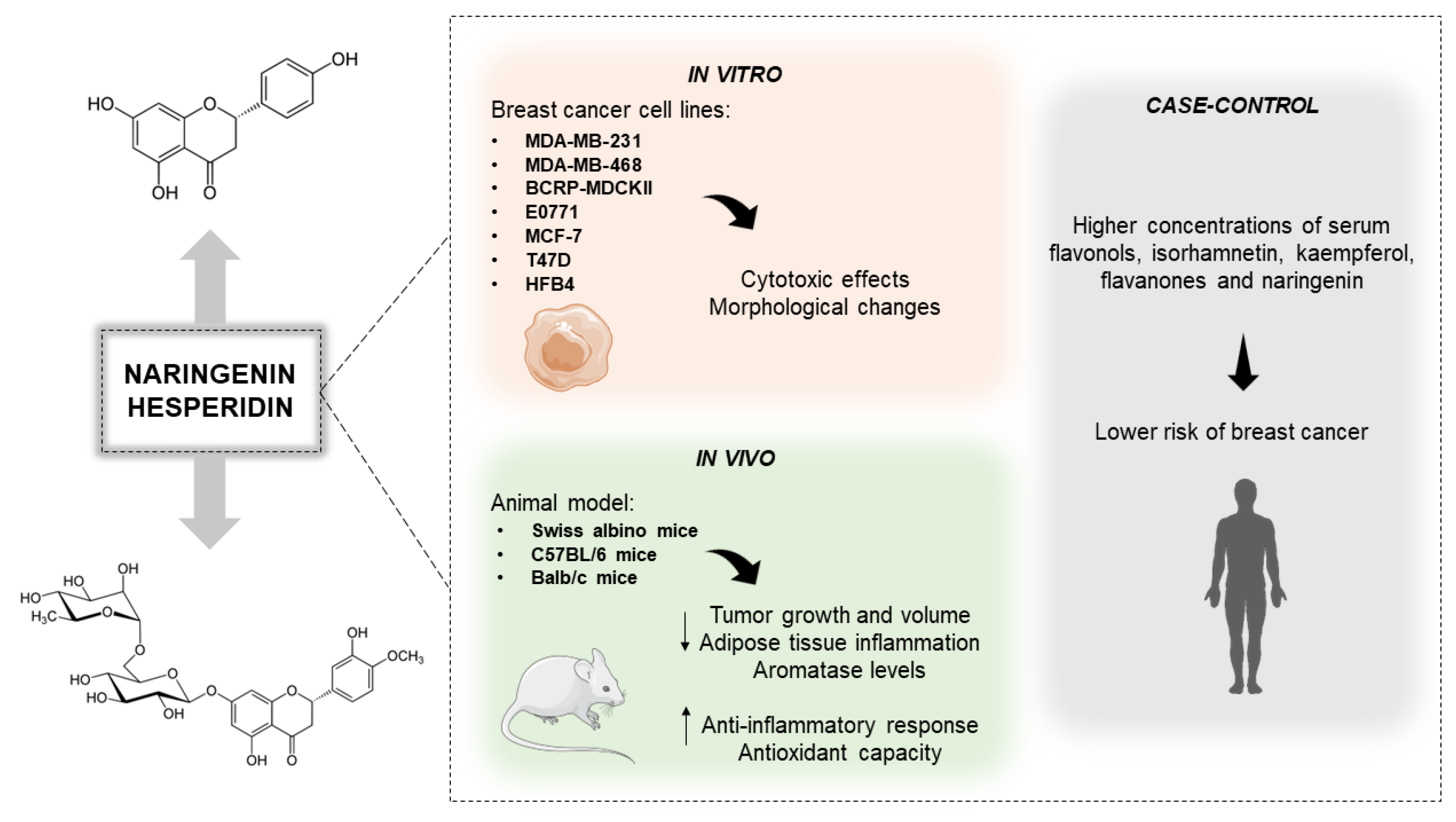
Naringenin and Hesperidin as Promising Alternatives for Prevention and Co-Adjuvant Therapy for Breast Cancer
Abstract
1. Introduction
2. Data Analysis Methodology
3. Naringenin and Hesperidin: An Overview
3.1. Citrus Fruits and Flavanones
3.2. Sites of Interaction and Structure-Activity Relationship by Naringenin and Hesperidin
3.3. Anti-Breast Cancer Role of the Citrus-Derivated Compounds Naringenin and Hesperidin
3.4. Naringenin and Hesperidin on Modulation of Epigenetics and Estrogens Mechanisms
3.5. Induction of Cell Death via Regulation of Apoptotic Signaling Pathways by Naringenin and Hesperidin
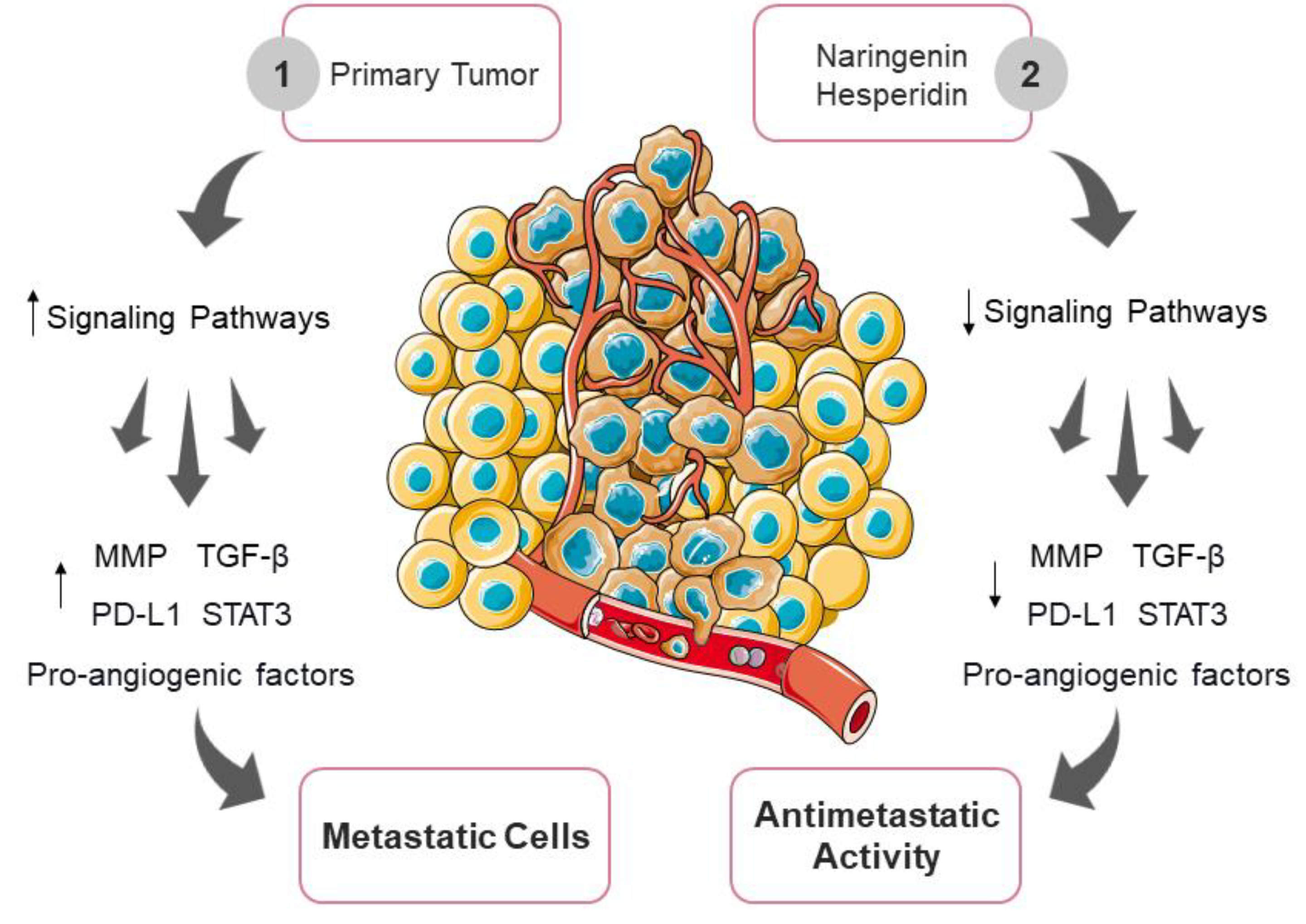
3.6. Inhibition of Tumor Invasion and Metastasis by Naringenin and Hesperidin
3.7. Nanotechnology as a Potentiator of Naringenin and Hesperidin Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.L.; Cuzick, J.; Phillips, K.-A. Key Steps for Effective Breast Cancer Prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; Carvalho, D.P.; Fortunato, R.S. The Role of Oxidative Stress on Breast Cancer Development and Therapy. Tumour Biol. 2016, 37, 4281–4291. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Cai, Q.; Shu, X.O.; Nechuta, S.J. The Role of Biomarkers of Oxidative Stress in Breast Cancer Risk and Prognosis: A Systematic Review of the Epidemiologic Literature. J. Women’s Health 2017, 26, 467–482. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe Vera (L.) Webb.: Natural Sources of Antioxidants—A Review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Embuscado, M.E. Spices and Herbs: Natural Sources of Antioxidants—A Mini Review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Ramana, K.V.; Reddy, A.B.M.; Majeti, N.V.R.K.; Singhal, S.S. Therapeutic Potential of Natural Antioxidants. Oxid. Med. Cell. Longev. 2018, 2018, 9471051. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-L.; Liu, H.-X. The Double-Edged Roles of ROS in Cancer Prevention and Therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential Mechanisms of Quercetin in Cancer Prevention: Focus on Cellular and Molecular Targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef]
- Sanches, V.L.; de Souza Mesquita, L.M.; Viganó, J.; Contieri, L.S.; Pizani, R.; Chaves, J.; da Silva, L.C.; de Souza, M.C.; Breitkreitz, M.C.; Rostagno, M.A. Insights on the Extraction and Analysis of Phenolic Compounds from Citrus Fruits: Green Perspectives and Current Status. Crit. Rev. Anal. Chem. 2022, 1–27. [Google Scholar] [CrossRef]
- Griñan-Lison, C.; Blaya-Cánovas, J.L.; López-Tejada, A.; Ávalos-Moreno, M.; Navarro-Ocón, A.; Cara, F.E.; González-González, A.; Lorente, J.A.; Marchal, J.A.; Granados-Principal, S. Antioxidants for the Treatment of Breast Cancer: Are We There Yet? Antioxidants 2021, 10, 205. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative Stress and Its Role in Cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative Stress and Dietary Phytochemicals: Role in Cancer Chemoprevention and Treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Younas, M.; Hano, C.; Giglioli-Guivarc’h, N.; Abbasi, B.H. Mechanistic Evaluation of Phytochemicals in Breast Cancer Remedy: Current Understanding and Future Perspectives. RSC Adv. 2018, 8, 29714–29744. [Google Scholar] [CrossRef]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the Origin and Evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernández Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, L.; Maugeri, A.; Cirmi, S.; Lombardo, G.E.; Russo, C.; Gangemi, S.; Calapai, G.; Navarra, M. Citrus Fruits and Their Flavonoids in Inflammatory Bowel Disease: An Overview. Nat. Prod. Res. 2020, 34, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.-J.; Chen, J.-B.; Cao, J.-P.; Li, X.; Sun, C.-D. Citrus Flavonoids and Their Antioxidant Evaluation. Crit. Rev. Food Sci. Nutr. 2022, 62, 3833–3854. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Shariati, M.A.; Imran, M.; Bashir, K.; Khan, S.A.; Mitra, S.; Emran, T.B.; Badalova, K.; Uddin, M.S.; Mubarak, M.S.; et al. Comprehensive Review on Naringenin and Naringin Polyphenols as a Potent Anticancer Agent. Environ. Sci. Pollut. Res. Int. 2022, 29, 31025–31041. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Brockmueller, A.; Zhai, K.; Abdellatif, B.; Siddiqui, M.; Biringer, K.; Kudela, E.; Pec, M.; et al. Flavonoids as an Effective Sensitizer for Anti-Cancer Therapy: Insights into Multi-Faceted Mechanisms and Applicability towards Individualized Patient Profiles. EPMA J. 2021, 12, 155–176. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F. A Mechanistic Review of the Anticancer Potential of Hesperidin, a Natural Flavonoid from Citrus Fruits. Nutr. Res. 2021, 92, 21–31. [Google Scholar] [CrossRef]
