The Effect of Storage and Pasteurization (Thermal and High-Pressure) Conditions on the Stability of Phycocyanobilin and Phycobiliproteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Arthrospira Platensis (Spirulina) Protein Concentrate (SPC)
2.3. Preparation of Phycobiliproteins (PBP) Extract
2.4. Preparation of Phycocyanobilin (PCB) Extract
2.5. Buffer and Stock Solution Preparation
2.6. LC-MS Method Development and Analysis of PCB Extract and Biliverdin
2.7. Spectral Stability of PBP Extract, PCB Extract, and Biliverdin after High Pressure
2.8. Spectral Stability of PBP Extract, PCB Extract, and Biliverdin under High-Temperature Conditions
2.9. Color Deterioration Kinetics of PBP Extract under High Temperature and Pressure
2.10. The Stability of Biliverdin and PCB Extract at Room Temperature
2.11. PCB Extract Aggregation at Various pH Levels
2.12. Verification of PCB Extract Oxidation Mechanism at pH 7 and 8
2.13. Statistical Analysis
3. Results
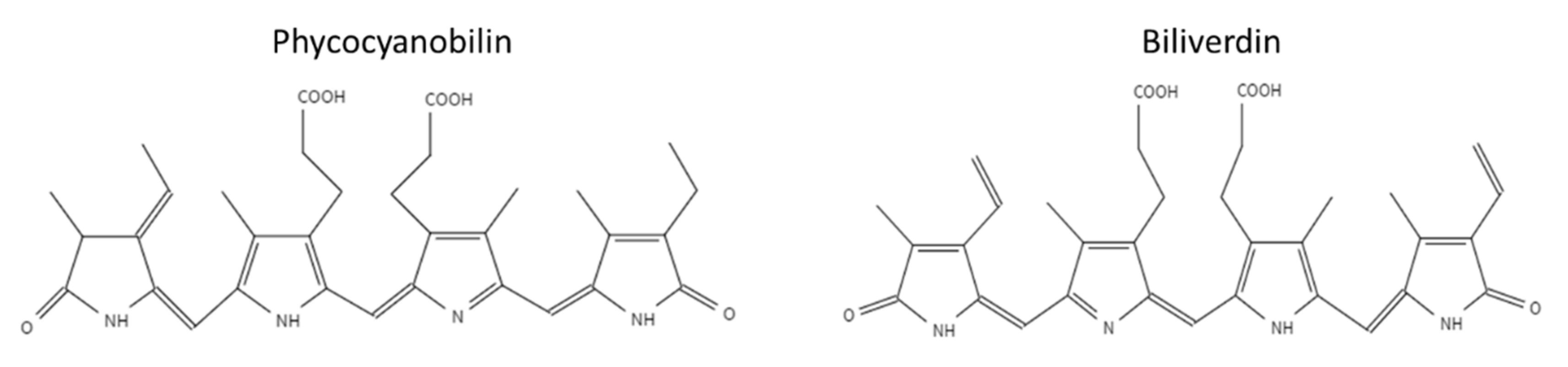
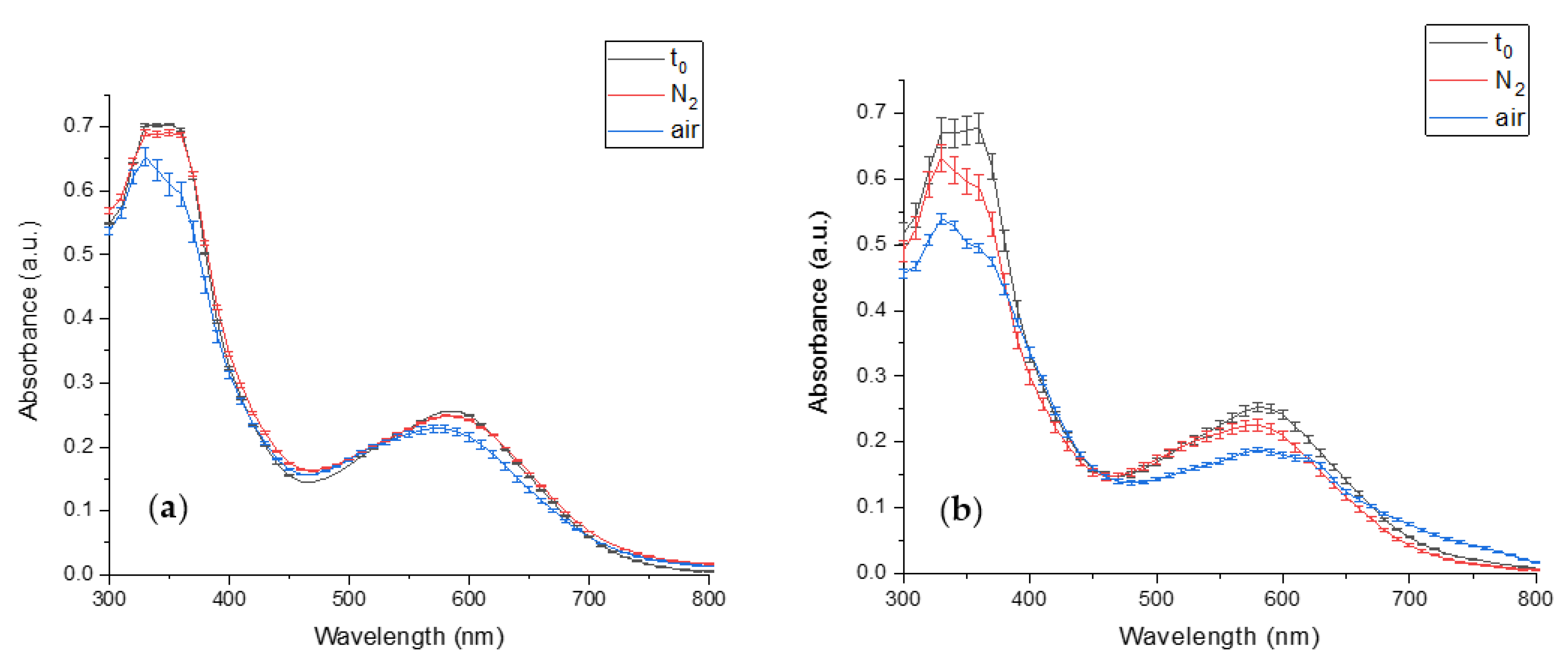
3.1. Characterization of PBP and PCB Extracts
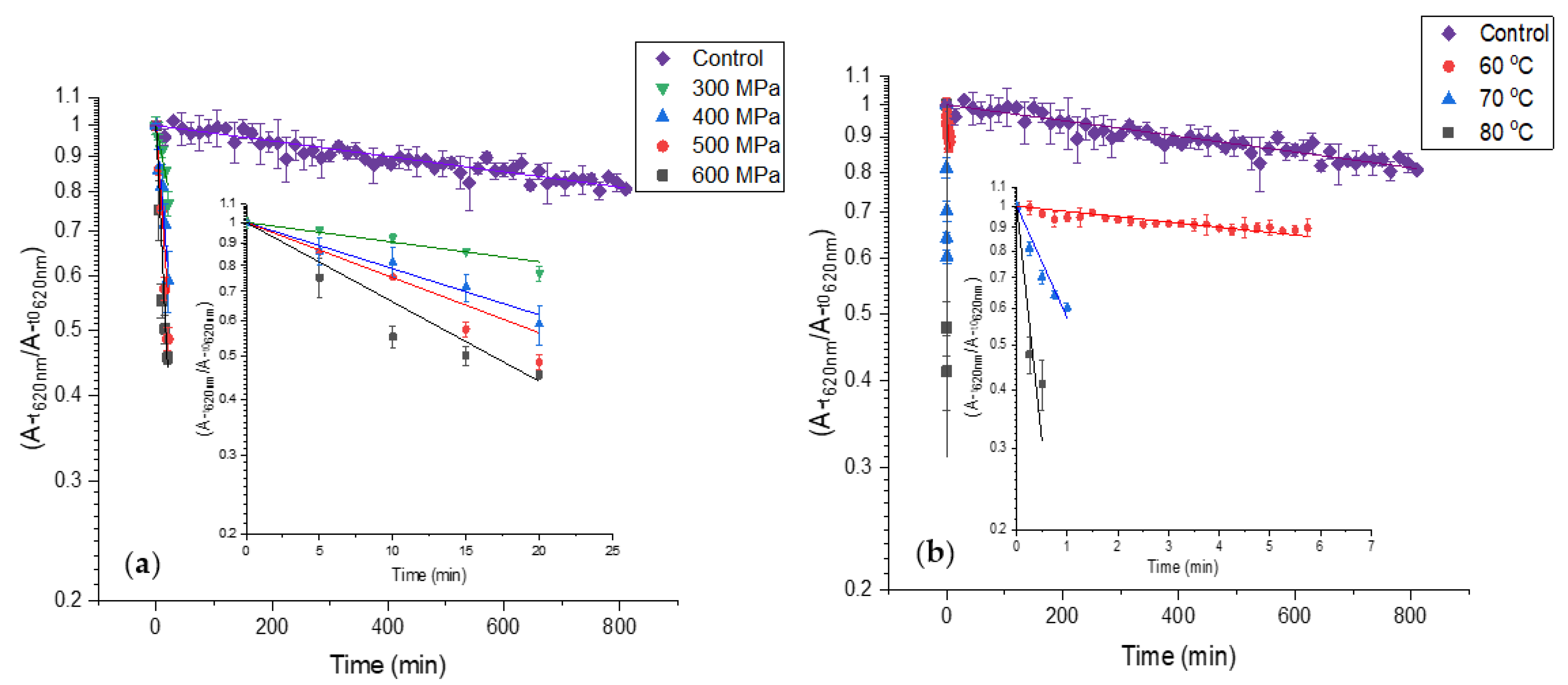
3.2. The Stability of PBP Extract and PCB Extract under Various Pasteurization Conditions
3.3. The Kinetics of PBP Extract Color Deterioration at Different Pasteurization Treatments
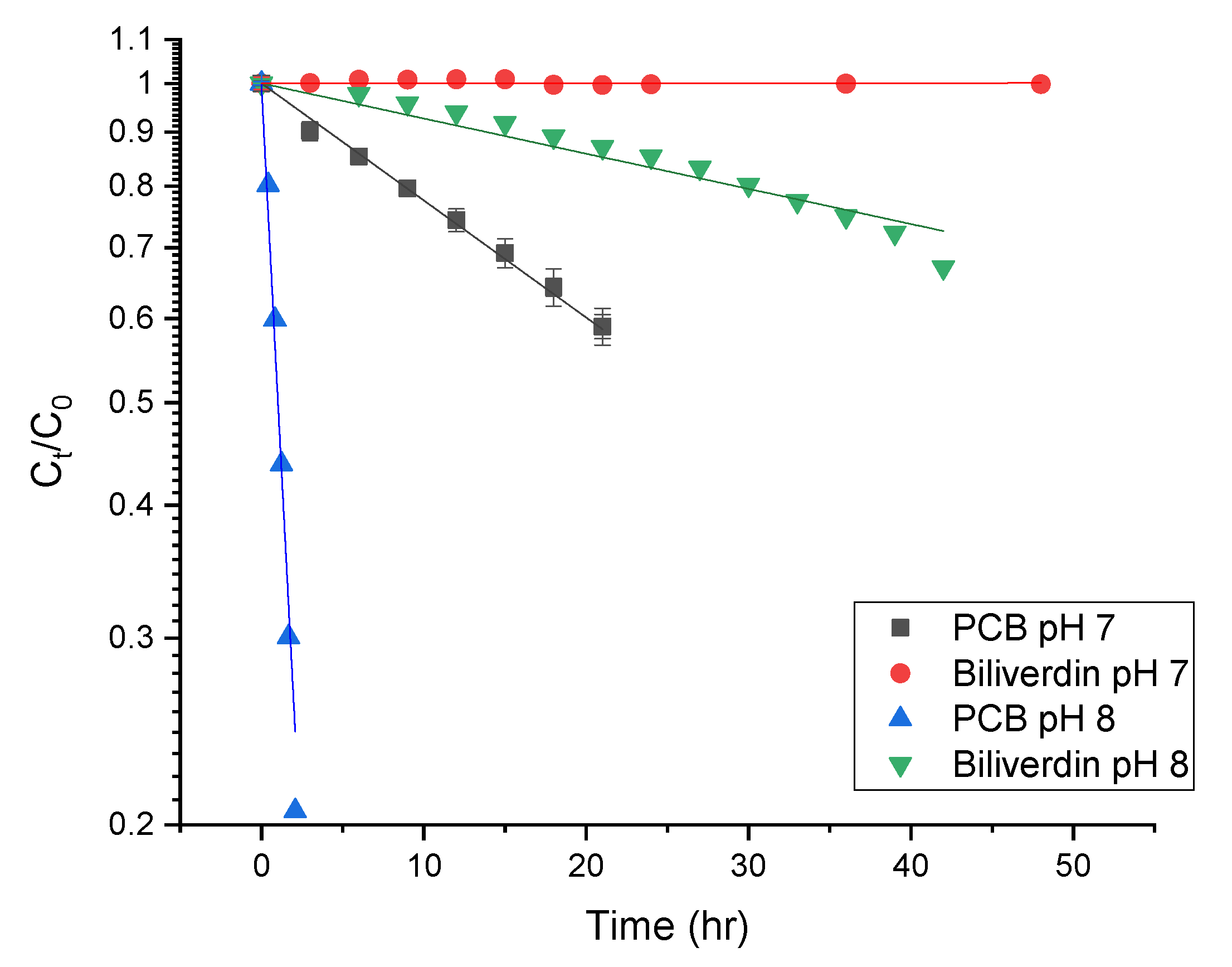
3.4. The Effect of pH on PCB Extract Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newsome, A.G.; Culver, C.A.; Van Breemen, R.B. Nature’s palette: The search for natural blue colorants. J. Agric. Food Chem. 2014, 62, 6498–6511. [Google Scholar] [CrossRef] [PubMed]
- Pez Jaeschke, D.; Rocha Teixeira, I.; Damasceno Ferreira Marczak, L.; Domeneghini Mercali, G. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Maddaly, R. The beneficial effects of spirulina focusing on its immunomodulatory and antioxidant properties. Nutr. Diet. Suppl. 2010, 2, 73. [Google Scholar] [CrossRef] [Green Version]
