Pressurized Liquid Extraction for the Recovery of Bioactive Compounds from Seaweeds for Food Industry Application: A Review
Abstract
:1. Introduction
1.1. Seaweeds as a Bioactive Compound Matrix
1.2. Extraction Techniques Applied in Seaweeds Matrices
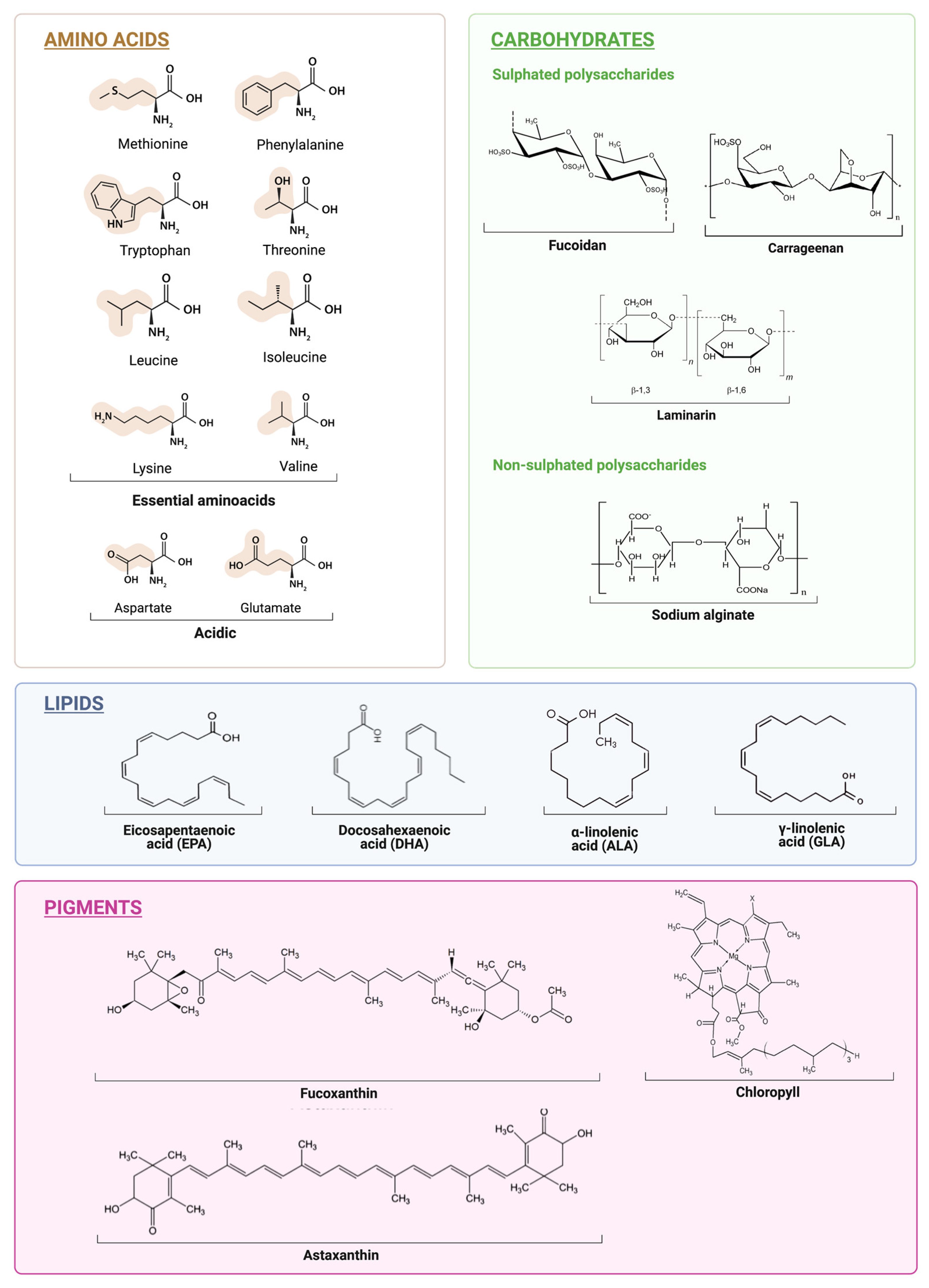
2. Matrix Components
2.1. Proteins
2.2. Carbohydrates
2.3. Lipids
2.4. Pigments
2.5. Metals
3. Pressurized Liquid Extraction (PLE)
3.1. General Aspects of PLE
3.2. Sample Pre-Treatments
- −
- Drying: the objective of this step is to remove water from the matrix, increasing extraction efficiency [23]. Air-drying, oven heating or lyophilization can all be used, with the latter being the most advantageous because it takes less time and does not degrade the compounds. Indeed, when non-polar solvents are used, this step is critical and a desiccant is commonly included in the PLE cell [22].
- −
- Homogenization: by grinding, the sample should be distributed in a homogeneous manner [22].
- −
- Sieving: this step increases the surface area of the analyte as well as the diffusion of the analyte from the matrix to the solvent [23]. This step yielded a similar particle size in which 2 mm is commonly used for PLE [22]. After sieving you can carry out grinding of the separated portion with greater particle size.
- −
- Dispersion with an inert material: this step is recommended for some samples to avoid aggregation of particles that may lead to alteration in the extraction efficiency [23].
3.3. Relevant Parameters in PLE
3.3.1. Solvent
3.3.2. Temperature and Pressure
3.3.3. Time and Number of Cycles
3.4. Post-Extraction Treatment (Clean-Up)
- −
- On-cell clean-up technique: the solvent is passed through the cell to elute interferences prior to the extraction step. This reduces the extraction time required and enables the process to be automated. On the other hand, the analyte must have a different polarity than the compounds that cause the interference. Moreover, finding a solvent capable of eliminating the interferences without causing damage to the analytes can be difficult [78].
