Insight into Antioxidant Activity and Peptide Profile of Jinhua Ham Broth Peptides at Different Cooking Times
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. FAAs Analysis
2.4. Peptide Content Determination
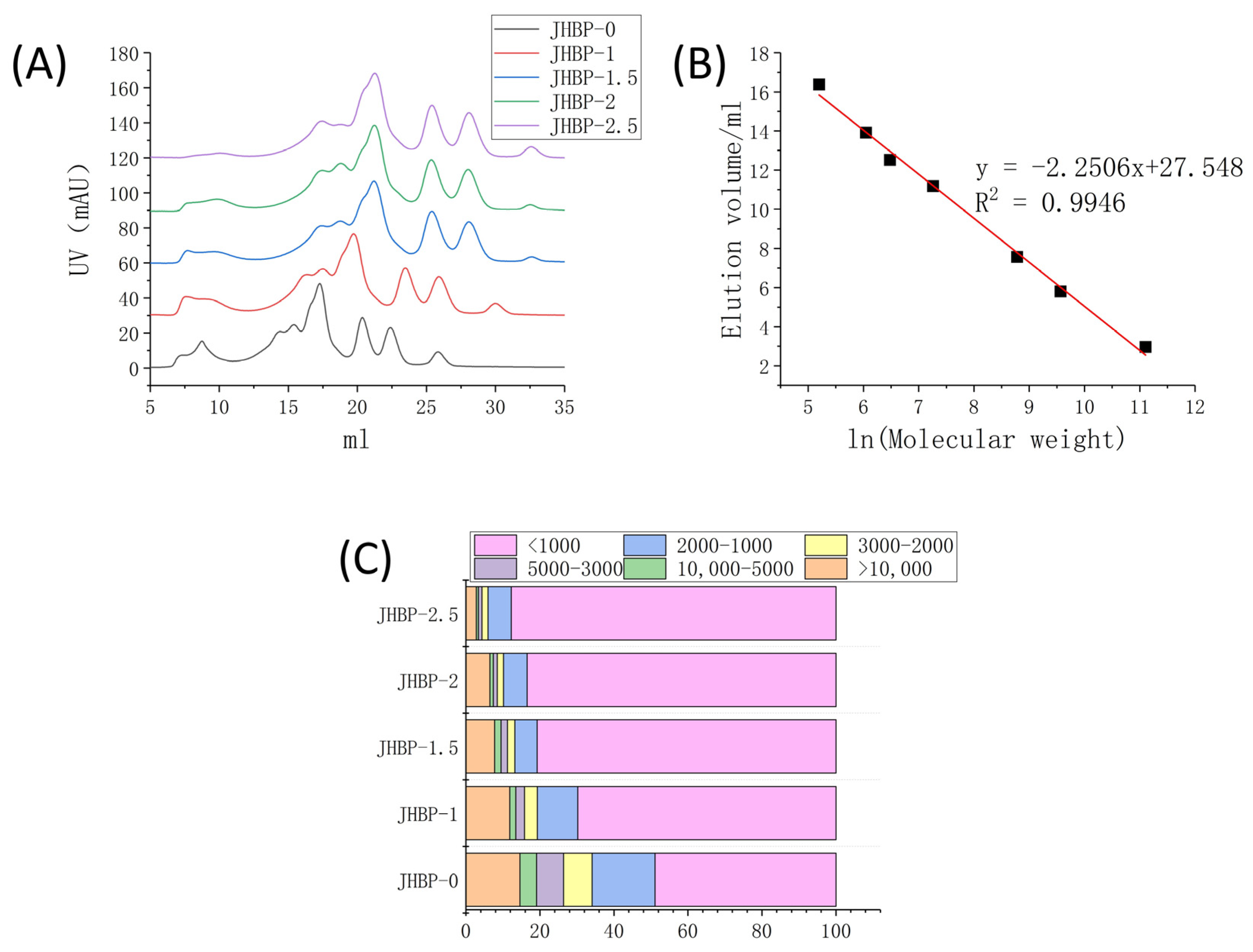
2.5. Determination of Peptide Molecular Weight (MW) Distribution
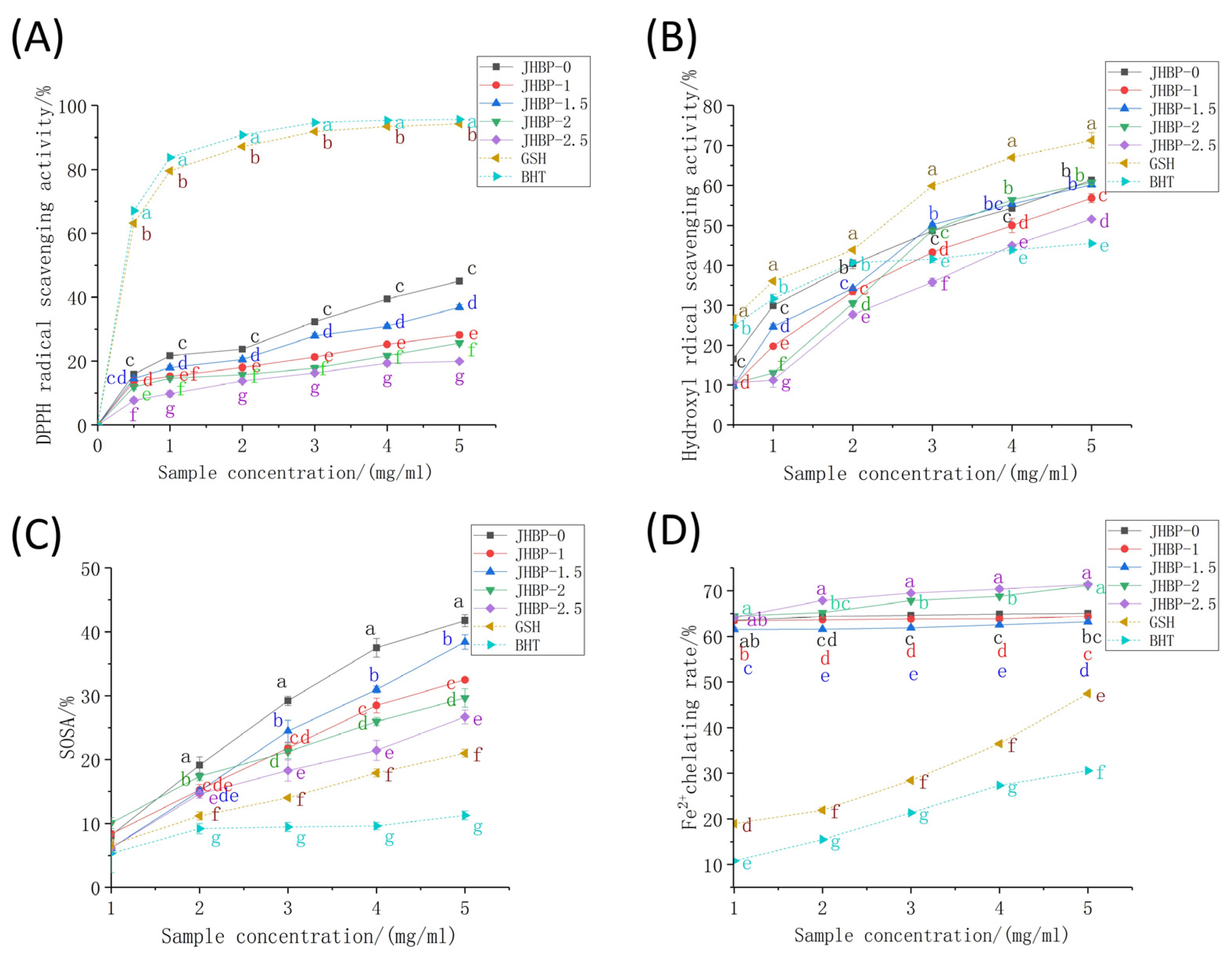
2.6. DPPH Free Radical Scavenging Ability
2.7. Hydroxyl Radical Scavenging Efficacy (•OH)
2.8. Superoxide Anion Radical Scavenging Action (SOSA)
2.9. Fe2+ Chelating Capacity
2.10. Ferric-Reducing Antioxidant Power (FRAP)
2.11. ABTS+ Radical Scavenging Capacity
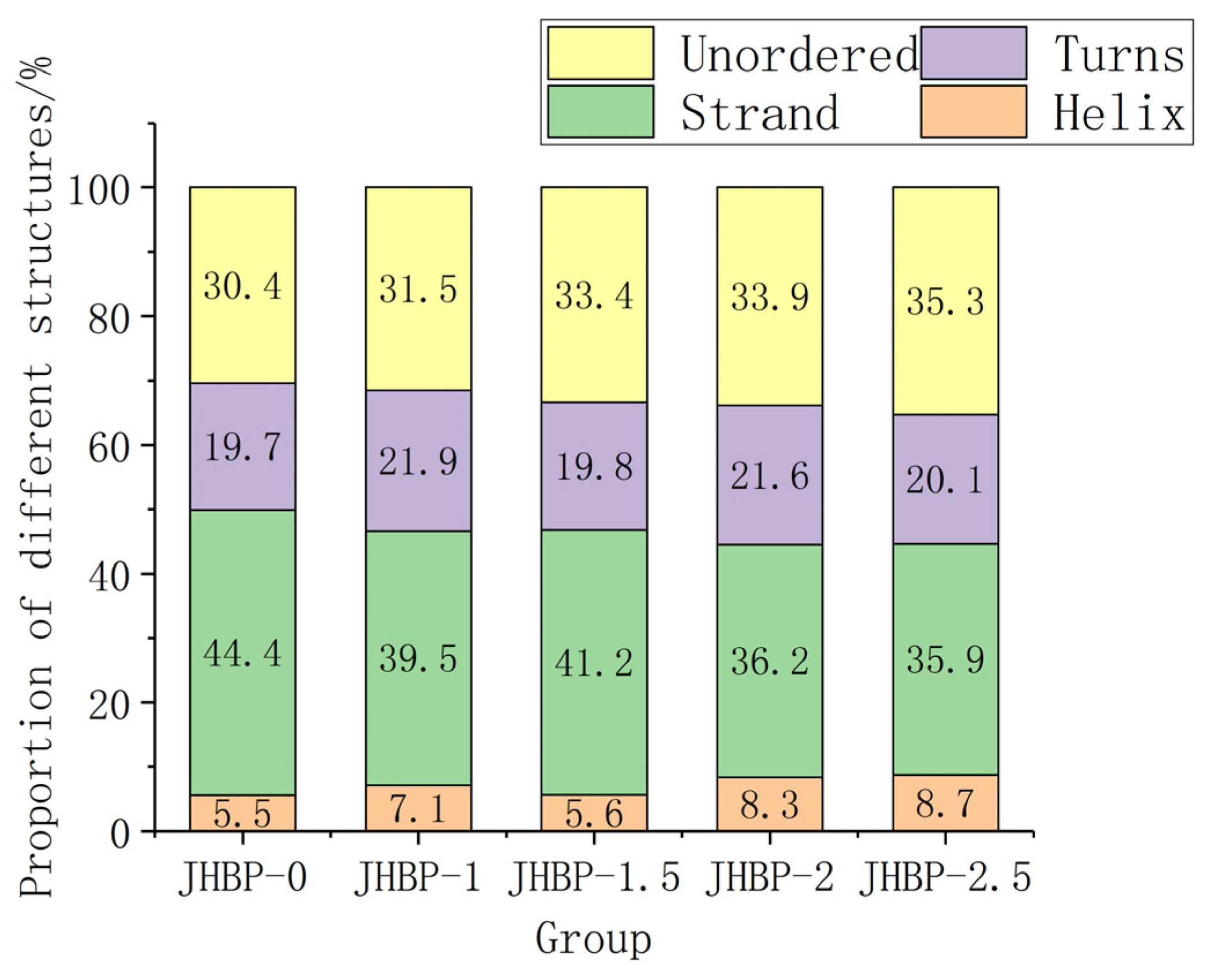
2.12. CD Spectra of the Peptides
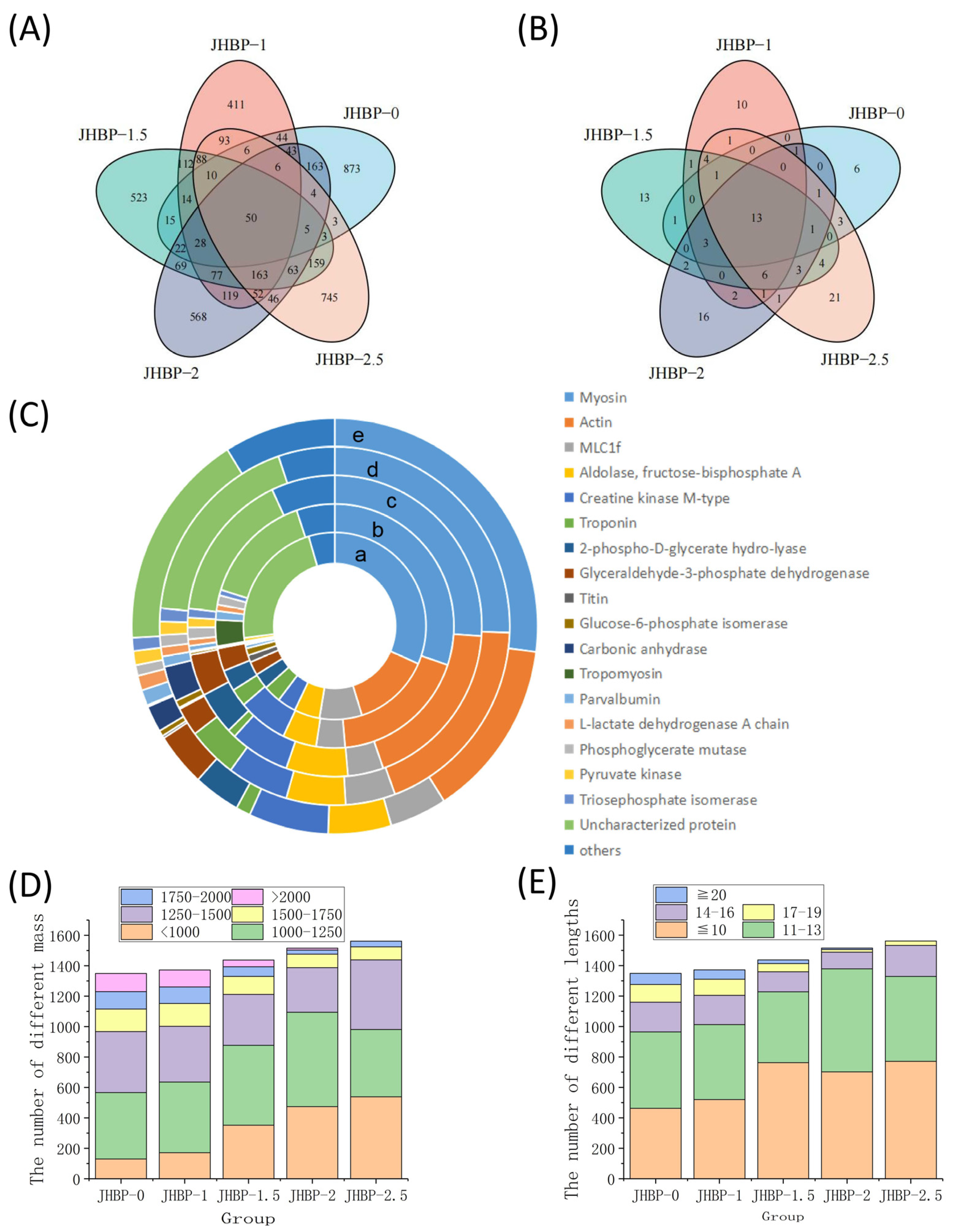
2.13. Characterization of Peptide Sequences
2.14. Statistical Analysis
3. Results and Discussion
3.1. FAAs
3.2. Peptide Content in Jinhua Ham Broth
3.3. Peptide Molecular Weight (MW) Distribution
3.4. Antioxidation Activity of JHBP
3.5. Secondary Structure of Peptides
3.6. Peptidomics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toldra, F.; Gallego, M.; Reig, M.; Aristoy, M.; Mora, L. Bioactive peptides generated in the processing of dry-cured ham. Food Chem. 2020, 321, 126689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhen, Z.; Zhang, W.; Zeng, T.; Zhou, G. Effect of intensifying high-temperature ripening on proteolysis, lipolysis and flavor of Jinhua ham. J. Sci. Food Agric. 2009, 89, 834–842. [Google Scholar] [CrossRef]