- El-Kersh, D.M.; Ezzat, S.M.; Salama, M.M.; Mahrous, E.A.; Attia, Y.M.; Ahmed, M.S.; Elmazar, M.M. Anti-Estrogenic and Anti-Aromatase Activities of Citrus Peels Major Compounds in Breast Cancer. Sci. Rep. 2021, 11, 7121. [Google Scholar] [CrossRef] [PubMed]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Yip, Y.M. Therapeutic Potential of Bioactive Flavonoids from Citrus Fruit Peels toward Obesity and Diabetes Mellitus. Future Pharmacol. 2023, 3, 14–37. [Google Scholar] [CrossRef]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; Asad, M.H.H.B. Citrus Flavonoids as Potential Therapeutic Agents: A Review. Phytother. Res. 2022, 36, 1417–1441. [Google Scholar] [CrossRef]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and Metabolic Syndrome: A Review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Marunaka, Y.; Marunaka, R.; Sun, H.; Yamamoto, T.; Kanamura, N.; Inui, T.; Taruno, A. Actions of Quercetin, a Polyphenol, on Blood Pressure. Molecules 2017, 22, 209. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Schluesener, H. Health-Promoting Effects of the Citrus Flavanone Hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent for Obesity. Drug Des. Devel. Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef]
- Man, M.-Q.; Yang, B.; Elias, P.M. Benefits of Hesperidin for Cutaneous Functions. Evid. Based. Complement. Alternat. Med. 2019, 2019, 2676307. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of Their Molecular Mechanisms and Experimental Models: Hesperidin and Hesperetin as Antioxidant and Anti-Inflammatory Agents. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.; Sultana, H.; Sultana, T.; Al Amin, M.; Aktar, S.; Ali, M.C.; Rahim, Z.B.; Hossain, M.A.; Al Mamun, A.; Amin, M.N.; et al. Chemotherapeutic Potential of Hesperetin for Cancer Treatment, with Mechanistic Insights: A Comprehensive Review. Heliyon 2022, 8, e08815. [Google Scholar] [CrossRef]
- Neba Ambe, G.N.N.; Breda, C.; Bhambra, A.S.; Arroo, R.R.J. Effect of the Citrus Flavone Nobiletin on Circadian Rhythms and Metabolic Syndrome. Molecules 2022, 27, 7727. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef]
- Huang, J.; Chang, Z.; Lu, Q.; Chen, X.; Najafi, M. Nobiletin as an Inducer of Programmed Cell Death in Cancer: A Review. Apoptosis 2022, 27, 297–310. [Google Scholar] [CrossRef]
- Islam, A.; Islam, M.S.; Rahman, M.K.; Uddin, M.N.; Akanda, M.R. The Pharmacological and Biological Roles of Eriodictyol. Arch. Pharm. Res. 2020, 43, 582–592. [Google Scholar] [CrossRef]
- Deng, Z.; Hassan, S.; Rafiq, M.; Li, H.; He, Y.; Cai, Y.; Kang, X.; Liu, Z.; Yan, T. Pharmacological Activity of Eriodictyol: The Major Natural Polyphenolic Flavanone. Evid. Based. Complement. Alternat. Med. 2020, 2020, 6681352. [Google Scholar] [CrossRef] [PubMed]
- Quetglas-Llabrés, M.M.; Quispe, C.; Herrera-Bravo, J.; Catarino, M.D.; Pereira, O.R.; Cardoso, S.M.; Dua, K.; Chellappan, D.K.; Pabreja, K.; Satija, S.; et al. Pharmacological Properties of Bergapten: Mechanistic and Therapeutic Aspects. Oxid. Med. Cell. Longev. 2022, 2022, 8615242. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, L.; Liu, K.; Cao, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. Bergapten: A Review of Its Pharmacology, Pharmacokinetics, and Toxicity. Phytother. Res. 2021, 35, 6131–6147. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Ghasemipour Afshar, E. Tangeretin: A Mechanistic Review of Its Pharmacological and Therapeutic Effects. J. Basic Clin. Physiol. Pharmacol. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Luqman, S.; Meena, A. Prospects of Tangeretin as a Modulator of Cancer Targets/Pathways. Pharmacol. Res. 2020, 161, 105202. [Google Scholar] [CrossRef] [PubMed]
- Tayarani-Najaran, Z.; Tayarani-Najaran, N.; Eghbali, S. A Review of Auraptene as an Anticancer Agent. Front. Pharmacol. 2021, 12, 698352. [Google Scholar] [CrossRef]
- Bibak, B.; Shakeri, F.; Barreto, G.E.; Keshavarzi, Z.; Sathyapalan, T.; Sahebkar, A. A Review of the Pharmacological and Therapeutic Effects of Auraptene. Biofactors 2019, 45, 867–879. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Auraptene and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 399–407. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, C.; Luo, T.; Wang, J.; Tang, Y.; Chen, Z.; Yu, L. Limonin: A Review of Its Pharmacology, Toxicity, and Pharmacokinetics. Molecules 2019, 24, 3679. [Google Scholar] [CrossRef]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a Potential Immunomodulator in Therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Goyal, A.; Verma, A.; Dubey, N.; Raghav, J.; Agrawal, A. Naringenin: A Prospective Therapeutic Agent for Alzheimer’s and Parkinson’s Disease. J. Food Biochem. 2022, 46, e14415. [Google Scholar] [CrossRef] [PubMed]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.-L.; et al. Naringenin: A Potential Flavonoid Phytochemical for Cancer Therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef] [PubMed]
- Heidary Moghaddam, R.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and Naringin in Cardiovascular Disease Prevention: A Preclinical Review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ma, J.; Fan, Y.; Wang, X.; Zheng, S.; Feng, J.; Li, J.; Fan, Z.; Li, G.; Ye, Q. Naringenin: A Promising Therapeutic Agent against Organ Fibrosis. Oxid. Med. Cell. Longev. 2021, 2021, 1210675. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Muriel, P. Beneficial Effects of Naringenin in Liver Diseases: Molecular Mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Chen, R.; Qi, Q.-L.; Wang, M.-T.; Li, Q.-Y. Therapeutic Potential of Naringin: An Overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A Systematic Review of the Preventive and Therapeutic Effects of Naringin against Human Malignancies. Front. Pharmacol. 2021, 12, 639840. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Liu, S.; Lou, Y.; Li, Y.; Zhang, J.; Li, P.; Yang, B.; Gu, Q. Review of Phytochemical and Nutritional Characteristics and Food Applications of Citrus L. Fruits. Front. Nutr. 2022, 9, 968604. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2021, 12, 29. [Google Scholar] [CrossRef]