- −
- Liquid chromatography techniques: they are used to remove interferences from complex matrices. The most used techniques are normal phase liquid extraction (NPLE) and gel permeation chromatography (GPC). On the one hand, NPLE is used as a preparative chromatography in a glass column filled in-house with the stationary phase [78], whereas GPC is a purifying technique based in the separation depending on the molecular size of the compounds. The main advantages of GPC are its ability to be automated and the clean-up capacity maintenance for months. Divinylbenzene-linked polystyrene gel is the main material used for GPC [22].
4. Combinatorial Approaches of PLE with New Extraction Methodologies
4.1. PLE Combined with SPE
4.2. PLE Combined with UAE
4.3. PLE Combined with SFE-CO2
4.4. PLE Combined with EAE
5. Evaluation of Pressurized Liquid Extraction (PLE) Applications
5.1. Technological Function and Functional Ingredients
| Product | Seaweed | Form | Technological/Nutritional Function | Ref. |
|---|---|---|---|---|
| Pasta | Undaria pinnatifida | Powder | Fucoxanthin was not altered | [102] |
| Pasta | Sargassum marginatum | Powder | Lower cooking loss Better gluten network when 2.5% of seaweed was added | [103] |
| Beef patty | Undaria pinnatifida | Dried and ground | Lower cooking loss Better texture Higher quantities of mineral and fiber when 3% of seaweed was added | [104] |
| Chicken breast meat | Undaria pinnatifida | Carotenoid pigment Fucoxanthin | Red and yellow color were increased Inhibition of lipid peroxidation after cooking | [98] |
| Restructured poultry steak | Hymanthalia elongata | Powder | Lower cooking loss | [105] |
| Pork emulsion meat | Hymanthalia elongata, Undaria pinnatifida, Porphyra umbilicalis | Dried and ground | Higher antioxidant ability Omega-3 was increased Higher mineral profile Higher amino acid profile (P. umbilicalis) | [93] |
| Low-fat Frankfurters | Hymanthalia elongata | Dried and ground | Higher levels of w-3 Higher quantity of fiber | [106] |
| Cod | Fucus vesiculosus | Extract and subfractions of phlorotannin | Inhibition of lipid oxidation in fish model systems | [107] |
| Fish oil-enriched granola bar | Fucus vesiculosus | Phlorotannin extract | Inhibition of lipid oxidation | [108] |
| Canola oil stored under favorable oxidation conditions | Fucus vesiculosus, Ascophyllum nodosum, Bifurcaria bifurcata | Phenolic compounds | Reduction of lipid oxidation | [109] |
| Pork homogenates | Laminaria digitata | Fucoidan extract | Reduction of lipid oxidation Oxidation of myoglobin | [110] |
| Fish oil-enriched mayonnaise | Fucus vesiculosus | Phenolic compounds | Prevention of lipid oxidation | [111] |
| Fish oil-enriched milk | Fucus vesiculosus | Phlorotannin | Prevention of lipid oxidation | [112] |
| Cookies | Fucus vesiculosus, Ascophyllum nodosum, Bifurcaria bifurcata | Phenolic compounds | Antioxidant effect | [113] |
| Bread | Sargassum fulvellum | Powder | The shelf life is increased Less hardness and gumminess | [114] |
| Bread | Kappaphycus alvarezii | Powder | High dietary fiber content | [115] |
5.2. Application as Innovative Food Packaging
6. Advantages and Drawbacks of the Application of PLE as an Extraction Technique of the Bioactive Compounds from Seaweeds
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gutiérrez Cuesta, A.; García, G.; Rivera, H.; Suárez, A.; Delange, M. Algas marinas, fuente potencial de macronutrientes. Item Type Journal Contribution. Rev. Investig. Mar. 2017, 37, 14. Available online: http://hdl.handle.net/1834/12438 (accessed on 17 July 2022).