- Yin, Y.; Pereira, J.; Zhou, L.; Lorenzo, J.M.; Tian, X.; Zhang, W. Insight into the effects of sous vide on cathepsin B and L activities, protein degradation and the ultrastructure of beef. Foods 2020, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Toldra, F. The relevance of dipeptides and tripeptides in the bioactivity and taste of dry-cured ham. Food Prod. Process. Nutr. 2019, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.; Zhang, W. A review of antioxidant peptides derived from meat muscle and by-products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Liu, R.; Cao, S.; Zhang, W.; Zhou, G. Meat protein based bioactive peptides and their potential functional activity: A review. Int. J. Food Sci. Technol. 2019, 54, 1956–1966. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Naveena, B.M.; Jo, C.; Sakata, R.; Zhou, G.; Banerjee, R.; Nishiumi, T. Technological demands of meat processing-an asian perspective. Meat Sci. 2017, 132, 35–44. [Google Scholar] [CrossRef]
- Wang, Z. Chinese Medicinal Diet Dictionary; Traditional Chinese Medicine Classics Press: Beijing, China, 2017; p. 837. [Google Scholar]
- Dai, Y.; Zhang, Q.; Wang, L.; Liu, Y.; Li, X.; Dai, R. Changes in shear parameters, protein degradation and ultrastructure of pork following water bath and ohmic cooking. Food Bioprocess Technol. 2014, 7, 1393–1403. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, W.; Kang, Z.; Zhou, G.; Xu, X. Stability of an antioxidant peptide extracted from Jinhua ham. Meat Sci. 2014, 96, 783–789. [Google Scholar] [CrossRef]
- Xing, L.; Hu, Y.; Hu, H.; Ge, Q.; Zhou, G.; Zhang, W. Purification and identification of antioxidative peptides from dry-cured Xuanwei ham. Food Chem. 2016, 194, 951–958. [Google Scholar] [CrossRef]
