Associations between Serum Folate Concentrations and Functional Disability in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Serum Folate Assessment
2.3. Self-Reported Functional Disability Assessment
2.4. Other Covariates
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Participants
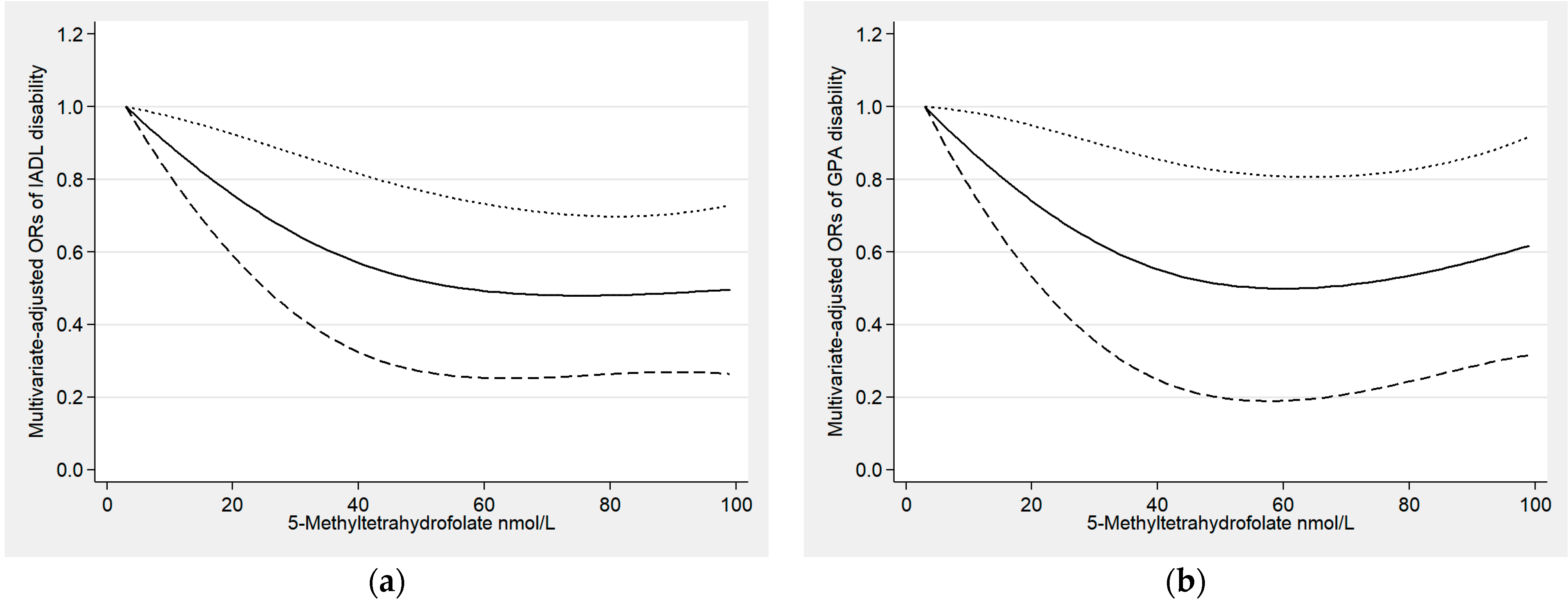
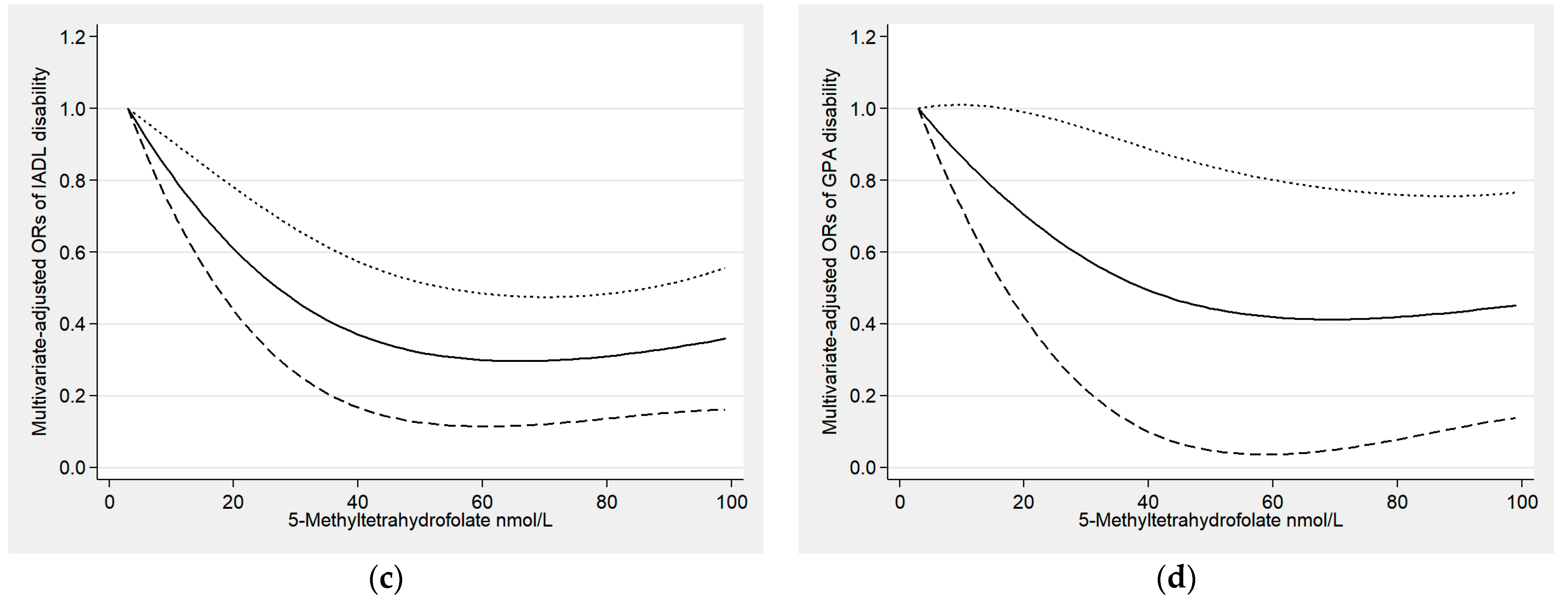
3.2. Relationships between Serum Folate and Functional Disability
3.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verbrugge, L.M.; Jette, A.M. The disablement process. Soc. Sci. Med. 1994, 38, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.S.; Oliveira, L.S.; Castro, F.D.; Gois-Santos, V.T.; Lemos, L.M.; Ribeiro Mdo, C.; Cuevas, L.E.; Gurgel, R.Q. Functional Activity Limitation and Quality of Life of Leprosy Cases in an Endemic Area in Northeastern Brazil. PLoS Negl. Trop. Dis. 2015, 9, e0003900. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.S.; Ettner, S.L.; Morrison, R.S.; Du, Q.; Sarkisian, C.A. Disability and decline in physical function associated with hospital use at end of life. J. Gen. Intern. Med. 2012, 27, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.W.; Chen, W.L.; Peng, T.C.; Chiang, S.T.; Yang, H.F.; Sun, Y.S.; Chan, J.Y.; Kao, T.W. All-cause mortality risk in elderly individuals with disabilities: A retrospective observational study. BMJ Open 2016, 6, e011164. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.W.; Fulmer, T.; Fried, L. Preparing for Better Health and Health Care for an Aging Population. JAMA 2016, 316, 1643–1644. [Google Scholar] [CrossRef] [PubMed]
- Courtney-Long, E.A.; Carroll, D.D.; Zhang, Q.C.; Stevens, A.C.; Griffin-Blake, S.; Armour, B.S.; Campbell, V.A. Prevalence of Disability and Disability Type Among Adults--United States, 2013. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.E.; Merkin, S.S.; Crimmins, E.M.; Karlamangla, A.S. Disability trends among older Americans: National Health And Nutrition Examination Surveys, 1988–1994 and 1999–2004. Am. J. Public Health 2010, 100, 100–107. [Google Scholar] [CrossRef]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Bennett, D.A.; Morris, M.C. Dietary Patterns and Self-reported Incident Disability in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 1331–1337. [Google Scholar] [CrossRef]