- Kiran Raj, T.; Ranjithkumar, R.; Kanthesh, B.M.; Gopenath, T.S. C-Phycocyanin of Spirulina Plantesis Inhibits Nsp12 Required for Replication of Sars-Cov-2: A Novel Finding in-Silico. Int. J. Pharm. Sci. Res. 2020, 11, 4271. [Google Scholar]
- Li, W.; Su, H.N.; Pu, Y.; Chen, J.; Liu, L.N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Dufossé, L. Microbial Pigments From Bacteria, Yeasts, Fungi, and Microalgae for the Food and Feed Industries. In Natural and Artificial Flavoring Agents and Food; Academic Press: Cambridge, MA, USA, 2018; pp. 113–132. [Google Scholar]
- Chronakis, I.S.; Galatanu, A.N.; Nylander, T.; Lindman, B. The behaviour of protein preparations from blue-green algae (Spirulina platensis strain Pacifica) at the air/water interface. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 181–192. [Google Scholar] [CrossRef]
- Patil, G.; Chethana, S.; Madhusudhan, M.C.; Raghavarao, K.S.M.S. Fractionation and purification of the phycobiliproteins from Spirulina platensis. Bioresour. Technol. 2008, 99, 7393–7396. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. Evid.-Based Complement. Altern. Med. 2016, 2016, 7803846. [Google Scholar]
- Trost, B.M. Comprehensive Organic Synthesis: Selectivity Strategy and Efficiency in Modern Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 1991; Volume 7. [Google Scholar]
- Chaiklahan, R.; Chirasuwan, N.; Loha, V.; Tia, S.; Bunnag, B. Separation and purification of phycocyanin from Spirulina sp. using a membrane process. Bioresour. Technol. 2011, 102, 7159–7164. [Google Scholar] [CrossRef] [PubMed]
- Weesepoel, Y.; Gruppen, H.; Vincken, J.P. Preliminary UHPLC-PDA-ESI-MS screening of light-accelerated autoxidation products. Food Chem. 2015, 173, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Kozitsyna, N.Y.; Vargaftik, M.N.; Moiseev, I.I. Vinylic, allylic and homoallylic oxidations of alkenes via π- and σ-organopalladium complexes. J. Organomet. Chem. 2000, 593–594, 274–291. [Google Scholar] [CrossRef]