- Xing, L.; Liu, R.; Gao, X.; Zheng, J.; Wang, C.; Zhou, G.; Zhang, W. The proteomics homology of antioxidant peptides extracted from dry-cured Xuanwei and Jinhua ham. Food Chem. 2018, 266, 420–426. [Google Scholar] [CrossRef]
- Xing, L.; Fu, L.; Toldra, F.; Teng, S.; Yin, Y.; Zhang, W. The stability of dry-cured ham-derived peptides and its anti-inflammatory effect in RAW264.7 macrophage cells. Int. J. Food Sci. Technol. 2022, 58. [Google Scholar] [CrossRef]
- Erickson, M.C. Lipid extraction from channel catfish muscle-comparison of solvent systems. J. Food Sci 1993, 58, 84–89. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Zhang, G.; Tian, Y.; Kong, F.; Xiong, S.; Zhao, S.; Jia, D.; Manyande, A.; Du, H. Identification of novel antioxidant peptides from snakehead (Channa argus) soup generated during gastrointestinal digestion and insights into the anti-oxidation mechanisms. Food Chem. 2021, 337, 127921. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, L.; Le, T.T.; Larsen, L.B.; Su, G.; Liang, Y.; Li, B. Digestibility of glyoxal-glycated beta-casein and beta-lactoglobulin and distribution of peptide-bound advanced glycation end products in gastrointestinal digests. J. Agric. Food Chem. 2017, 65, 5778–5788. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ning, P.; Jiao, Y.; Xu, Z.; Cheng, Y. Extraction of antioxidant peptides from rice dreg protein hydrolysate via an angling method. Food Chem. 2021, 337, 128069. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Feng, M.; Sun, J. Influence of mixed starters on the degradation of proteins and the formation of peptides with antioxidant activities in dry fermented sausages. Food Control 2021, 123, 107743. [Google Scholar] [CrossRef]