- Krok-Schoen, J.L.; Archdeacon Price, A.; Luo, M.; Kelly, O.J.; Taylor, C.A. Low Dietary Protein Intakes and Associated Dietary Patterns and Functional Limitations in an Aging Population: A NHANES analysis. J. Nutr. Health Aging 2019, 23, 338–347. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Y.; Wang, W.; Zhang, D. Association between coffee consumption and functional disability in older US adults. Br. J. Nutr. 2021, 125, 695–702. [Google Scholar] [CrossRef]
- Shea, M.K.; Kritchevsky, S.B.; Loeser, R.F.; Booth, S.L. Vitamin K Status and Mobility Limitation and Disability in Older Adults: The Health, Aging, and Body Composition Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 792–797. [Google Scholar] [CrossRef] [PubMed]
- García-Esquinas, E.; Carrasco-Rios, M.; Ortolá, R.; Sotos Prieto, M.; Pérez-Gómez, B.; Gutiérrez-González, E.; Banegas, J.R.; Queipo, R.; Olmedo, P.; Gil, F.; et al. Selenium and impaired physical function in US and Spanish older adults. Redox Biol. 2021, 38, 101819. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Ghanavati, M.; Ashtary-Larky, D.; Bagheri, R.; Rezaei Kelishadi, M.; Nazarian, B.; Nordvall, M.; Wong, A.; Dutheil, F.; Suzuki, K.; et al. Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2021, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ferrucci, L.; Sun, K.; Walston, J.; Varadhan, R.; Guralnik, J.M.; Fried, L.P. Oxidative stress and severe walking disability among older women. Am. J. Med. 2007, 120, 1084–1089. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef]
- Kuo, H.K.; Liao, K.C.; Leveille, S.G.; Bean, J.F.; Yen, C.J.; Chen, J.H.; Yu, Y.H.; Tai, T.Y. Relationship of homocysteine levels to quadriceps strength, gait speed, and late-life disability in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 434–439. [Google Scholar] [CrossRef]
- Swart, K.M.; van Schoor, N.M.; Heymans, M.W.; Schaap, L.A.; den Heijer, M.; Lips, P. Elevated homocysteine levels are associated with low muscle strength and functional limitations in older persons. J. Nutr. Health Aging 2013, 17, 578–584. [Google Scholar] [CrossRef]
- Struijk, E.A.; Lana, A.; Guallar-Castillón, P.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Intake of B vitamins and impairment in physical function in older adults. Clin. Nutr. 2018, 37, 1271–1278. [Google Scholar] [CrossRef]