- Wu, H.L.; Wang, G.H.; Xiang, W.Z.; Li, T.; He, H. Stability and Antioxidant Activity of Food-Grade Phycocyanin Isolated from Spirulina platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef] [Green Version]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Martelli, G.; Folli, C.; Visai, L.; Daglia, M.; Ferrari, D. Thermal stability improvement of blue colorant C-Phycocyanin from Spirulina platensis for food industry applications. Process Biochem. 2014, 49, 154–159. [Google Scholar] [CrossRef]
- Antelo, F.S.; Costa, J.A.V.; Kalil, S.J. Thermal degradation kinetics of the phycocyanin from Spirulina platensis. Biochem. Eng. J. 2008, 41, 43–47. [Google Scholar] [CrossRef]
- Faieta, M.; Neri, L.; Sacchetti, G.; Di Michele, A.; Pittia, P. Role of saccharides on thermal stability of phycocyanin in aqueous solutions. Food Res. Int. 2020, 132, 109093. [Google Scholar] [CrossRef]
- Mújica-Paz, H.; Valdez-Fragoso, A.; Samson, C.T.; Welti-Chanes, J.; Torres, J.A. High-Pressure Processing Technologies for the Pasteurization and Sterilization of Foods. Food Bioprocess Technol. 2011, 4, 969–985. [Google Scholar] [CrossRef]
- Muntean, M.-V.; Marian, O.; Barbieru, V.; Cătunescu, G.M.; Ranta, O.; Drocas, I.; Terhes, S. High Pressure Processing in Food Industry—Characteristics and Applications. Agric. Agric. Sci. Procedia 2016, 10, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, D.G.; Angelova, G.V. High pressure processing for foods preserving. Biotechnol. Biotechnol. Equip. 2010, 24, 1940–1945. [Google Scholar] [CrossRef] [Green Version]
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High pressure processing of carrot juice: Effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2020, 307, 125549. [Google Scholar] [CrossRef]
- Stinco, C.M.; Szczepańska, J.; Marszałek, K.; Pinto, C.A.; Inácio, R.S.; Mapelli-Brahm, P.; Barba, F.J.; Lorenzo, J.M.; Saraiva, J.A.; Meléndez-Martínez, A.J. Effect of high-pressure processing on carotenoids profile, colour, microbial and enzymatic stability of cloudy carrot juice. Food Chem. 2019, 299, 125112. [Google Scholar] [CrossRef]