- Rotola-Pukkila, M.K.; Pihlajaviita, S.T.; Kaimainen, M.T.; Hopia, A.I. Concentration of umami compounds in pork meat and cooking juice with different cooking times and temperatures. J. Food Sci. 2015, 80, C2711–C2716. [Google Scholar] [CrossRef]
- Sasaki, K.; Motoyama, M.; Mitsumoto, M. Changes in the amounts of water-soluble umami-related substances in porcine longissimus and biceps femoris muscles during moist heat cooking. Meat Sci. 2007, 77, 167–172. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, W.; Sun, L.; Liu, Y.; Li, M.; Zhao, G. Characterization of protein changes and development of flavor components induced by thermal modulation during the cooking of chicken meat. J. Food Process. Preserv. 2019, 43, e13949. [Google Scholar] [CrossRef]
- Fu, L.; Xing, L.; Hao, Y.; Yang, Z.; Teng, S.; Wei, L.; Zhang, W. The anti-inflammatory effects of dry-cured ham derived peptides in RAW264.7 macrophage cells. J. Funct. Foods 2021, 87, 104827. [Google Scholar] [CrossRef]
- Song, Y.; Huang, F.; Li, X.; Han, D.; Zhang, C. Effects of different wet heating methods on the water distribution, microstructure and protein denaturation of pork steaks. Int. J. Food Sci. Technol. 2021, 56, 4627–4638. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Jin, G.; Duan, J.; Zhang, Y.; Cao, J. The texture change of chinese traditional pig trotter with soy sauce during stewing processing: Based on a thermal degradation model of collagen fibers. Foods 2022, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, A.; Gambacorta, E.; Perna, A. Antioxidative and antihypertensive activities of pig meat before and after cooking and in vitro gastrointestinal digestion: Comparison between Italian autochthonous pig Suino Nero Lucano and a modern crossbred pig. Food Chem. 2016, 212, 590–595. [Google Scholar] [CrossRef]
- Jensen, I.; Dort, J.; Eilertsen, K. Proximate composition, antihypertensive and antioxidative properties of the semimembranosus muscle from pork and beef after cooking and in vitro digestion. Meat Sci. 2014, 96, 916–921. [Google Scholar] [CrossRef]
- Remanan, M.K.; Wu, J. Antioxidant activity in cooked and simulated digested eggs. Food Funct. 2014, 5, 1464–1474. [Google Scholar] [CrossRef]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Han, R.; Cheng, H.; Feng, R.; Li, D.; Lai, W.; Zhang, J.; Skibsted, L.H. beta-Carotene As a Lipophilic Scavenger of Nitric Oxide. J. Phys. Chem B 2014, 118, 11659–11666. [Google Scholar] [CrossRef]
- Schmid, S.; Adjobo-Hermans, M.J.W.; Kohze, R.; Enderle, T.; Brock, R.; Milletti, F. Identification of short hydrophobic cell-penetrating peptides for cytosolic peptide delivery by rational design. Bioconjug. Chem. 2017, 28, 382–389. [Google Scholar] [CrossRef]
- Lacou, L.; Leonil, J.; Gagnaire, V. Functional properties of peptides: From single peptide solutions to a mixture of peptides in food products. Food Hydrocoll. 2016, 57, 187–199. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Zhu, Y.; Wang, Y.; He, S.; Zhang, T. Enhancing the in vitro antioxidant capacities via the interaction of amino acids. Emir. J. Food Agric. 2018, 30, 224–231. [Google Scholar]