- Behrouzi, P.; Grootswagers, P.; Keizer, P.L.C.; Smeets, E.; Feskens, E.J.M.; de Groot, L.; van Eeuwijk, F.A. Dietary Intakes of Vegetable Protein, Folate, and Vitamins B-6 and B-12 Are Partially Correlated with Physical Functioning of Dutch Older Adults Using Copula Graphical Models. J. Nutr. 2020, 150, 634–643. [Google Scholar] [CrossRef]
- Swart, K.M.; Ham, A.C.; van Wijngaarden, J.P.; Enneman, A.W.; van Dijk, S.C.; Sohl, E.; Brouwer-Brolsma, E.M.; van der Zwaluw, N.L.; Zillikens, M.C.; Dhonukshe-Rutten, R.A.; et al. A Randomized Controlled Trial to Examine the Effect of 2-Year Vitamin B12 and Folic Acid Supplementation on Physical Performance, Strength, and Falling: Additional Findings from the B-PROOF Study. Calcif. Tissue Int. 2016, 98, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.M.; Bailey, R.; O’Connor, D.L. Folate. Adv. Nutr. (Bethesda Md.) 2013, 4, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Aung, K.C.; Feng, L.; Scherer, S.C.; Yap, K.B. Homocysteine, folate, vitamin B-12, and physical function in older adults: Cross-sectional findings from the Singapore Longitudinal Ageing Study. Am. J. Clin. Nutr. 2012, 96, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Mañas, L.R.; Andrés, P.; Gaspar, M.J.; Agudo, F.R.; Jiménez, A.; Pascual, T. Functional and psychic deterioration in elderly people may be aggravated by folate deficiency. J. Nutr. 1996, 126, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Bartali, B.; Frongillo, E.A.; Guralnik, J.M.; Stipanuk, M.H.; Allore, H.G.; Cherubini, A.; Bandinelli, S.; Ferrucci, L.; Gill, T.M. Serum micronutrient concentrations and decline in physical function among older persons. JAMA 2008, 299, 308–315. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/ (accessed on 2 December 2022).
- Luo, J.; Ge, H.; Sun, J.; Hao, K.; Yao, W.; Zhang, D. Associations of Dietary ω-3, ω-6 Fatty Acids Consumption with Sleep Disorders and Sleep Duration among Adults. Nutrients 2021, 13, 1475. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey. 2011-2012 Data Documentation, Codebook, and Frequencies. Folate Forms—Total & Individual—Serum (FOLFMS_G). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/FOLFMS_G.htm (accessed on 2 December 2022).