- Blikra, M.J.; Altintzoglou, T.; Løvdal, T.; Rognså, G.; Skipnes, D.; Skåra, T.; Sivertsvik, M.; Noriega Fernández, E. Seaweed products for the future: Using current tools to develop a sustainable food industry. Trends Food Sci. Technol. 2021, 118, 765–776. [Google Scholar] [CrossRef]
- Demarco, M.; Oliveira de Moraes, J.; Matos, Â.P.; Derner, R.B.; de Farias Neves, F.; Tribuzi, G. Digestibility, bioaccessibility and bioactivity of compounds from algae. Trends Food Sci. Technol. 2022, 121, 114–128. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Evaluation of the chemical composition and nutritional potential of brown macroalgae commercialised in China. Algal Res. 2022, 64, 102683. [Google Scholar] [CrossRef]
- Melo, T.; Alves, E.; Azevedo, V.; Martins, A.S.; Neves, B.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Lipidomics as a new approach for the bioprospecting of marine macroalgae—Unraveling the polar lipid and fatty acid composition of chondrus crispus. Algal Res. 2015, 8, 181–191. [Google Scholar] [CrossRef]
- Otero, P.; López-Martínez, M.I.; García-Risco, M.R. Application of pressurized liquid extraction (PLE) to obtain bioactive fatty acids and phenols from Laminaria ochroleuca collected in Galicia (NW Spain). J. Pharm. Biomed. Anal. 2019, 164, 86–92. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.W.; Jeon, J.S.; Jung, Y.J.; Kim, W.R.; Kim, C.Y.; Um, B.H. Determination of major phlorotannins in Eisenia bicyclis using hydrophilic interaction chromatography: Seasonal variation and extraction characteristics. Food Chem. 2013, 138, 2399–2406. [Google Scholar] [CrossRef]
- Shin, T.; Ahn, M.; Hyun, J.W.; Kim, S.H.; Moon, C. Antioxidant marine algae phlorotannins and radioprotection: A review of experimental evidence. Acta Histochem. 2014, 116, 669–674. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Vallejo, F.; Gil-Izquierdo, Á.; Andrade, P.B.; Valentão, P.; Pereira, D.M.; Ferreres, F. Profiling phlorotannins from Fucus spp. of the Northern Portuguese coastline: Chemical approach by HPLC-DAD-ESI/MSn and UPLC-ESI-QTOF/MS. Algal Res. 2018, 29, 113–120. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeon, Y.J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Périno, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Tiwari, B. Conventional extraction techniques: Solvent extraction. In Sustainable Seaweed Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 171–189. [Google Scholar] [CrossRef]
- Pereira, T.; Barroso, S.; Mendes, S.; Amaral, R.A.; Dias, J.R.; Baptista, T.; Saraiva, J.A.; Alves, N.M.; Gil, M.M. Optimization of phycobiliprotein pigments extraction from red algae Gracilaria gracilis for substitution of synthetic food colorants. Food Chem. 2020, 321, 126688. [Google Scholar] [CrossRef]
- Mena-García, A.; Ruiz-Matute, A.I.; Soria, A.C.; Sanz, M.L. Green techniques for extraction of bioactive carbohydrates. TrAC Trends Anal. Chem. 2019, 119, 115612. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Lourenço-Lopes, C.; Otero, P.; Carpena, M.; Gonzalez Pereira, A.; Echave, J.; Soria-Lopez, A.; Chamorro, F.; Prieto, M.A.; Simal-Gandara, J. Application of Green Extraction Techniques for Natural Additives Production. In Natural Food Additives; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Sabeena, S.F.; Alagarsamy, S.; Sattari, Z.; Al-Haddad, S.; Fakhraldeen, S.; Al-Ghunaim, A.; Al-Yamani, F. Enzyme-assisted extraction of bioactive compounds from brown seaweeds and characterization. J. Appl. Phycol. 2020, 32, 615–629. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction of biomolecules from seaweeds. In Seaweed Sustainability: Food and Non-Food Applications. Manchester; Academic Press: Cambridge, MA, USA, 2015; pp. 243–269. [Google Scholar] [CrossRef]
- Nieto, A.; Borrull, F.; Pocurull, E.; Marcé, R.M. Pressurized liquid extraction: A useful technique to extract pharmaceuticals and personal-care products from sewage sludge. TrAC Trends Anal. Chem. 2010, 29, 752–764. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Revilla-Ruiz, P.; Hernández-Méndez, J. Pressurized liquid extraction in the analysis of food and biological samples. J. Chromatogr. A 2005, 1089, 1–17. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Ortega-Barbosa, J.P.; del Pilar Sanchez-Camargo, A.; Rodríguez-Varela, L.I.; Parada-Alfonso, F. Pressurized Liquid Extraction of Bioactives. In Comprehensive Foodomics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 754–770. ISBN 9780128163955. [Google Scholar]
- Pavkovich, A.M.; Bell, D.S. Extraction. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Ashour, M.; Kamel, A.E.-W. Enhance Growth and Biochemical Composition of Nannochloropsis oceanica, Cultured under Nutrient Limitation, Using Commercial Agricultural Fertilizers. J. Mar. Sci. Res. Dev. 2017, 07, 233. [Google Scholar] [CrossRef] [Green Version]