- Udenigwe, C.C.; Aluko, R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, H.; Winkler-Moser, J.K. Antioxidant activity of amino acids in soybean oil at frying temperature: Structural effects and synergism with tocopherols. Food Chem. 2017, 221, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xia, T.; Duan, W.; Zhang, Z.; Li, Y.; Fang, B.; Xia, M.; Wang, M. Effects of organic acids, amino acids and phenolic compounds on antioxidant characteristic of zhenjiang aromatic vinegar. Molecules 2019, 24, 3799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Nokihara, K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J. Agric. Food Chem. 1996, 44, 2619–2623. [Google Scholar] [CrossRef]
- Hernandez-Ledesma, B.; Davalos, A.; Bartolome, B.; Amigo, L. Preparation of antioxidant enzymatic hydrolysates from (alpha-lactalbumin and beta-lactoglobulin. Identification of active peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 588–593. [Google Scholar] [CrossRef]
- Yuan, H.; Lv, J.; Gong, J.; Xiao, G.; Zhu, R.; Li, L.; Qiu, J. Secondary structures and their effects on antioxidant capacity of antioxidant peptides in yogurt. Int. J. Food Prop. 2018, 21, 2167–2180. [Google Scholar] [CrossRef] [Green Version]
| JHBP-0/ (mg/50 g ham) | JHBP-1/ (mg/100 mL Broth) | JHBP-1.5/ (mg/100 mL Broth) | JHBP-2/ (mg/100 mL Broth) | JHBP-2.5/ (mg/100 mL Broth) | |
|---|---|---|---|---|---|