- Fazili, Z.; Sternberg, M.R.; Potischman, N.; Wang, C.Y.; Storandt, R.J.; Yeung, L.; Yamini, S.; Gahche, J.J.; Juan, W.; Qi, Y.P.; et al. Demographic, Physiologic, and Lifestyle Characteristics Observed with Serum Total Folate Differ Among Folate Forms: Cross-Sectional Data from Fasting Samples in the NHANES 2011–2016. J. Nutr. 2020, 150, 851–860. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Sternberg, M.R.; Fazili, Z.; Lacher, D.A.; Zhang, M.; Johnson, C.L.; Hamner, H.C.; Bailey, R.L.; Rader, J.I.; Yamini, S.; et al. Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Examination Survey 2011–2012. Br. J. Nutr. 2015, 113, 1965–1977. [Google Scholar] [CrossRef]
- Hannisdal, R.; Ueland, P.M.; Svardal, A. Liquid chromatography-tandem mass spectrometry analysis of folate and folate catabolites in human serum. Clin. Chem. 2009, 55, 1147–1154. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, J.; Li, Z.; Zhang, D. The relationship between serum folate and grip strength in American adults. Arch. Osteoporos. 2021, 16, 97. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey. 2011-2012 Data Documentation, Codebook, and Frequencies. Physical Functioning (PFQ_G). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/PFQ_G.htm (accessed on 2 December 2022).
- Chen, Y.Y.; Wang, C.C.; Kao, T.W.; Wu, C.J.; Chen, Y.J.; Lai, C.H.; Zhou, Y.C.; Chen, W.L. The relationship between lead and cadmium levels and functional dependence among elderly participants. Environ. Sci. Pollut. Res. Int. 2020, 27, 5932–5940. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Saudek, C.D.; Brancati, F.L.; Selvin, E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: Results from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Diabetes Care 2010, 33, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.L.C.; Wang, D.; Gottesman, R.F.; Selvin, E. Prevalence of Disability Associated With Head Injury With Loss of Consciousness in Adults in the United States: A Population-Based Study. Neurology 2021, 97, e124–e135. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, X. Obesity and functional disability in elderly Americans. J. Am. Geriatr. Soc. 2008, 56, 689–694. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Z.; Zhou, C.; Li, Q.; He, P.; Zhang, Y.; Li, H.; Liu, C.; Liang, M.; Wang, X.; et al. Relationship of several serum folate forms with the risk of mortality: A prospective cohort study. Clin. Nutr. 2021, 40, 4255–4262. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, C.; Zhang, Z.; Li, Q.; He, P.; Zhang, Y.; Li, H.; Liu, C.; Fan Hou, F.; Qin, X. Relationship of several serum folate forms with kidney function and albuminuria: Cross-sectional data from the National Health and Nutrition Examination Surveys (NHANES) 2011–2018. Br. J. Nutr. 2022, 127, 1050–1059. [Google Scholar] [CrossRef]
- Gana, W.; De Luca, A.; Debacq, C.; Poitau, F.; Poupin, P.; Aidoud, A.; Fougère, B. Analysis of the Impact of Selected Vitamins Deficiencies on the Risk of Disability in Older People. Nutrients 2021, 13, 3163. [Google Scholar] [CrossRef]
- Novochadlo, M.; Goldim, M.P.; Bonfante, S.; Joaquim, L.; Mathias, K.; Metzker, K.; Machado, R.S.; Lanzzarin, E.; Bernades, G.; Bagio, E.; et al. Folic acid alleviates the blood brain barrier permeability and oxidative stress and prevents cognitive decline in sepsis-surviving rats. Microvasc. Res. 2021, 137, 104193. [Google Scholar] [CrossRef]
- Bartali, B.; Curto, T.; Maserejian, N.N.; Araujo, A.B. Intake of antioxidants and subsequent decline in physical function in a racially/ethnically diverse population. J. Nutr. Health Aging 2015, 19, 542–547. [Google Scholar] [CrossRef]
- Bahmani, F.; Karamali, M.; Shakeri, H.; Asemi, Z. The effects of folate supplementation on inflammatory factors and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Clin. Endocrinol. 2014, 81, 582–587. [Google Scholar] [CrossRef]