- Terefe, N.S.; Kleintschek, T.; Gamage, T.; Fanning, K.J.; Netzel, G.; Versteeg, C.; Netzel, M. Comparative effects of thermal and high pressure processing on phenolic phytochemicals in different strawberry cultivars. Innov. Food Sci. Emerg. Technol. 2013, 19, 57–65. [Google Scholar] [CrossRef]
- Martínez-Monteagudo, S.I.; Balasubramaniam, V.M. High Pressure Processing of Food Principles, Technology and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Foguel, D.; Weber, G. Pressure-induced dissociation and denaturation of allophycocyanin at subzero temperatures. J. Biol. Chem. 1995, 270, 28759–28766. [Google Scholar] [CrossRef] [Green Version]
- Faieta, M.; Neri, L.; Di Michele, A.; Di Mattia, C.D.; Pittia, P. High hydrostatic pressure treatment of Arthrospira (Spirulina) platensis extracts and the baroprotective effect of sugars on phycobiliproteins. Innov. Food Sci. Emerg. Technol. 2021, 70, 102693. [Google Scholar] [CrossRef]
- Zhang, Z.; Cho, S.; Dadmohammadi, Y.; Li, Y.; Abbaspourrad, A. Improvement of the storage stability of C-phycocyanin in beverages by high-pressure processing. Food Hydrocoll. 2021, 110, 106055. [Google Scholar] [CrossRef]
- Fratelli, C.; Burck, M.; Amarante, M.C.A.; Braga, A.R.C. Antioxidant potential of nature’s “something blue”: Something new in the marriage of biological activity and extraction methods applied to C-phycocyanin. Trends Food Sci. Technol. 2021, 107, 309–323. [Google Scholar] [CrossRef]
- Sharma, R.; Nath, P.C.; Vanitha, K.; Tiwari, O.N.; Bandyopadhyay, T.K.; Bhunia, B. Analysis of kinetics, thermodynamics, colour and antioxidant properties. Food Biosci. 2021, 44, 101354. [Google Scholar] [CrossRef]
- Shkolnikov Lozober, H.; Okun, Z.; Shpigelman, A. The impact of high-pressure homogenization on thermal gelation of Arthrospira platensis (Spirulina) protein concentrate. Innov. Food Sci. Emerg. Technol. 2021, 74, 102857. [Google Scholar] [CrossRef]