- Rehman, M.F.; Batool, A.I.; Qadir, R.; Aslam, M. Hesperidin and Naringenin. In A Centum of Valuable Plant Bioactives; Elsevier: Amsterdam, The Netherlands, 2021; pp. 403–444. [Google Scholar]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, Pharmacodynamic and Formulations Aspects of Naringenin: An Update. Life Sci. 2018, 215, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, F.; Lian, Y.; Xiao, H.; Zheng, J. Biosynthesis of Citrus Flavonoids and Their Health Effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Lucas-Abellán, C.; Pérez-Abril, M.; Castillo, J.; Serrano, A.; Mercader, M.T.; Fortea, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Effect of Temperature, PH, β- and HP-β-Cds on the Solubility and Stability of Flavanones: Naringenin and Hesperetin. Lebenson. Wiss. Technol. 2019, 108, 233–239. [Google Scholar] [CrossRef]
- Venkateswara Rao, P.; Kiran, S.; Rohini, P.; Bhagyasree, P. Flavonoid: A Review on Naringenin. J. Pharmacogn. Phytochem. 2017, 6, 27. [Google Scholar]
- Aalikhani, M.; Safdari, Y.; Jahanshahi, M.; Alikhani, M.; Khalili, M. Comparison between Hesperidin, Coumarin, and Deferoxamine Iron Chelation and Antioxidant Activity against Excessive Iron in the Iron Overloaded Mice. Front. Neurosci. 2021, 15, 811080. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Martorell, M.; Capó, X.; Tur, J.A.; Pons, A.; Sureda, A. Potential Anti-Inflammatory Effects of Hesperidin from the Genus Citrus. Curr. Med. Chem. 2018, 25, 4929–4945. [Google Scholar] [CrossRef]
- Pla-Pagà, L.; Companys, J.; Calderón-Pérez, L.; Llauradó, E.; Solà, R.; Valls, R.M.; Pedret, A. Effects of Hesperidin Consumption on Cardiovascular Risk Biomarkers: A Systematic Review of Animal Studies and Human Randomized Clinical Trials. Nutr. Rev. 2019, 77, 845–864. [Google Scholar] [CrossRef]
- Albuquerque de Oliveira Mendes, L.; Ponciano, C.S.; Depieri Cataneo, A.H.; Wowk, P.F.; Bordignon, J.; Silva, H.; Vieira de Almeida, M.; Ávila, E.P. The Anti-Zika Virus and Anti-Tumoral Activity of the Citrus Flavanone Lipophilic Naringenin-Based Compounds. Chem. Biol. Interact. 2020, 331, 109218. [Google Scholar] [CrossRef]
- Hosseinzadeh, E.; Hassanzadeh, A.; Marofi, F.; Alivand, M.R.; Solali, S. Flavonoid-Based Cancer Therapy: An Updated Review. Anticancer Agents Med. Chem. 2020, 20, 1398–1414. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Liras, P. Comparative Molecular Mechanisms of Biosynthesis of Naringenin and Related Chalcones in Actinobacteria and Plants: Relevance for the Obtention of Potent Bioactive Metabolites. Antibiotics 2022, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Sordon, S.; Popłoński, J.; Milczarek, M.; Stachowicz, M.; Tronina, T.; Kucharska, A.Z.; Wietrzyk, J.; Huszcza, E. Structure-Antioxidant-Antiproliferative Activity Relationships of Natural C7 and C7-C8 Hydroxylated Flavones and Flavanones. Antioxidants 2019, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, L.; Zhang, P.; Liu, T.; Zhou, L.; Yang, G.; Lin, R.; Zhang, J. Solubilities of Naringin and Naringenin in Different Solvents and Dissociation Constants of Naringenin. J. Chem. Eng. Data 2015, 60, 932–940. [Google Scholar] [CrossRef]
- Sangpheak, W.; Kicuntod, J.; Schuster, R.; Rungrotmongkol, T.; Wolschann, P.; Kungwan, N.; Viernstein, H.; Mueller, M.; Pongsawasdi, P. Physical Properties and Biological Activities of Hesperetin and Naringenin in Complex with Methylated β-Cyclodextrin. Beilstein J. Org. Chem. 2015, 11, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, N.; Shareghi, B.; Farhadian, S.; Hosseini-Koupaei, M. A Comparative Study of the Interaction of Naringenin with Lysozyme by Multi-Spectroscopic Methods, Activity Comparisons, and Molecular Modeling Procedures. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 271, 120931. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, Y.; Liu, H.; Xu, Y.; Wang, X.; Zhang, C.; Ma, X. Comparative Studies on the Interaction of Nine Flavonoids with Trypsin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 238, 118440. [Google Scholar] [CrossRef]
- Malik, N.; Dhiman, P.; Khatkar, A. Mechanistic Approach towards Interaction of Newly Synthesized Hesperidin Derivatives against Xanthine Oxidase. Int. J. Biol. Macromol. 2019, 135, 864–876. [Google Scholar] [CrossRef]
- Choi, S.; Yu, S.; Lee, J.; Kim, W. Effects of Neohesperidin Dihydrochalcone (NHDC) on Oxidative Phosphorylation, Cytokine Production, and Lipid Deposition. Foods 2021, 10, 1408. [Google Scholar] [CrossRef]
- Zhang, F.-X.; Yuan, Y.-L.-L.; Cui, S.-S.; Li, M.; Tan, X.; Qiu, Z.-C.; Li, R.-M. Dissection of the Potential Pharmacological Function of Neohesperidin Dihydrochalcone—A Food Additive—By in Vivo Substances Profiling and Network Pharmacology. Food Funct. 2021, 12, 4325–4336. [Google Scholar] [CrossRef]