- Zanella, L.; Vianello, F. Microalgae of the genus Nannochloropsis: Chemical composition and functional implications for human nutrition. J. Funct. Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- Pandeirada, C.O.; Maricato, É.; Ferreira, S.S.; Correia, V.G.; Pinheiro, B.A.; Evtuguin, D.V.; Palma, A.S.; Correia, A.; Vilanova, M.; Coimbra, M.A.; et al. Structural analysis and potential immunostimulatory activity of Nannochloropsis oculata polysaccharides. Carbohydr. Polym. 2019, 222, 114962. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Kim, S.M. Characterization and immunomodulatory activities of polysaccharides extracted from green alga Chlorella ellipsoidea. Int. J. Biol. Macromol. 2017, 95, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Priscila Barros de Medeiros, V.; Karoline Almeida da Costa, W.; Tavares da Silva, R.; Colombo Pimentel, T.; Magnani, M. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food Sci. Nutr. 2021, 62, 4929–4950. [Google Scholar] [CrossRef]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerød, H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Pora, B.L.R.; Dong, K.; Hasjim, J. Health benefits of docosahexaenoic acid and its bioavailability: A review. Food Sci. Nutr. 2021, 9, 5229–5243. [Google Scholar] [CrossRef]
- Molino, A.; Martino, M.; Larocca, V.; Di Sanzo, G.; Spagnoletta, A.; Marino, T.; Karatza, D.; Iovine, A.; Mehariya, S.; Musmarra, D. Eicosapentaenoic acid extraction from nannochloropsis gaditana using carbon dioxide at supercritical conditions. Mar. Drugs 2019, 17, 132. [Google Scholar] [CrossRef] [Green Version]
- Freire, I.; Cortina-Burgueño, A.; Grille, P.; Arizcun Arizcun, M.; Abellán, E.; Segura, M.; Witt Sousa, F.; Otero, A. Nannochloropsis limnetica: A freshwater microalga for marine aquaculture. Aquaculture 2016, 459, 124–130. [Google Scholar] [CrossRef]
- Mitra, M.; Patidar, S.K.; George, B.; Shah, F.; Mishra, S. A euryhaline nannochloropsis gaditana with potential for nutraceutical (EPA) and biodiesel production. Algal Res. 2015, 8, 161–167. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef]
- Krienitz, L.; Wirth, M. The high content of polyunsaturated fatty acids in Nannochloropsis limnetica (Eustigmatophyceae) and its implication for food web interactions, freshwater aquaculture and biotechnology. Limnologica 2006, 36, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.; Mesa-Herrera, F.; Marín, R. Dha and its elaborated modulation of antioxidant defenses of the brain: Implications in aging and ad neurodegeneration. Antioxidants 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a sustainable source of edible proteins and bioactive peptides—Current trends and future prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef] [PubMed]
- Skjånes, K.; Aesoy, R.; Herfindal, L.; Skomedal, H. Bioactive peptides from microalgae: Focus on anti-cancer and immunomodulating activity. Physiol. Plant. 2021, 173, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Barkia, I.; Al-Haj, L.; Abdul Hamid, A.; Zakaria, M.; Saari, N.; Zadjali, F. Indigenous marine diatoms as novel sources of bioactive peptides with antihypertensive and antioxidant properties. Int. J. Food Sci. Technol. 2019, 54, 1514–1522. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Xiao, J. Polyphenols: Absorption, bioavailability, and metabolomics. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 45–67. ISBN 9780128135723. [Google Scholar]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Factories 2020, 19, 201. [Google Scholar] [CrossRef]
- Tarento, T.D.C.; McClure, D.D.; Vasiljevski, E.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. Microalgae as a source of vitamin K1. Algal Res. 2018, 36, 77–87. [Google Scholar] [CrossRef]
- Raja, K.; Kadirvel, V.; Subramaniyan, T. Seaweeds, an aquatic plant-based protein for sustainable nutrition—A review. Futur. Foods 2022, 5, 100142. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarkar, S.; Manna, M.S.; Gayen, K.; Bhowmick, T.K. Extraction of carbohydrates and proteins from algal resources using supercritical and subcritical fluids for high-quality products. In Innovative and Emerging Technologies in the Bio-Marine Food Sector; Academic Press: Cambridge, MA, USA, 2022; pp. 249–275. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T.; Englyst, H.N. Prebiotic digestion and fermentation. Am. J. Clin. Nutr. 2001, 73 (Suppl. 2), 415–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravana, P.S.; Cho, Y.J.; Park, Y.B.; Woo, H.C.; Chun, B.S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Abdel Aziz, M.S.; Salama, H.E. Developing multifunctional edible coatings based on alginate for active food packaging. Int. J. Biol. Macromol. 2021, 190, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll. 2021, 112, 106332. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Kim, S.K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods 2013, 5, 16–27. [Google Scholar] [CrossRef]
- Chen, K.; Roca, M. Cooking effects on bioaccessibility of chlorophyll pigments of the main edible seaweeds. Food Chem. 2019, 295, 101–109. [Google Scholar] [CrossRef]
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Comprehensive chlorophyll composition in the main edible seaweeds. Food Chem. 2017, 228, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.P.; Baskaran, V. Polysaccharide (laminaran and fucoidan), fucoxanthin and lipids as functional components from brown algae (Padina tetrastromatica) modulates adipogenesis and thermogenesis in diet-induced obesity in C57BL6 mice. Algal Res. 2021, 54, 102187. [Google Scholar] [CrossRef]