- Kamble, S.P.; Gaikar, R.B.; Padalia, R.B.; Shinde, K.D. Extraction and purification of C-phycocyanin from dry Spirulina powder and evaluating its antioxidant, anticoagulation and prevention of DNA damage activity. J. Appl. Pharm. Sci. 2013, 3, 149–153. [Google Scholar]
- Patil, G.; Chethana, S.; Sridevi, A.S.; Raghavarao, K.S.M.S. Method to obtain C-phycocyanin of high purity. J. Chromatogr. A 2006, 1127, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Chirug, L.; Okun, Z.; Ramon, O.; Shpigelman, A. Iron ions as mediators in pectin-flavonols interactions. Food Hydrocoll. 2018, 84, 441–449. [Google Scholar] [CrossRef]
- Safi, C.; Charton, M.; Pignolet, O.; Silvestre, F.; Vaca-Garcia, C.; Pontalier, P.-Y. Influence of microalgae cell wall characteristics on protein extractability and determination of nitrogen-to-protein conversion factors. J. Appl. Phycol. 2013, 25, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Roda-Serrat, M.C.; Christensen, K.V.; El-Houri, R.B.; Fretté, X.; Christensen, L.P. Fast cleavage of phycocyanobilin from phycocyanin for use in food colouring. Food Chem. 2018, 240, 655–661. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, R.J.; Reinhart, G.D. Baroresistant buffer mixtures for biochemical analyses. Anal. Biochem. 2005, 341, 69–76. [Google Scholar] [CrossRef]
- Shkolnikov, H.; Belochvostov, V.; Okun, Z.; Shpigelman, A. The effect of pressure on the kinetics of polyphenolics degradation—Implications to hyperbaric storage using Epigallocatechin-gallate as a model. Innov. Food Sci. Emerg. Technol. 2020, 59, 102273. [Google Scholar] [CrossRef]
- Pearson, E. Histochemistry: Theoretical and Applied 1980; Churchill Livingstone: New York, NY, USA, 1980. [Google Scholar]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef]
- Garrett, J.M.; Stairs, R.A.; Annett, R.G. Thermal Denaturation and Coagulation of Whey Proteins: Effect of Sugars. J. Dairy Sci. 1988, 71, 10–16. [Google Scholar] [CrossRef]