- Yang, X.; Wang, T.; Guo, J.; Sun, M.; Wong, M.W.; Huang, D. Dietary Flavonoids Scavenge Hypochlorous Acid via Chlorination on A- and C-Rings as Primary Reaction Sites: Structure and Reactivity Relationship. J. Agric. Food Chem. 2019, 67, 4346–4354. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Ayob, Z.; Mohd Bohari, S.P.; Abd Samad, A.; Jamil, S. Cytotoxic Activities against Breast Cancer Cells of Local Justicia Gendarussa Crude Extracts. Evid. Based. Complement. Alternat. Med. 2014, 2014, 732980. [Google Scholar] [CrossRef]
- Fan, X.; Bai, J.; Zhao, S.; Hu, M.; Sun, Y.; Wang, B.; Ji, M.; Jin, J.; Wang, X.; Hu, J.; et al. Evaluation of Inhibitory Effects of Flavonoids on Breast Cancer Resistance Protein (BCRP): From Library Screening to Biological Evaluation to Structure-Activity Relationship. Toxicol. In Vitro 2019, 61, 104642. [Google Scholar] [CrossRef]
- Ke, J.-Y.; Banh, T.; Hsiao, Y.-H.; Cole, R.M.; Straka, S.R.; Yee, L.D.; Belury, M.A. Citrus Flavonoid Naringenin Reduces Mammary Tumor Cell Viability, Adipose Mass, and Adipose Inflammation in Obese Ovariectomized Mice. Mol. Nutr. Food Res. 2017, 61, 1600934. [Google Scholar] [CrossRef] [PubMed]
- Al-Rikabi, R.; Al-Shmgani, H.; Dewir, Y.H.; El-Hendawy, S. In Vivo and in Vitro Evaluation of the Protective Effects of Hesperidin in Lipopolysaccharide-Induced Inflammation and Cytotoxicity of Cell. Molecules 2020, 25, 478. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-L.; Zhan, X.-X.; Zuo, L.-S.-Y.; Mo, X.-F.; Zhang, X.; Liu, K.-Y.; Li, L.; Zhang, C.-X. Associations between Serum Concentration of Flavonoids and Breast Cancer Risk among Chinese Women. Eur. J. Nutr. 2021, 60, 1347–1362. [Google Scholar] [CrossRef]
- Vo, A.T.; Millis, R.M. Epigenetics and Breast Cancers. Obstet. Gynecol. Int. 2012, 2012, 602720. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Belwal, T.; Efferth, T.; Farooqi, A.A.; Sanches-Silva, A.; Vacca, R.A.; Nabavi, S.F.; Khan, F.; Prasad Devkota, H.; Barreca, D.; et al. Targeting Epigenetics in Cancer: Therapeutic Potential of Flavonoids. Crit. Rev. Food Sci. Nutr. 2021, 61, 1616–1639. [Google Scholar] [CrossRef]
- Jiang, W.; Xia, T.; Liu, C.; Li, J.; Zhang, W.; Sun, C. Remodeling the Epigenetic Landscape of Cancer-Application Potential of Flavonoids in the Prevention and Treatment of Cancer. Front. Oncol. 2021, 11, 705903. [Google Scholar] [CrossRef]
- Lee, H.-S.; Herceg, Z. The Epigenome and Cancer Prevention: A Complex Story of Dietary Supplementation. Cancer Lett. 2014, 342, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Chiou, Y.-S.; Chen, L.-H.; Ho, C.-T. Breast Cancer Chemoprevention by Dietary Natural Phenolic Compounds: Specific Epigenetic Related Molecular Targets. Mol. Nutr. Food Res. 2015, 59, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Saha Roy, S.; Vadlamudi, R.K. Role of Estrogen Receptor Signaling in Breast Cancer Metastasis. Int. J. Breast Cancer 2012, 2012, 654698. [Google Scholar] [CrossRef] [PubMed]
- Hervouet, E.; Cartron, P.-F.; Jouvenot, M.; Delage-Mourroux, R. Epigenetic Regulation of Estrogen Signaling in Breast Cancer. Epigenetics 2013, 8, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, L.; Zhang, Y.; Nakata, Y.; Chan, H.L.; Morey, L. Epigenetic Mechanisms in Breast Cancer Therapy and Resistance. Nat. Commun. 2021, 12, 1786. [Google Scholar] [CrossRef]
- Yuan, L.; Cai, Y.; Zhang, L.; Liu, S.; Li, P.; Li, X. Promoting Apoptosis, a Promising Way to Treat Breast Cancer with Natural Products: A Comprehensive Review. Front. Pharmacol. 2021, 12, 801662. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, B.; Liu, J.; Wu, X.; Luo, N.; Wang, X.; Zheng, X.; Pan, X. Combinatorial Anti-Proliferative Effects of Tamoxifen and Naringenin: The Role of Four Estrogen Receptor Subtypes. Toxicology 2018, 410, 231–246. [Google Scholar] [CrossRef]
- Hatkevich, T.; Ramos, J.; Santos-Sanchez, I.; Patel, Y.M. A Naringenin-Tamoxifen Combination Impairs Cell Proliferation and Survival of MCF-7 Breast Cancer Cells. Exp. Cell Res. 2014, 327, 331–339. [Google Scholar] [CrossRef]
- Eanes, L.; Patel, Y.M. Inhibition of the MAPK Pathway Alone Is Insufficient to Account for All of the Cytotoxic Effects of Naringenin in MCF-7 Breast Cancer Cells. Biochim. Open 2016, 3, 64–71. [Google Scholar] [CrossRef]
- van Duursen, M.B.M. Modulation of Estrogen Synthesis and Metabolism by Phytoestrogensin Vitroand the Implications for Women’s Health. Toxicol. Res. 2017, 6, 772–794. [Google Scholar] [CrossRef]
- Hermawan, A.; Ikawati, M.; Jenie, R.I.; Khumaira, A.; Putri, H.; Nurhayati, I.P.; Angraini, S.M.; Muflikhasari, H.A. Identification of Potential Therapeutic Target of Naringenin in Breast Cancer Stem Cells Inhibition by Bioinformatics and in Vitro Studies. Saudi Pharm. J. 2021, 29, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Fu, W.; Wang, J.; Kang, D.; Xu, L.; Zhao, Y.; Liu, A.-L.; Du, G.-H. Identification of Estrogen Receptor α Antagonists from Natural Products via in Vitro and in Silico Approaches. Oxid. Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Kim, S.; Park, T.I. Naringenin: A Partial Agonist on Estrogen Receptor in T47D-KBluc Breast Cancer Cells. Int. J. Clin. Exp. Med. 2013, 6, 890–899. [Google Scholar] [PubMed]