- Siddique, M.A.M.; Hossain, M.S.; Islam, M.M.; Rahman, M.; Kibria, G. Heavy metals and metalloids in edible seaweeds of Saint Martin’s Island, Bay of Bengal, and their potential health risks. Mar. Pollut. Bull. 2022, 181, 113866. [Google Scholar] [CrossRef]
- Peng, Z.; Guo, Z.; Wang, Z.; Zhang, R.; Wu, Q.; Gao, H.; Wang, Y.; Shen, Z.; Lek, S.; Xiao, J. Species-specific bioaccumulation and health risk assessment of heavy metal in seaweeds in tropic coasts of South China Sea. Sci. Total Environ. 2022, 832, 155031. [Google Scholar] [CrossRef] [PubMed]
- Kibria, G.; Haroon, A.K.; Rose, G.; Hossain, M.M.; Nugegoda, D. Pollution Risks, Impacts and Management: Social, Economic, and Enviromental Perspectives; Scientific Publishers: Jodhpur, India, 2021; 833p, ISBN 9789389184969. [Google Scholar]
- Herrero, M.; de Paula Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- MS, J.; Soylak, M. Deep eutectic solvents-based adsorbents in enviromental analysis. TrAC Trends Anal. Chem. 2022, 157, 116762. [Google Scholar] [CrossRef]
- Vo Dinh, T.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Plaza, M.; Marina, M.L. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2019, 116, 236–247. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.B.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E.C. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef]
- Mustafa, A.; Trevino, L.M.; Turner, C. Pressurized hot ethanol extraction of carotenoids from carrot by-products. Molecules 2012, 17, 1809–1818. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros-Vivas, D.; Alvarez-Rivera, G.; Ibánez, E.; Parada-Alfonso, F.; Cifuentes, A. Integrated strategy for the extraction and profiling of bioactive metabolites from Passiflora mollissima seeds combining pressurized-liquid extraction and gas/liquid chromatography–high resolution mass spectrometry. J. Chromatogr. A 2019, 1595, 144–157. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Mendiola, J.A.; Rezaei, K.; Ibáñez, E. Pressurized limonene as an alternative bio-solvent for the extraction of lipids from marine microorganisms. J. Supercrit. Fluids 2014, 92, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- del Pilar Sánchez-Camargo, A.; Pleite, N.; Herrero, M.; Cifuentes, A.; Ibáñez, E.; Gilbert-López, B. New approaches for the selective extraction of bioactive compounds employing bio-based solvents and pressurized green processes. J. Supercrit. Fluids 2017, 128, 112–120. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Barranco, A.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Development of new green processes for the recovery of bioactives from Phaeodactylum tricornutum. Food Res. Int. 2017, 99, 1056–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Y.F.; Kim, S.M.; Lee, W.J.; Um, B.H. Pressurized liquid method for fucoxanthin extraction from Eisenia bicyclis (Kjellman) Setchell. J. Biosci. Bioeng. 2011, 111, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Anaëlle, T.; Serrano Leon, E.; Laurent, V.; Elena, I.; Mendiola, J.A.; Stéphane, C.; Nelly, K.; Stéphane, L.B.; Luc, M.; Valérie, S.P. Green improved processes to extract bioactive phenolic compounds from brown macroalgae using Sargassum muticum as model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef]
- Park, J.S.; Han, J.M.; Surendhiran, D.; Chun, B.S. Physicochemical and biofunctional properties of Sargassum thunbergii extracts obtained from subcritical water extraction and conventional solvent extraction. J. Supercrit. Fluids 2022, 182, 105535. [Google Scholar] [CrossRef]
- Lin, E.T.; Lee, Y.C.; Wang, H.M.D.; Chiu, C.Y.; Chang, Y.K.; Huang, C.Y.; Chang, C.C.; Tsai, P.C.; Chang, J.S. Efficient fucoidan extraction and purification from Sargassum cristaefolium and preclinical dermal biological activity assessments of the purified fucoidans. J. Taiwan Inst. Chem. Eng. 2022, 137, 104294. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Chun, B.S. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep. Purif. Technol. 2018, 196, 309–317. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Hayes, M.; Kerry, J.P.; O’Brien, N.M. The effect of solvents on the antioxidant activity in Caco-2 cells of Irish brown seaweed extracts prepared using accelerated solvent extraction (ASE®). J. Funct. Foods 2013, 5, 940–948. [Google Scholar] [CrossRef]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; García-Risco, M.R. Pressurized Liquid Extraction (PLE) as an innovative green technology for the effective enrichment of galician algae extracts with high quality fatty acids and antimicrobial and antioxidant properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef] [Green Version]
- Fontanals, N.; Pocurull, E.; Borrull, F.; Marcé, R.M. Clean-up techniques in the pressurized liquid extraction of abiotic environmental solid samples. Trends Environ. Anal. Chem. 2021, 29, e00111. [Google Scholar] [CrossRef]
- da Silva, L.C.; Souza, M.C.; Sumere, B.R.; Silva, L.G.S.; da Cunha, D.T.; Barbero, G.F.; Bezerra, R.M.N.; Rostagno, M.A. Simultaneous extraction and separation of bioactive compounds from apple pomace using pressurized liquids coupled on-line with solid-phase extraction. Food Chem. 2020, 318, 126450. [Google Scholar] [CrossRef]