- Scheer, H.; Kufer, W. Conformational Studies on C-Phycocyanin from Spirulina platensis. Z. Für Naturforsch. C 1977, 32, 513–519. [Google Scholar] [CrossRef]
- Böcker, L.; Hostettler, T.; Diener, M.; Eder, S.; Demuth, T.; Adamcik, J.; Reineke, K.; Leeb, E.; Nyström, L.; Mathys, A. Time-temperature-resolved functional and structural changes of phycocyanin extracted from Arthrospira platensis/Spirulina. Food Chem. 2020, 316, 126374. [Google Scholar] [CrossRef]
- Knorr, D.; Heinz, V.; Buckow, R. High pressure application for food biopolymers. Biochim. Biophys. Acta Proteins Proteom. 2006, 1764, 619–631. [Google Scholar] [CrossRef]
- Cooper, A. Thermal difference spectra of aromatic amino acids in water. FEBS Lett. 1974, 48, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Nilamsari, A.M.; Yunanda, A.; Hadiyanto, H. Thermal degradation kinetics of phycocyanin encapsulation as an antioxidant agent. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012055. [Google Scholar] [CrossRef]
- Hadiyanto; Christwardana, M.; Sutanto, H.; Suzery, M.; Amelia, D.; Aritonang, R.F. Kinetic study on the effects of sugar addition on the thermal degradation of phycocyanin from Spirulina sp. Food Biosci. 2018, 22, 85–90. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Lavelli, V. Circular food supply chains—Impact on value addition and safety. Trends Food Sci. Technol. 2021, 114, 323–332. [Google Scholar] [CrossRef]
- Abalde, J.; Betancourt, L.; Torres, E.; Cid, A.; Barwell, C. Purification and characterization of phycocyanin from the marine cyanobacterium Synechococcus sp. IO9201. Plant Sci. 1998, 136, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Minic, S.L.; Milčić, M.; Stanić-Vučinić, D.; Radibratović, M.; Sotiroudis, T.G.; Nikolić, M.; Ćirković Veličković, T. Phycocyanobilin, a bioactive tetrapyrrolic compound of blue-green alga Spirulina, binds with high affinity and competes with bilirubin for binding on human serum albumin. RSC Adv. 2015, 5, 61787–61798. [Google Scholar] [CrossRef] [Green Version]