- Bulzomi, P.; Bolli, A.; Galluzzo, P.; Acconcia, F.; Ascenzi, P.; Marino, M. The Naringenin-Induced Proapoptotic Effect in Breast Cancer Cell Lines Holds out against a High Bisphenol a Background. IUBMB Life 2012, 64, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-H.; Chen, W.-H.; Juan-Lu, C.; Hsieh, S.-C.; Lin, S.-C.; Mai, R.-T.; Chen, S.-Y. Hesperidin and Chlorogenic Acid Synergistically Inhibit the Growth of Breast Cancer Cells via Estrogen Receptor/Mitochondrial Pathway. Life 2021, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.S.; Niazi, A.; Moghadam, A.; Afsharifar, A. Experimental, Molecular Docking and Molecular Dynamic Studies of Natural Products Targeting Overexpressed Receptors in Breast Cancer. PLoS ONE 2022, 17, e0267961. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Vaux, D.L. Cell Death in the Origin and Treatment of Cancer. Mol. Cell 2020, 78, 1045–1054. [Google Scholar] [CrossRef]
- Hossain, R.; Jain, D.; Khan, R.A.; Islam, M.T.; Mubarak, M.S.; Saikat, A.S.M. Natural-Derived Molecules as a Potential Adjuvant in Chemotherapy: Normal Cell Protectors and Cancer Cell Sensitizers. Anticancer. Agents Med. Chem. 2022, 22, 836–850. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting Cancer Signaling Pathways by Natural Products: Exploring Promising Anti-Cancer Agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular Mechanisms behind the Biological Effects of Hesperidin and Hesperetin for the Prevention of Cancer and Cardiovascular Diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef]
- Mir, I.A.; Tiku, A.B. Chemopreventive and Therapeutic Potential of “Naringenin”, a Flavanone Present in Citrus Fruits. Nutr. Cancer 2015, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jin, G.; Ge, Y.; Guo, Z. Naringenin Inhibits Migration of Breast Cancer Cells via Inflammatory and Apoptosis Cell Signaling Pathways. Inflammopharmacology 2019, 27, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Zaim, Ö.; Doğanlar, O.; Zreigh, M.M.; Doğanlar, Z.B.; Özcan, H. Synthesis, Cancer-Selective Antiproliferative and Apoptotic Effects of Some (±)-Naringenin Cycloaminoethyl Derivatives. Chem. Biodivers. 2018, 15, e1800016. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Kong, S.; Zhao, S.; Tang, Q. Naringenin Inhibits Human Breast Cancer Cells (MDA-MB-231) by Inducing Programmed Cell Death, Caspase Stimulation, G2/M Phase Cell Cycle Arrest and Suppresses Cancer Metastasis. Cell. Mol. Biol. 2021, 67, 8–13. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Dong, T.; Shen, J.; Gao, X.; Zhou, J. Naringenin Has a Chemoprotective Effect in MDA-MB-231 Breast Cancer Cells via Inhibition of Caspase-3 and -9 Activities. Oncol. Lett. 2018, 17, 1217–1222. [Google Scholar] [CrossRef]
- Yousuf, M.; Shamsi, A.; Khan, S.; Khan, P.; Shahwan, M.; Elasbali, A.M.; Haque, Q.M.R.; Hassan, M.I. Naringenin as a Potential Inhibitor of Human Cyclin-Dependent Kinase 6: Molecular and Structural Insights into Anti-Cancer Therapeutics. Int. J. Biol. Macromol. 2022, 213, 944–954. [Google Scholar] [CrossRef]
- Pateliya, B.; Burade, V.; Goswami, S. Enhanced Antitumor Activity of Doxorubicin by Naringenin and Metformin in Breast Carcinoma: An Experimental Study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1949–1961. [Google Scholar] [CrossRef]
- Filho, J.C.C.; Sarria, A.L.F.; Becceneri, A.B.; Fuzer, A.M.; Batalhão, J.R.; da Silva, C.M.P.; Carlos, R.M.; Vieira, P.C.; Fernandes, J.B.; Cominetti, M.R. Copper (II) and 2,2’-Bipyridine Complexation Improves Chemopreventive Effects of Naringenin against Breast Tumor Cells. PLoS ONE 2014, 9, e107058. [Google Scholar] [CrossRef]
- Ajji, P.K.; Walder, K.; Puri, M. Combination of Balsamin and Flavonoids Induce Apoptotic Effects in Liver and Breast Cancer Cells. Front. Pharmacol. 2020, 11, 574496. [Google Scholar] [CrossRef]
- Fazary, A.E.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Amer, M.E.; Nasr, M.S.M.; Abuamara, T.M.M.; Badr, D.A.; Ju, Y.-H.; Mohamed, A.F. Bioactivity Studies of Hesperidin and XAV939. ACS Omega 2021, 6, 20042–20052. [Google Scholar] [CrossRef]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, Bioactive Components of Propolis, Exhibit Cytotoxic Activity and Induce Cell Cycle Arrest and Apoptosis in Human Breast Cancer Cells MDA-MB-231 and MCF-7—A Comparative Study. Cell. Mol. Biol. 2018, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Magura, J.; Moodley, R.; Mackraj, I. The Effect of Hesperidin and Luteolin Isolated from Eriocephalus Africanus on Apoptosis, Cell Cycle and MiRNA Expression in MCF-7. J. Biomol. Struct. Dyn. 2022, 40, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Tiriveedhi, V. Perplexing Role of P-Glycoprotein in Tumor Microenvironment. Front. Oncol. 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Febriansah, R.; Dyaningtyas, D.P.; Nurulita, N.A.; Meiyanto, E.; Nugroho, A.E. Hesperidin as a Preventive Resistance Agent in MCF-7 Breast Cancer Cells Line Resistance to Doxorubicin. Asian Pac. J. Trop. Biomed. 2014, 4, 228–233. [Google Scholar] [CrossRef]
- Korga-Plewko, A.; Michalczyk, M.; Adamczuk, G.; Humeniuk, E.; Ostrowska-Lesko, M.; Jozefczyk, A.; Iwan, M.; Wojcik, M.; Dudka, J. Apigenin and Hesperidin Downregulate DNA Repair Genes in MCF-7 Breast Cancer Cells and Augment Doxorubicin Toxicity. Molecules 2020, 25, 4421. [Google Scholar] [CrossRef]