- Souza, M.C.; Silva, L.C.; Chaves, J.O.; Salvador, M.P.; Sanches, V.L.; da Cunha, D.T.; Foster Carneiro, T.; Rostagno, M.A. Simultaneous extraction and separation of compounds from mate (Ilex paraguariensis) leaves by pressurized liquid extraction coupled with solid-phase extraction and in-line UV detection. Food Chem. Mol. Sci. 2021, 2, 100008. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.O.; Sanches, V.L.; Viganó, J.; de Souza Mesquita, L.M.; de Souza, M.C.; da Silva, L.C.; Acunha, T.; Faccioli, L.H.; Rostagno, M.A. Integration of pressurized liquid extraction and in-line solid-phase extraction to simultaneously extract and concentrate phenolic compounds from lemon peel (Citrus limon L.). Food Res. Int. 2022, 157, 111252. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Plaza, M.; Šnóblová, M.; Lojková, L. Development of new efficient method for isolation of phenolics from sea algae prior to their rapid resolution liquid chromatographic–tandem mass spectrometric determination. J. Pharm. Biomed. Anal. 2017, 135, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Viganó, J.; de Paula Assis, B.F.; Náthia-Neves, G.; dos Santos, P.; Meireles, M.A.A.; Veggi, P.C.; Martínez, J. Extraction of bioactive compounds from defatted passion fruit bagasse (Passiflora edulis sp.) applying pressurized liquids assisted by ultrasound. Ultrason. Sonochem. 2020, 64, 104999. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.B.; de Aguiar, A.C.; Rostagno, M.A. Extraction of natural products using supercritical fluids and pressurized liquids assisted by ultrasound: Current status and trends. Ultrason. Sonochem. 2021, 74, 105584. [Google Scholar] [CrossRef]
- Kraujalis, P.; Kraujalienė, V.; Kazernavičiūtė, R.; Venskutonis, P.R. Supercritical carbon dioxide and pressurized liquid extraction of valuable ingredients from Viburnum opulus pomace and berries and evaluation of product characteristics. J. Supercrit. Fluids 2017, 122, 99–108. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Kraujalis, P.; Tamkutė, L.; Urbonavičienė, D.; Viškelis, P.; Venskutonis, P.R. Recovery of bioactive substances from rowanberry pomace by consecutive extraction with supercritical carbon dioxide and pressurized solvents. J. Ind. Eng. Chem. 2020, 85, 152–160. [Google Scholar] [CrossRef]
- Viganó, J.; Zabot, G.L.; Martínez, J. Supercritical fluid and pressurized liquid extractions of phytonutrients from passion fruit by-products: Economic evaluation of sequential multi-stage and single-stage processes. J. Supercrit. Fluids 2017, 122, 88–98. [Google Scholar] [CrossRef]
- Das, S.; Nadar, S.S.; Rathod, V.K. Integrated strategies for enzyme assisted extraction of bioactive molecules: A review. Int. J. Biol. Macromol. 2021, 191, 899–917. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.D.P.; Montero, L.; Stiger-Pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerg-ing extraction technologies, chemical modifications and bioactive properties. In Critical Reviews in Food Science and Nutrition; Taylor and Francis Ltd.: London, UK, 2021. [Google Scholar] [CrossRef]
- Cofrades, S.; López-López, I.; Solas, M.T.; Bravo, L.; Jiménez-Colmenero, F. Influence of different types and proportions of added edible seaweeds on characteristics of low-salt gel/emulsion meat systems. Meat Sci. 2008, 79, 767–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajauria, G.; Cornish, L.; Ometto, F.; Msuya, F.E.; Villa, R. Identification and selection of algae for food, feed, and fuel applications. In Seaweed Sustainability; Academic Press: Cambridge, MA, USA, 2015; pp. 315–345. [Google Scholar] [CrossRef]
- López-López, I.; Bastida, S.; Ruiz-Capillas, C.; Bravo, L.; Larrea, M.T.; Sánchez-Muniz, F.; Cofrades, S.; Jiménez-Colmenero, F. Composition and antioxidant capacity of low-salt meat emulsion model systems containing edible seaweeds. Meat Sci. 2009, 83, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Prabhasankar, P. Marine foods as functional ingredients in bakery and pasta products. Food Res. Int. 2010, 43, 1975–1980. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Hayes, M. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev. Int. 2016, 32, 15–45. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef]
- Mahadevan, K. Seaweeds: A sustainable food source. In Seaweed Sustainability: Food and Non-Food Applications; Elsevier Inc.: Manchester, UK, 2015; ISBN 9780124199583. [Google Scholar]
- Rey-Crespo, F.; López-Alonso, M.; Miranda, M. The use of seaweed from the Galician coast as a mineral supplement in organic dairy cattle. Animal 2014, 8, 580–586. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Rathgeber, B.; Stratton, G.; Thomas, N.; Evans, F.; Critchley, A.; Hafting, J.; Prithiviraj, B. Feed supplementation with red seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens. Poult. Sci. 2014, 93, 2991–3001. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N. Influence of Indian brown seaweed (Sargassum marginatum) as an ingredient on quality, biofunctional, and microstructure characteristics of pasta. Food Sci. Technol. Int. 2009, 15, 471–479. [Google Scholar] [CrossRef]
- López-López, I.; Cofrades, S.; Yakan, A.; Solas, M.T.; Jiménez-Colmenero, F. Frozen storage characteristics of low-salt and low-fat beef patties as affected by Wakame addition and replacing pork backfat with olive oil-in-water emulsion. Food Res. Int. 2010, 43, 1244–1254. [Google Scholar] [CrossRef]
