DNA visualized by atomic force microscopy. Appl. Surf. Sci. 2007, 253, 4311–4316. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Xu, W.; Jin, W.; Zhang, C.; Liang, H.; Shah, B.R.; Li, B. Environment induced self-aggregation behavior of κ-carrageenan/lysozyme complex. Bioact. Carbohydr. Diet. Fibre 2015, 6, 75–82. [Google Scholar] [CrossRef]
- Xu, W.; Jin, W.; Li, Z.; Liang, H.; Wang, Y.; Shah, B.R.; Li, Y.; Li, B. Synthesis and characterization of nanoparticles based on negatively charged xanthan gum and lysozyme. Food Res. Int. 2015, 71, 83–90. [Google Scholar] [CrossRef]
- Zhu, K.K.; Ye, T.; Liu, J.J. Nanogels fabricated by lysozyme and sodium carboxymethyl cellulose for 5-fluorouracil controlled release. Int. J. Pharm. 2013, 441, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Zema, P.; Pilosof, A.M. On the binding of folic acid to food proteins performing as vitamin micro/nanocarriers. Food Hydrocoll. 2018, 79, 509–517. [Google Scholar] [CrossRef]
- Ahmad, M.; Qureshi, S.; Maqsood, S.; Gani, A.; Masoodi, F.A. Micro-encapsulation of folic acid using horse chestnut starch and β-cyclodextrin: Microcapsule characterization, release behavior & antioxidant potential during GI tract conditions. Food Hydrocoll. 2017, 66, 154–160. [Google Scholar]
- Liu, J.; Jiang, L.; Zhang, Y.; Du, Z.; Qiu, X.; Kong, L.; Zhang, H. Binding behaviors and structural characteristics of ternary complexes of β-lactoglobulin, curcumin, and fatty acids. RSC Adv. 2017, 7, 45960–45967. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Improved chemical stability and cellular antioxidant activity of resveratrol in zein nanoparticle with bovine serum albumin-caffeic acid conjugate. Food Chem. 2018, 261, 283–291. [Google Scholar] [CrossRef]