- Hermawan, A.; Khumaira, A.; Ikawati, M.; Putri, H.; Jenie, R.I.; Angraini, S.M.; Muflikhasari, H.A. Identification of Key Genes of Hesperidin in Inhibition of Breast Cancer Stem Cells by Functional Network Analysis. Comput. Biol. Chem. 2021, 90, 107427. [Google Scholar] [CrossRef]
- Patel, P.; Shah, J. Protective Effects of Hesperidin through Attenuation of Ki67 Expression against DMBA-Induced Breast Cancer in Female Rats. Life Sci. 2021, 285, 119957. [Google Scholar] [CrossRef]
- Omar, H.A.; Mohamed, W.R.; Arafa, E.-S.A.; Shehata, B.A.; El Sherbiny, G.A.; Arab, H.H.; Elgendy, A.N.A.M. Hesperidin Alleviates Cisplatin-Induced Hepatotoxicity in Rats without Inhibiting Its Antitumor Activity. Pharmacol. Rep. 2016, 68, 349–356. [Google Scholar] [CrossRef]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The Lingering Mysteries of Metastatic Recurrence in Breast Cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Tahir, F.; Fakhar, M.; Butt, G.; Colombo Pimentel, T.; Wu, N.; Yulaevna, I.M.; Attar, R. Antimetastatic Effects of Citrus-Derived Bioactive Ingredients: Mechanistic Insights. Cell. Mol. Biol. 2021, 67, 178–186. [Google Scholar] [CrossRef]
- Casey, S.C.; Amedei, A.; Aquilano, K.; Azmi, A.S.; Benencia, F.; Bhakta, D.; Bilsland, A.E.; Boosani, C.S.; Chen, S.; Ciriolo, M.R.; et al. Cancer Prevention and Therapy through the Modulation of the Tumor Microenvironment. Semin. Cancer Biol. 2015, 35, S199–S223. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Rezaei Tavirani, M.; Deravi, N.; Mahboobi Rabbani, M.I.; Zarghi, A. Naringenin Enhances the Anti-Cancer Effect of Cyclophosphamide against MDA-MB-231 Breast Cancer Cells via Targeting the STAT3 Signaling Pathway. Iran. J. Pharm. Res. 2020, 19, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, N.; Zheng, Y.; Yang, B.; Wang, S.; Wang, X.; Pan, B.; Wang, Z. Naringenin in Si-Ni-San Formula Inhibits Chronic Psychological Stress-Induced Breast Cancer Growth and Metastasis by Modulating Estrogen Metabolism through FXR/EST Pathway. J. Adv. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Daroqui, M.C.; Vazquez, P.; Bal de Kier Joffé, E.; Bakin, A.V.; Puricelli, L.I. TGF-β Autocrine Pathway and MAPK Signaling Promote Cell Invasiveness and in Vivo Mammary Adenocarcinoma Tumor Progression. Oncol. Rep. 2012, 28, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Hiyoshi, H.; Ito, I.; Iida, K.; Nakajima, Y.; Nagasawa, K.; Yanagisawa, J. Identification of a Novel Compound That Suppresses Breast Cancer Invasiveness by Inhibiting Transforming Growth Factor-β Signaling via Estrogen Receptor α. J. Cancer 2014, 5, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Dong, W.; Zeng, W.; Zhang, L.; Zhang, C.; Qiu, Y.; Wang, L.; Yin, X.; Zhang, C.; Liang, W. Naringenin Prevents TGF-Β1 Secretion from Breast Cancer and Suppresses Pulmonary Metastasis by In-Hibiting PKC Activation. Breast Cancer Res. 2016, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Jia, M.; Gao, J.; Liu, X.; Guo, W.; Zhang, H. Effects of Dietary Patterns Combined with Dietary Phytochemicals on Breast Cancer Metastasis. Life Sci. 2021, 264, 118720. [Google Scholar] [CrossRef] [PubMed]
- Alsuliman, A.; Colak, D.; Al-Harazi, O.; Fitwi, H.; Tulbah, A.; Al-Tweigeri, T.; Al-Alwan, M.; Ghebeh, H. Bidirectional Crosstalk between PD-L1 Expression and Epithelial to Mesenchymal Transition: Significance in Claudin-Low Breast Cancer Cells. Mol. Cancer 2015, 14, 149. [Google Scholar] [CrossRef]
- Kongtawelert, P.; Wudtiwai, B.; Shwe, T.H.; Pothacharoen, P.; Phitak, T. Inhibitory Effect of Hesperidin on the Expression of Programmed Death Ligand (PD-L1) in Breast Cancer. Molecules 2020, 25, 252. [Google Scholar] [CrossRef]
- Amalina, N.; Nurhayati, I.P.; Meiyanto, E. Doxorubicin Induces Lamellipodia Formation and Cell Migration. Indones. J. Cancer Chemoprevention 2017, 8, 61. [Google Scholar] [CrossRef]
- Suzery, M.; Cahyono, B.; Amalina, N.D. Citrus Sinensis (L) Peels Extract Inhibits Metastasis of Breast Cancer Cells by Targeting the Downregulation Matrix Metalloproteinases-9. Open Access Maced. J. Med. Sci. 2021, 9, 464–469. [Google Scholar] [CrossRef]
- Barani, M.; Bilal, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanotechnology in Ovarian Cancer: Diagnosis and Treatment. Life Sci. 2021, 266, 118914. [Google Scholar] [CrossRef] [PubMed]
- Aiello, P.; Consalvi, S.; Poce, G.; Raguzzini, A.; Toti, E.; Palmery, M.; Biava, M.; Bernardi, M.; Kamal, M.A.; Perry, G.; et al. Dietary Flavonoids: Nano Delivery and Nanoparticles for Cancer Therapy. Semin. Cancer Biol. 2021, 69, 150–165. [Google Scholar] [CrossRef]
- Liu, C.H.; Grodzinski, P. Nanotechnology for Cancer Imaging: Advances, Challenges, and Clinical Op-Portunities. Radiol. Imaging Cancer 2021, 3, e200052. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids Nanoparticles in Cancer: Treatment, Prevention and Clinical Prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef]
- Ali, S.H.; Sulaiman, G.M.; Al-Halbosiy, M.M.F.; Jabir, M.S.; Hameed, A.H. Fabrication of Hesperidin Nanoparticles Loaded by Poly Lactic Co-Glycolic Acid for Improved Therapeutic Efficiency and Cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 378–394. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef]