| Asp | 26.71 ± 0.36 Df | 33.07 ± 0.39 Cf | 35.73 ± 0.85 Cf | 57.82 ± 3.00 Bf | 65.85 ± 0.76 Af |
| Thr | 23.50 ± 0.39 Dh | 28.71 ± 0.40 Ch | 30.14 ± 0.68 Ch | 48.65 ± 1.86 Bg | 56.30 ± 0.65 Ah |
| Ser | 25.63 ± 0.51 Bfg | 31.01 ± 0.31 Bg | 32.69 ± 1.20 Bg | 57.42 ± 7.42 Af | 61.38 ± 0.93 Ag |
| Glu | 67.43 ± 0.78 Eb | 83.88 ± 1.33 Db | 88.89 ± 2.05 Cb | 138.02 ± 0.97 Bb | 162.95 ± 2.40 Ab |
| Gly | 16.06 ± 0.42 Dj | 19.46 ± 0.26 Bk | 20.16 ± 0.67 Bk | 34.21 ± 3.20 Akj | 37.60 ± 0.49 Ak |
| Ala | 45.16 ± 0.67 Dc | 54.89 ± 1.34 Cc | 57.48 ± 1.36 Cc | 91.03 ± 2.96 Bc | 107.15 ± 1.51 Ac |
| Val | 32.48 ± 0.49 De | 38.11 ± 0.70 Ce | 40.21 ± 1.00 Ce | 63.50 ± 1.86 Be | 75.63 ± 1.62 Ae |
| Met | 13.15 ± 0.57 Dk | 15.35 ± 0.07 Cm | 15.89 ± 0.24 Cl | 25.26 ± 0.57 Bl | 30.46 ± 0.37 Al |
| Ile | 21.61 ± 0.34 Di | 24.38 ± 0.47 Cj | 25.61 ± 0.12 Ci | 41.36 ± 1.50 Bi | 49.88 ± 0.86 Ai |
| Leu | 40.91 ± 0.79 Dd | 46.18 ± 0.60 Cd | 48.44 ± 0.27 Cd | 78.62 ± 2.06 Bd | 94.38 ± 1.84 Ad |
| Tyr | 15.35 ± 0.90 Ej | 19.73 ± 0.21 Dk | 22.67 ± 0.49 Cj | 35.88 ± 0.86 Bj | 43.79 ± 1.23 Aj |
| Phe | 24.02 ± 1.52 Dgh | 28.63 ± 0.55 Ch | 29.49 ± 1.67 Ch | 47.51 ± 0.79 Bgh | 57.22 ± 0.81 Ah |
| Lys | 70.67 ± 1.34 Da | 94.26 ± 1.14 Ca | 96.67 ± 1.44 Ca | 152.49 ± 1.88 Ba | 190.77 ± 4.52 Aa |
| His | 13.52 ± 0.30 Dk | 17.77 ± 0.11 Cl | 18.13 ± 0.13 Ck | 30.39 ± 1.60 Bkl | 36.28 ± 0.96 Ak |
| Arg | 33.74 ± 1.35 De | 45.12 ± 0.33 Cd | 46.63 ± 0.76 Cd | 74.09 ± 1.32 Bd | 94.25 ± 3.09 Ad |
| Pro | 21.61 ± 0.62 Di | 26.79 ± 0.89 Ci | 27.41 ± 0.45 Ci | 42.52 ± 1.48 Bhi | 49.98 ± 0.63 Ai |