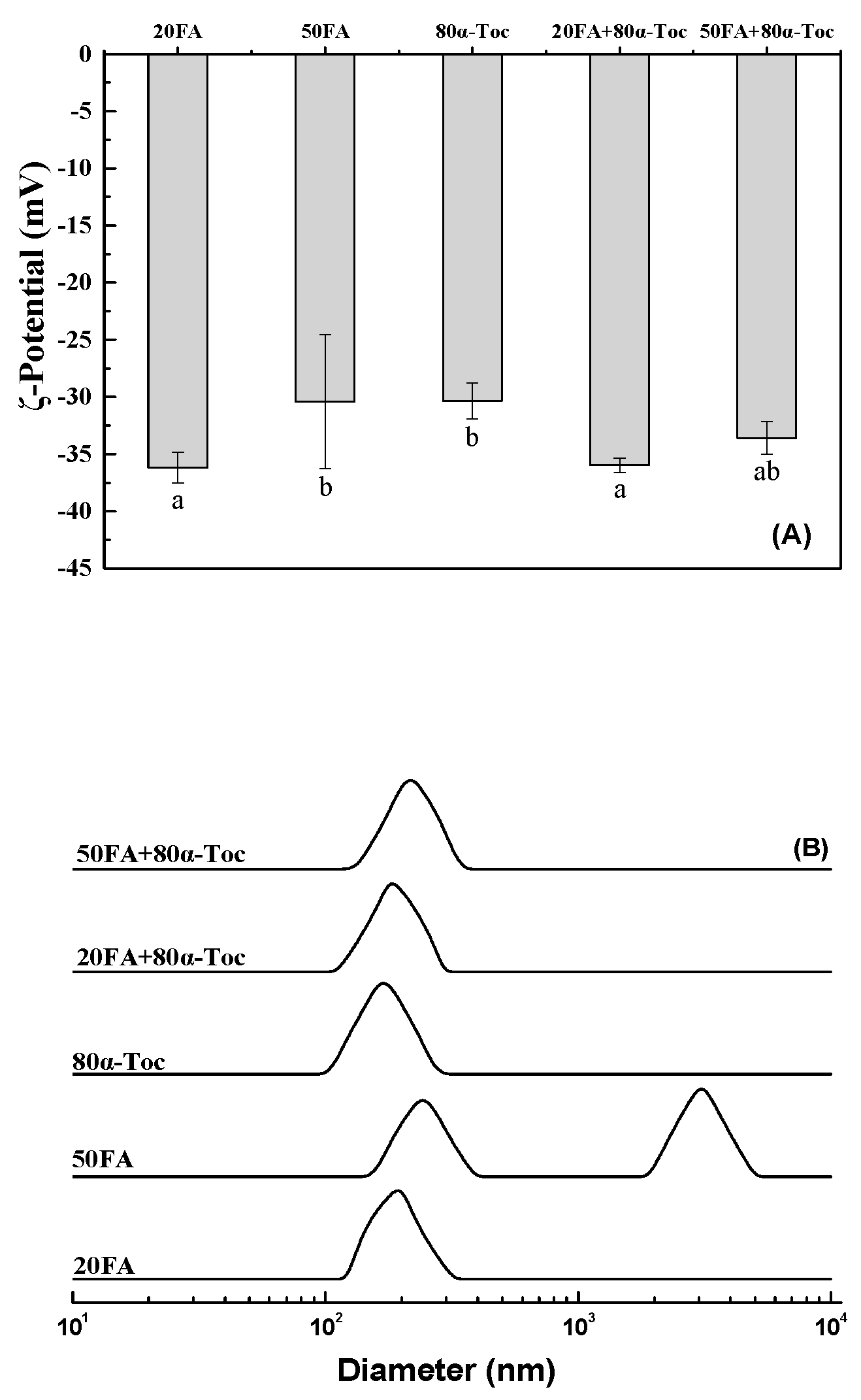

| Folic Acid (μg/mL) | α-Tocopherol (μg/mL) | Mean Diameter (nm) | PDI |
|---|---|---|---|
| 20 | 0 | 168.16 ± 5.10 a | 0.19 ± 0.020 ab |
| 50 | 0 | 2196.51 ± 138.48 d | 0.34 ± 0.02 c |
| 0 | 80 | 168.21 ± 5.92 ab | 0.18 ± 0.01 a |
| 20 | 80 | 197.80 ± 4.12 b | 0.17 ± 0.04 a |
| 50 | 80 | 233.14 ± 0.32 c | 0.20 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Gao, T.; Cheng, H.; Li, N.; Huang, W.; Liang, L. Encapsulation of Folic Acid and α-Tocopherol in Lysozyme Particles and Their Bioaccessibility in the Presence of DNA. Antioxidants 2023, 12, 564. https://doi.org/10.3390/antiox12030564
Ma L, Gao T, Cheng H, Li N, Huang W, Liang L. Encapsulation of Folic Acid and α-Tocopherol in Lysozyme Particles and Their Bioaccessibility in the Presence of DNA. Antioxidants. 2023; 12(3):564. https://doi.org/10.3390/antiox12030564
Chicago/Turabian StyleMa, Lingling, Tiecheng Gao, Hao Cheng, Ning Li, Weining Huang, and Li Liang. 2023. "Encapsulation of Folic Acid and α-Tocopherol in Lysozyme Particles and Their Bioaccessibility in the Presence of DNA" Antioxidants 12, no. 3: 564. https://doi.org/10.3390/antiox12030564
APA StyleMa, L., Gao, T., Cheng, H., Li, N., Huang, W., & Liang, L. (2023). Encapsulation of Folic Acid and α-Tocopherol in Lysozyme Particles and Their Bioaccessibility in the Presence of DNA. Antioxidants, 12(3), 564. https://doi.org/10.3390/antiox12030564