- Wadhwa, R.; Paudel, K.R.; Chin, L.H.; Hon, C.M.; Madheswaran, T.; Gupta, G.; Panneerselvam, J.; Lakshmi, T.; Singh, S.K.; Gulati, M.; et al. Anti-Inflammatory and Anticancer Activities of Naringenin-Loaded Liquid Crystalline Nanoparticles in Vitro. J. Food Biochem. 2021, 45, e13572. [Google Scholar] [CrossRef]
- Askar, M.A.; El Shawi, O.E.; Abou Zaid, O.A.R.; Mansour, N.A.; Hanafy, A.M. Breast Cancer Suppression by Curcumin-Naringenin-Magnetic-Nano-Particles: In Vitro and in Vivo Studies. Tumour Biol. 2021, 43, 225–247. [Google Scholar] [CrossRef]
- Rajamani, S.; Radhakrishnan, A.; Sengodan, T.; Thangavelu, S. Augmented Anticancer Activity of Naringenin-Loaded TPGS Polymeric Nanosuspension for Drug Resistive MCF-7 Human Breast Cancer Cells. Drug Dev. Ind. Pharm. 2018, 44, 1752–1761. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Classification | Review Highlights |
|---|---|---|
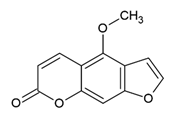 Bergapten 5-Methoxypsoralen | Polyphenol class: Other polyphenols Polyphenol sub-class: Furanocoumarins Family: Furanocoumarins | Anti-inflammatory, antimicrobial, antifungal, antiviral, anticancer, and antiosteoporosis [52]. Neuroprotection activity, effect on vitiligo and psoriasis, analgesic activity, immunosuppressive properties, and antidiabetics [53]. |
 Eriodictyol 5,7,3′,4′-Tetrahydroxyflavanone | Polyphenol class: Flavonoids Polyphenol sub-class: Flavanones Family: Flavanones | Antioxidant, anti-inflammatory, anticancer, neuroprotective, cardioprotective, hepatoprotective, anti-diabetic, and anti-obesity activity [50]. Skin protection, immunomodulatory, analgesic, antipyretic, antinociceptive, and miscellaneous activities [51]. |
 Auraptene 7-geranyloxycoumarin | Class: Phenol lipids Sub-class: Terpene Lactones Family: Terpene Lactones | Antitumor activity against BC, colorectal, ovarian, skin, gastric, esophageal, hepatic, and prostate cancer [56]. Cardioprotective, gastrointestinal protective, immune protective, and miscellaneous effects [57]. Effects on neurodegenerative diseases, periodontal disease, oncogenesis, cystic fibrosis, hypertension, and lipid profile [58]. |
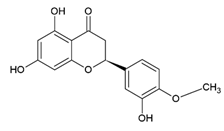 Hesperetin 5,7,3′-Trihydroxy-4′-methoxyflavanone | Polyphenol class: Flavonoids Polyphenol sub-class: Flavanones Family: Methoxyflavanones | Antioxidant and anti-inflammatory effects [45]. Anticancer activities against glioblastoma, breast, lung, prostate, colon, liver, pancreatic, kidney, gastric, oral, ovarian, and leukemia [46]. |
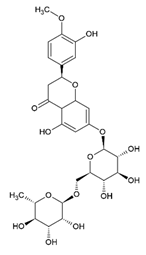 Hesperidin Hesperetin 7-O-rutinoside | Polyphenol class: Flavonoids Polyphenol sub-class: Flavanones Family: Flavanones | Effects on cardiovascular, neurological, psychiatric disorders, and antitumor activity [42]. Lipid metabolism, glucose metabolism, and inflammation activity [43]. Improvements in epidermal permeability barrier function, protection against UV irradiation, melanogenesis, acceleration of cutaneous wound healing, antioxidant [44]. |
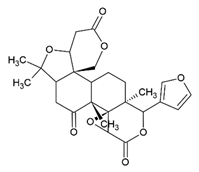 Limonin | Class: Prenol lipids Sub-class: Triterpenoids | Anticancer, anti-inflammatory, analgesic, antibacterial, antiviral, anti-insect, antioxidant, liver protection, neuroprotection, anti-osteoporosis, anti-obesity, and anti-allergy activities [59]. |
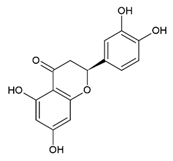
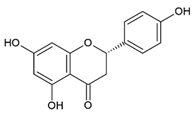 Naringenin 5,7,4′-Trihydroxyflavanone | Polyphenol class: Flavonoids Polyphenol sub-class: Flavanones Family: Flavanones | Immunomodulator [60], neuroprotective effects [61], anticancer activity against breast, colorectal, lung, liver, brain, leukemia, lymphoma, skin, cervical, prostate, pancreatic, gastric, oral, osteosarcoma, bladder, and ovarian cancer [62]. Effects on atherosclerosis, coronary artery disease, hypertension, cardiac hypertrophy, myocardial infarction, ischemic stroke [63], and fibrosis [64]. Antioxidant, antiviral, and antidiabetic [65]. |
 Naringin Naringenin 7-O-neohesperidoside | Polyphenol class: Flavonoids Polyphenol sub-class: Flavanones Family: Flavanones | Effect on obesity, diabetes, hypertension, cardiac toxicity, hypertrophy, remodeling, steatosis, hepatic protection, atherosclerosis, oxidative stress [66], metabolic syndrome, bone regeneration, genetic damage, central nervous system (CNS) diseases, anticancer and anti-inflammatory activity [67,68]. |
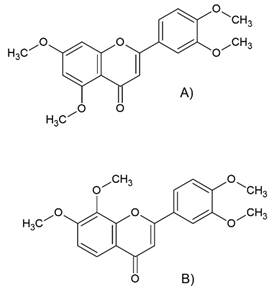 (A) 5,7,3′,4′-Tetra-methoxyflavone (B) 7,8,3′,4′-Tetra-methoxyflavone | Polyphenol class: Other polyphenols Polyphenol sub-class: Polymethoxyflavones Family: Polymethoxyflavones | Effects on circadian rhythm and metabolism [47] and neurodegenerative diseases [48]. Induces apoptosis via modulating the anti-tumor immunity, endoplasmic reticulum (ER) stress-mediated apoptosis, epigenetics modulators, protective autophagy, pyroptosis, ferroptosis, and anoikis cell death [49]. |
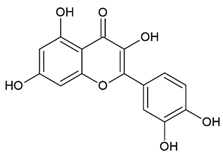 Quercetin 3,5,7,3′,4′-Pentahydroxyflavone | Polyphenol class: Flavonoids Polyphenol sub-class: Flavanols Family: Flavonols | Anti-cancer properties in BC, prostate cancer, ovarian cancer, lung cancer, colon cancer, hepatocellular carcinoma, lymphoma, and pancreatic cancer [15]. Effects on autoimmune diseases [35], metabolic syndrome [36], oxidative stress, and autophagy [37]. Anti-allergic [38], anti-inflammatory, anti-hypertensive [39], antiviral [40], and neuroprotective efficacy [41]. |