- Cofrades, S.; López-López, I.; Ruiz-Capillas, C.; Triki, M.; Jiménez-Colmenero, F. Quality characteristics of low-salt restructured poultry with microbial transglutaminase and seaweed. Meat Sci. 2011, 87, 373–380. [Google Scholar] [CrossRef] [PubMed]
- López-López, I.; Cofrades, S.; Ruiz-Capillas, C.; Jiménez-Colmenero, F. Design and nutritional properties of potential functional frankfurters based on lipid formulation, added seaweed and low salt content. Meat Sci. 2009, 83, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Thorkelsson, G.; Jacobsen, C.; Hamaguchi, P.Y.; Ólafsdóttir, G. Inhibition of haemoglobin-mediated lipid oxidation in washed cod muscle and cod protein isolates by Fucus vesiculosus extract and fractions. Food Chem. 2010, 123, 321–330. [Google Scholar] [CrossRef]
- Hermund, D.B.; Andersen, U.; Jónsdóttir, R.; Kristinsson, H.G.; Alasalvar, C.; Jacobsen, C. Oxidative Stability of Granola Bars Enriched with Multilayered Fish Oil Emulsion in the Presence of Novel Brown Seaweed Based Antioxidants. J. Agric. Food Chem. 2016, 64, 8359–8368. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate composition, phenolic content and in vitro antioxidant activity of aqueous extracts of the seaweeds Ascophyllum nodosum, Bifurcaria bifurcata and Fucus vesiculosus. Effect of addition of the extracts on the oxidative stability of canola oil under accelerated storage conditions. Food Res. Int. 2017, 99, 986–994. [Google Scholar] [CrossRef]
- Moroney, N.C.; O’Grady, M.N.; Lordan, S.; Stanton, C.; Kerry, J.P. Seaweed polysaccharides (laminarin and fucoidan) as functional ingredients in pork meat: An evaluation of anti-oxidative potential, thermal stability and bioaccessibility. Mar. Drugs 2015, 13, 2447–2464. [Google Scholar] [CrossRef] [Green Version]
- Honold, P.J.; Jacobsen, C.; Jónsdóttir, R.; Kristinsson, H.G.; Hermund, D.B. Potential seaweed-based food ingredients to inhibit lipid oxidation in fish-oil-enriched mayonnaise. Eur. Food Res. Technol. 2016, 242, 571–584. [Google Scholar] [CrossRef]
- Baun, D. General Rights Extraction, Characterization and Application of Antioxidants from the Nordic Brown Alga Fucus vesiculosus; National Food Institute (DTU Food), Technical University of Denmark (DTU): Lyngby, Denmark, 2016. [Google Scholar]
- Arufe, S.; Sineiro, J.; Moreira, R. Determination of thermal transitions of gluten-free chestnut flour doughs enriched with brown seaweed powders and antioxidant properties of baked cookies. Heliyon 2019, 5, e01805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quitral, V.; Sepúlveda, M.; Gamero-Vega, G.; Jiménez, P. Seaweeds in bakery and farinaceous foods: A mini-review. Int. J. Gastron. Food Sci. 2022, 28, 100403. [Google Scholar] [CrossRef]
- Komatsuzaki, N.; Arai, S.; Fujihara, S.; Shima, J.; Wijesekara, R.S.; Dileepa, M.; De Croos, S.T. Development of Novel Bread by Combining Seaweed Kappaphycus alvarezii from Sri Lanka and Saccharomyces cerevisiae Isolated from Nectarine. J. Agric. Sci. Technol. B 2019, 9, 339–346. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Marine algae as efficacious bioresources housing antimicrobial compounds for preserving foods—A review. Int. J. Food Microbiol. 2021, 358, 109416. [Google Scholar] [CrossRef]
- Dang, B.T.; Bui, X.T.; Tran, D.P.H.; Hao Ngo, H.; Nghiem, L.D.; Hoang, T.K.D.; Nguyen, P.T.; Nguyen, H.H.; Vo, T.K.Q.; Lin, C.; et al. Current application of algae derivatives for bioplastic production: A review. Bioresour. Technol. 2022, 347, 126698. [Google Scholar] [CrossRef]
- Pavkovich, A.M.; Bell, D.S. Extraction | Pressurized liquid extraction. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 78–83. ISBN 9780081019832. [Google Scholar]
- Calvo-Flores, F.G. Green Processes in Foodomics. Green Solvents for Sustainable Processes. In Comprehensive Foodomics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 690–709. ISBN 9780128163955. [Google Scholar]




| Compound | Algae | Dry Weight (%) | Functional Properties | Ref. | |
|---|---|---|---|---|---|
| Polysaccharides | Nannochloropsis oceanica | 8.33 | - Prebiotic activity 1* - Immuno-modulatory 1* - Low blood sugar and lipid levels in vitro 1 * | [26,27,28] | |
| Nannochloropsis oculata | 6.4 | ||||
| Nannochloropsis granulata | 14.9 | ||||
| Nannochloropsis limnetica | 10 | ||||
| Microchloropsis salina | 17.8–36.2 | ||||
| Microchloropsis gaditana | 21.7 | ||||
| Sulfated polysaccharides | Arthrospira platensis | 5–14.6 | -Antiviral - Anti-tumor - Anti-inflammatory - Immuno-modulatory | [29,30] | |
| Chlorella ellipsoidea | |||||
| Phaeodactylum sp. | |||||
| Schizochytrium spp. | |||||
| Thraustochytrium spp. | |||||
| Lipids | Nannochloropsis oceanica | 18.40–46.12 | - | [27,31,32,33,34,35,36] | |
| Nannochloropsis oculata | 8.2 | ||||
| Nannochloropsis granulate | 28.5 | ||||
| Nannochloropsis limnetica | 24 | ||||
| Microchloropsis salina | 6.2–26 | ||||
| Microchloropsis gaditana | 16.5 | ||||
| Essential fatty acids | EPA | Nannochloropsis oceanica | 2.34 | - Reduce cardiovascular disease - Improve mental health - Anti-inflammatory - Anti-diabetes - Anti-thrombotic activity | [26,27,31,32,33,34,35,36,37] |