- Settimo, L.; Bellman, K.; Knegtel, R.M.A. Comparison of the Accuracy of Experimental and Predicted pKa Values of Basic and Acidic Compounds. Pharm. Res. 2013, 31, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Bello, A.; Barreto-Palacios, V.; García-Segovia, P.; Mir-Bel, J.; Martínez-Monzó, J. Effect of pH on Color and Texture of Food Products. Food Eng. Rev. 2013, 5, 158–170. [Google Scholar] [CrossRef]



| Pressure (MPa) | CDR (1/min) × 102 |
| 0.1 | 0.0024 ± 0.0008 a |
| 300 | 1.4 ± 0.1 b |
| 400 | 1.7 ± 0.5 b |
| 500 | 3.4 ± 0.1 b |
| 600 | 4.7 ± 0.1 c |
| Temperature (°C) | CDR (1/min) × 102 |
| 25 | 0.0024 ± 0.0008 a |
| 60 | 2.0 ± 0.1 b |
| 70 | 58 ± 3 c |
| 80 | 227 ± 7 d |
| Solution | Degradation Rate (1/h) × 102 | Half-Life Time (h) × 103 |
|---|---|---|
| PCB extract pH 7 | 2.5 ± 0.2 a | 2.7 ± 0.2 a |
| PCB extract pH 8 | 9.4 ± 0.006 b | 0.74 ± 0.01 b |
| Biliverdin pH 7 | ND 2 | ND 2 |
| Biliverdin pH 8 | 0.76 ± 0.01 c | 9.1 ± 0.1 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkolnikov Lozober, H.; Okun, Z.; Parvari, G.; Shpigelman, A. The Effect of Storage and Pasteurization (Thermal and High-Pressure) Conditions on the Stability of Phycocyanobilin and Phycobiliproteins. Antioxidants 2023, 12, 568. https://doi.org/10.3390/antiox12030568
Shkolnikov Lozober H, Okun Z, Parvari G, Shpigelman A. The Effect of Storage and Pasteurization (Thermal and High-Pressure) Conditions on the Stability of Phycocyanobilin and Phycobiliproteins. Antioxidants. 2023; 12(3):568. https://doi.org/10.3390/antiox12030568
Chicago/Turabian StyleShkolnikov Lozober, Hani, Zoya Okun, Galit Parvari, and Avi Shpigelman. 2023. "The Effect of Storage and Pasteurization (Thermal and High-Pressure) Conditions on the Stability of Phycocyanobilin and Phycobiliproteins" Antioxidants 12, no. 3: 568. https://doi.org/10.3390/antiox12030568