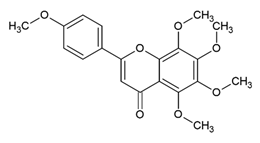 Tangeretin 5,6,7,8,4′-Pentamethoxyflavone | Polyphenol class: Flavonoids Polyphenol sub-class: Flavanes Family: methoxyflavones | Antitumor, neuroprotective, antidiabetic, hepatoprotective, immunomodulatory, melanogenesis, and antioxidant activities [54]. Induces apoptosis and autophagy and suppresses migration, invasion, and angiogenesis [55]. |
| Compounds | Type of Study | Experimental Aspects | Proposed Mechanism | Reference |
|---|---|---|---|---|
| Naringenin | In vitro and in vivo | MDA-MB-231 and MCF-10A cell lines and female Wistar rats (120–160 g) | ↓cell proliferation, tumor incidence and weight, TBARS, SOD, catalase, protein carbonyl, nitrate, GSH, vitamin C, vitamin E, GR, Bax, and Bad, ↑body weight (DMBA group) ↑G0/G1 and sub-G1 cell cycle, ↑caspase-3/-7, Apaf-1, VDAC, Bcl-2, cytochrome c, Bcl-xl, and procaspase-9 | [123] |
| In vitro | MCF-7, HT29, HeLa, DU145, and C8-D1A cell lines | For MCF-7: ↓cell proliferation, ↑expression P53 gene, Bax, cytochrome c, Apaf-1, and caspase-3 | [124] | |
| In vitro | MDA-MB-231 cell line | ↓cell proliferation, migration, invasion, and colony formation, ↑apoptosis, caspases-3/-8/-9, Bax, and ↓Bcl-2, ↑G2/M cell cycle | [125] | |
| In vitro | MDA-MB-231 cell line | ↓cell viability, colony formation, percentage of pH3-positive cells, and ↑apoptosis, caspase-3/-9, anti-PARP levels, LDH release and G2/M cell cycle | [126] | |
| In silico and in vitro | MCF-7 and A549 cell lines | ↓cell viability and colony formation, ↑apoptosis and binding affinity to CDK6 | [127] | |
| In vitro | MDA-MB-231 and MCF-10A cell lines | ↓cell proliferation, migration, colony number and size, pro-MMP9 activity, and ↑induce apoptosis/necrosis | [129] | |
| In vivo | Female Sprague Dawley rats (80–120 g) and female Balb/c mice (18–22 g) | ↓tumor weight, volume, and ↑tumor necrosis | [128] | |
| In vitro | MCF-7 and HepG2 cell lines | ↓cell viability, ↑apoptosis, caspase-3/-8, Bax, Bid, Bad, p53, ↑GRP78 and CHOP | [130] | |
| Hesperidin | In vitro | MDA-MB-231 and HepG2 cell lines | ↓cell viability, ↑caspase-3, Bax, and p53, ↓Bcl-2, ↓MMP1, ↑ROS, ↑G2/M cell cycle, apoptotic and nuclear fragmentation | [131] |
| In vitro | MCF-7 and MDA-MB-231 cell lines | ↓cell viability, cell cycle arrest, and ↑apoptosis | [132] | |
| In vitro | MCF-7 and HEK 293 cell lines | ↓cell viability, ↑number of apoptotic cells, ↑G0/G1 and sub-G1 cell cycle, ↑caspase-3/-9, ↑miR-16 and -34a, ↓miR-21, ↑Bax and ↓Bcl-2 | [133] | |
| In vitro | MCF-7-resistant doxorubicin cells (MCF-7/Dox) | ↓cell viability and expression of Pgp | [135] | |
| In vitro | MCF-7 cell line | ↓cell viability, ↑cells in sub-G1 phase, ↑early apoptosis, ↓GSH, ↑DNA damage, ↓expression of DNA repair genes | [136] | |
| In vitro | MCF-7 breast cancer cell line | ↓cell viability, mammosphere formation, colony formation, cell migration, ↑G0/G1 cell cycle, ↓p21, ↑cyclin D1, ↓ALDH1, ↓MMP9, ↑p53, and ↓Bcl-2 | [137] | |
| In vivo | Female Wistar rats | ↑Survival rate, ↑body weight, ↓tumor volume, tumor spread and invasion, ↓MDA, ↑GSH, ↑IL-1β, ↓IL-6, NF-κB, TNF-α, and Ki67 expression | [138] | |
| In vivo | Adult male Wistar rats (120–150 g) | ↓ALT, AST, TG, TC and MDA, ↑GSH, ↓hepatic NO, ↓NF-κB, ↑p-Akt expression | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madureira, M.B.; Concato, V.M.; Cruz, E.M.S.; Bitencourt de Morais, J.M.; Inoue, F.S.R.; Concimo Santos, N.; Gonçalves, M.D.; Cremer de Souza, M.; Basso Scandolara, T.; Fontana Mezoni, M.; et al. Naringenin and Hesperidin as Promising Alternatives for Prevention and Co-Adjuvant Therapy for Breast Cancer. Antioxidants 2023, 12, 586. https://doi.org/10.3390/antiox12030586
Madureira MB, Concato VM, Cruz EMS, Bitencourt de Morais JM, Inoue FSR, Concimo Santos N, Gonçalves MD, Cremer de Souza M, Basso Scandolara T, Fontana Mezoni M, et al. Naringenin and Hesperidin as Promising Alternatives for Prevention and Co-Adjuvant Therapy for Breast Cancer. Antioxidants. 2023; 12(3):586. https://doi.org/10.3390/antiox12030586
Chicago/Turabian StyleMadureira, Maria Beatriz, Virginia Marcia Concato, Ellen Mayara Souza Cruz, Juliana Maria Bitencourt de Morais, Fabricio Seidy Ribeiro Inoue, Natália Concimo Santos, Manoela Daniele Gonçalves, Milena Cremer de Souza, Thalita Basso Scandolara, Mariane Fontana Mezoni, and et al. 2023. "Naringenin and Hesperidin as Promising Alternatives for Prevention and Co-Adjuvant Therapy for Breast Cancer" Antioxidants 12, no. 3: 586. https://doi.org/10.3390/antiox12030586
APA StyleMadureira, M. B., Concato, V. M., Cruz, E. M. S., Bitencourt de Morais, J. M., Inoue, F. S. R., Concimo Santos, N., Gonçalves, M. D., Cremer de Souza, M., Basso Scandolara, T., Fontana Mezoni, M., Galvani, M., Rodrigues Ferreira Seiva, F., Panis, C., Miranda-Sapla, M. M., & Pavanelli, W. R. (2023). Naringenin and Hesperidin as Promising Alternatives for Prevention and Co-Adjuvant Therapy for Breast Cancer. Antioxidants, 12(3), 586. https://doi.org/10.3390/antiox12030586