| Nannochloropsis oculata | 2.33 | ||||
| Nannochloropsis limnetica | 2.81 | ||||
| Microchloropsis gaditana | 4.4–11 | ||||
| Phaeodactylum tricornutum | 0.7–6.1 3* | ||||
| Porosira glacialis | |||||
| Monodopsis subterranean | |||||
| Nannochloris spp. | |||||
| ARA | Ceramium rubrum | 24–77 3* | - Anti-inflammatory - Anti-cancer - Prevention of neurological disorders | [32,36] | |
| Parietochloris incisa | |||||
| Phormidium pseudoprsleyi | |||||
| Prphyridium purpureum | |||||
| DHA | Amphidiunum carterae | 17.5–30.2 3* | - Decreasing preterm birth - Improving cognitive - Prevention of cardiovascular disease - Promotion of eye health - Slowing Alzheimer’s disease | [36,38] | |
| Aurantochytrium spp. | |||||
| Phaeodactylum tricurnutum | |||||
| Schizochytrium spp. | |||||
| Thraustochytrium spp. | |||||
| Proteins | Nannochloropsis oceanica | 14.5 | - Gelling properties 2* - Foaming properties 2* | [26,27,39] | |
| Nannochloropsis oculata | 22.6 | ||||
| Nannochloropsis granulata | 45.8 | ||||
| Nannochloropsis limnetica | 37 | ||||
| Microchloropsis salina | 18.1–36.2 | ||||
| Microchloropsis gaditana | 47 | ||||
| Bioactive peptides | Chlorella elipsoidea | - | - Antihypertensive - Antibiotic - Antiviral | [40,41] | |
| D. salina | |||||
| Nitzschia sp. | |||||
| Bellerochae sp. | |||||
| Tetraselmis suecica | |||||
| Phenolic compounds | Chlorella vulgaris | 0.54–4.57 | - Antioxidant - Anti-inflammatory - Antimicrobial - Anti-cancer - Prevention of cardiovascular and neurodegenerative diseases | [42,43] | |
| Nannochloropsis sp. | |||||
| Phaedactylum tricornutum | |||||
| Scenedesmus obliquus | |||||
| Tetraselmis sp. | |||||
| Vitamins | Arthrospira | - | - Blood coagulation - Modulating inflammation - Neuroprotection, promoting eye and bone health | [44,45] | |
| Chlorella | |||||
| Isochrysis galbana | |||||
| Pavlova | |||||
| Porphyridium cruentum | |||||
| Tetraselmis sp. | |||||
| Solvent | Extracted Compound | Ref. |
|---|---|---|
| Water | Phenolic compounds//Di-, triterpenes//Proteins//Polysaccharides | [24,62] |
| Water + ionic liquids | Carrageenan//Alginates | [24,63] |
| Water + eutectic solvents | Carrageenan//Alginates | [24,63] |
| Ethanol | Polyphenols//Carotenoids//Alkaloids//Lipids | [24,64,65,66] |
| Aqueous ethanol | Polyphenols//Carotenoids//Alkaloids//Lipids | [24,64,65,66] |
| Ethyl acetate | Polyphenols//Carotenoids//Alkaloids//Lipids | [24,64,65,66] |
| Ethyl lactate | Fatty acids | [24,67,68] |
| (+)-limonene | Fatty acids | [24,67,68] |
| Seaweed | Compound | Solvent | T (°C) | P (bar) | t (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Saccharina japonica (B) | Alginate | NaOH 0.1%; H2Od; H2O | 80; 110; 140 | 5; 25; 50 | 5; 12 | 3–27.21 | [49] |
| Fucoidan | NaOH 0.1%; H2Od; CH2O2 0.1% | 80; 110; 125; 150 | 5; 25; 50 | 5; 25 | 2.5–15.7 | [49,62] | |
| TPC | H2O | 200 | 50 | 15 | 39.52 | ||
| Eisinia bicyclis (B) | Fucoxanthin | EtOH 90% | 110 | 103.42 | 5 | 0.39 | [71] |
| Laminaria ochroleuca (B) | Fatty acids | Hexane; ethyl acetate; EtOH; EtOH 50% | 120 | 100 | 10 | 7.42–47.16 | [7] |
| Sargassum muticum (B) | Phlorotannin | EtOH- H2O 95:5; 75:25; 25:75. | 120; 160 | 103.42 | 20 | 0.77–2.93 | [61,72] |
| Sargassum thumbergii (B) | Phlorotannin | H2O | 180 | 30 | 30 | 1.35 | [73] |
| Sargassum cristaelefolium (B) | Fucoidan | H2O | 121 | 1.0133 | 20 | 12.60 | [74] |
| Kappaphycus alvarezii (R) | Carrageenan | H2O | 150 | 50 | 5 | 71 | [75] |
| Ascophyllum nodosum (B) | TPC | H2O; EtOH: H2O 60:40; EtOH: H2O 80:20; MeOH: H2O 60:40 | 90; 100; 120 | 68.95 | 90 s | 34.5- 114 | [76] |
| Fucus serratus (B) | TPC | H2O; EtOH: H2O 60:40; EtOH: H2O; 80:20; MeOH: H2O 60:40; | 90; 100; 120 | 68.95 | 90 s | 19.7–40.7 | [76] |
| Fucus vesiculosus (B) | TPC | H2O; EtOH: H2O; 60:40; EtOH: H2O; 80:20; MeOH: H2O; 60:40 | 90; 100; 120 | 68.95 | 90 s | 114.0–110 | [76] |
| Fatty acids | EtOH: H2O; 50:50 | 120; 160 | 100 | 10 | 34.85–57.19 | [77] | |
| Ulva lactuca (G) | Fatty acids | EtOH: H2O; 50:50 | 80; 120; 160 | 100 | 10 | 34.85; 41.49; 57.19 | [77] |
| Ulva intestinalis (G) | Fatty acids | EtOH: H2O; 50:50 | 80; 120; 160 | 100 | 10 | 34.85; 41.49; 57.19 | [77] |
| Himanthalia elongata (B) | Fatty acids | EtOH: H2O; 50:50 | 80; 120; 160 | 100 | 10 | 34.85; 41.49; 57.19 | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Vazquez, A.; Carpena, M.; Barciela, P.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Pressurized Liquid Extraction for the Recovery of Bioactive Compounds from Seaweeds for Food Industry Application: A Review. Antioxidants 2023, 12, 612. https://doi.org/10.3390/antiox12030612
Perez-Vazquez A, Carpena M, Barciela P, Cassani L, Simal-Gandara J, Prieto MA. Pressurized Liquid Extraction for the Recovery of Bioactive Compounds from Seaweeds for Food Industry Application: A Review. Antioxidants. 2023; 12(3):612. https://doi.org/10.3390/antiox12030612
Chicago/Turabian StylePerez-Vazquez, Ana, Maria Carpena, Paula Barciela, Lucia Cassani, Jesus Simal-Gandara, and Miguel A. Prieto. 2023. "Pressurized Liquid Extraction for the Recovery of Bioactive Compounds from Seaweeds for Food Industry Application: A Review" Antioxidants 12, no. 3: 612. https://doi.org/10.3390/antiox12030612