| Total | 491.54 ± 4.96 D | 607.33 ± 7.05 C | 636.22 ± 6.32 C | 1018.76 ± 18.98 B | 1213.86 ± 20.20 A |
| Group | Crude Extracts g/100 g ham | Peptide Content in Crude Extracts/% | Peptide Extraction/% |
|---|---|---|---|
| JHBP-0 | 9.77 ± 0.88 d | 89.99 ± 0.65 a | 8.80 ± 0.80 d |
| JHBP-1 | 12.19 ± 0.30 c | 87.59 ± 0.97 b | 10.67 ± 0.24 c |
| JHBP-1.5 | 14.33 ± 0.33 b | 87.21 ± 1.28 b | 12.50 ± 0.15 b |
| JHBP-2 | 15.66 ± 041 a | 86.71 ± 1.38 b | 13.58 ± 0.19 a |
| JHBP-2.5 | 15.94 ± 0.46 a | 86.87 ± 0.90 b | 13.85 ± 0.28 a |
| Group | FRAP (μmol Fe2+/g) | ABTS+ (μmol TE/g) |
|---|---|---|
| JHBP-0 | 29.58 ± 3.01 a | 261.88 ± 4.22 e |
| JHBP-1 | 19.18 ± 1.00 c | 272.74 ± 0.68 d |
| JHBP-1.5 | 25.06 ± 2.91 ab | 317.99 ± 4.05 c |
| JHBP-2 | 22.09 ± 3.79 bc | 374.05 ± 3.44 b |
| JHBP-2.5 | 20.30 ± 1.02 c | 390.02 ± 0.53 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Cai, J.; Boateng, E.F.; Xing, L.; Zhang, W. Insight into Antioxidant Activity and Peptide Profile of Jinhua Ham Broth Peptides at Different Cooking Times. Antioxidants 2023, 12, 606. https://doi.org/10.3390/antiox12030606
Yang Z, Cai J, Boateng EF, Xing L, Zhang W. Insight into Antioxidant Activity and Peptide Profile of Jinhua Ham Broth Peptides at Different Cooking Times. Antioxidants. 2023; 12(3):606. https://doi.org/10.3390/antiox12030606
Chicago/Turabian StyleYang, Ziyi, Jiaming Cai, Evans Frimpong Boateng, Lujuan Xing, and Wangang Zhang. 2023. "Insight into Antioxidant Activity and Peptide Profile of Jinhua Ham Broth Peptides at Different Cooking Times" Antioxidants 12, no. 3: 606. https://doi.org/10.3390/antiox12030606