- Shidfar, F.; Homayounfar, R.; Fereshtehnejad, S.M.; Kalani, A. Effect of folate supplementation on serum homocysteine and plasma total antioxidant capacity in hypercholesterolemic adults under lovastatin treatment: A double-blind randomized controlled clinical trial. Arch. Med. Res. 2009, 40, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Waly, M.I.; Taranikanti, V.; Guizani, N.; Ali, A.; Rahman, M.S.; Al-Attabi, Z.; Al-Malky, R.N.; Al-Maskari, S.N.M.; Al-Ruqaishi, B.R.S.; et al. Folate/Vitamin B12 Supplementation Combats Oxidative Stress-Associated Carcinogenesis in a Rat Model of Colon Cancer. Nutr. Cancer 2019, 71, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Wijerathne, C.U.B.; Au-Yeung, K.K.W.; Siow, Y.L.; O, K. 5-Methyltetrahydrofolate Attenuates Oxidative Stress and Improves Kidney Function in Acute Kidney Injury through Activation of Nrf2 and Antioxidant Defense. Antioxidants 2022, 11, 1046. [Google Scholar] [CrossRef]
- Asbaghi, O.; Ashtary-Larky, D.; Bagheri, R.; Moosavian, S.P.; Nazarian, B.; Afrisham, R.; Kelishadi, M.R.; Wong, A.; Dutheil, F.; Suzuki, K.; et al. Effects of Folic Acid Supplementation on Inflammatory Markers: A Grade-Assessed Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2327. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, H.; Wu, Y.; Wang, W.; Zhang, D. The association between Dietary Inflammatory Index and disability in older adults. Clin. Nutr. 2021, 40, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.; Kritchevsky, S.B.; Newman, A.B.; Nicklas, B.J.; Simonsick, E.M.; Rubin, S.; Nevitt, M.; Visser, M.; Harris, T.; Pahor, M. Inflammatory markers and incident mobility limitation in the elderly. J. Am. Geriatr. Soc. 2004, 52, 1105–1113. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Cao, X.C.; Peng, J.Y.; Huang, S.L.; Chen, C.; Jia, S.Z.; Ni, J.Z.; Song, G.L. Reversal of Lipid Metabolism Dysregulation by Selenium and Folic Acid Co-Supplementation to Mitigate Pathology in Alzheimer’s Disease. Antioxidants 2022, 11, 829. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B(12), folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet. Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Kado, D.M.; Bucur, A.; Selhub, J.; Rowe, J.W.; Seeman, T. Homocysteine levels and decline in physical function: MacArthur Studies of Successful Aging. Am. J. Med. 2002, 113, 537–542. [Google Scholar] [CrossRef]
- Rolita, L.; Holtzer, R.; Wang, C.; Lipton, R.B.; Derby, C.A.; Verghese, J. Homocysteine and mobility in older adults. J. Am. Geriatr. Soc. 2010, 58, 545–550. [Google Scholar] [CrossRef]
- Veeranki, S.; Tyagi, S.C. Defective homocysteine metabolism: Potential implications for skeletal muscle malfunction. Int. J. Mol. Sci. 2013, 14, 15074–15091. [Google Scholar] [CrossRef]
- Tucker, L.A. Serum and Dietary Folate and Vitamin B(12) Levels Account for Differences in Cellular Aging: Evidence Based on Telomere Findings in 5581 U.S. Adults. Oxidative Med. Cell. Longev. 2019, 2019, 4358717. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Xu, R.; Huang, F.; Wang, Y.; Liu, Q.; Lv, Y.; Zhang, Q. Gender- and age-related differences in homocysteine concentration: A cross-sectional study of the general population of China. Sci. Rep. 2020, 10, 17401. [Google Scholar] [CrossRef]
- Cohen, E.; Margalit, I.; Shochat, T.; Goldberg, E.; Krause, I. Gender differences in homocysteine concentrations, a population-based cross-sectional study. Nutr. Metab. Cardiovasc. Dis. NMCD 2019, 29, 9–14. [Google Scholar] [CrossRef]
- Dierkes, J.; Jeckel, A.; Ambrosch, A.; Westphal, S.; Luley, C.; Boeing, H. Factors explaining the difference of total homocysteine between men and women in the European Investigation Into Cancer and Nutrition Potsdam study. Metab. Clin. Exp. 2001, 50, 640–645. [Google Scholar] [CrossRef]
- Prudova, A.; Albin, M.; Bauman, Z.; Lin, A.; Vitvitsky, V.; Banerjee, R. Testosterone regulation of homocysteine metabolism modulates redox status in human prostate cancer cells. Antioxid. Redox Signal. 2007, 9, 1875–1881. [Google Scholar] [CrossRef]
- Gaikwad, N.W. Mass spectrometry evidence for formation of estrogen-homocysteine conjugates: Estrogens can regulate homocysteine levels. Free Radic. Biol. Med. 2013, 65, 1447–1454. [Google Scholar] [CrossRef]
- Wu, Y.; Tomon, M.; Sumino, K. Methylenetetrahydrofolate reductase gene polymorphism and ischemic stroke: Sex difference in Japanese. Kobe J. Med. Sci. 2001, 47, 255–262. [Google Scholar]
- Cheng, F.W.; Gao, X.; Bao, L.; Mitchell, D.C.; Wood, C.; Sliwinski, M.J.; Smiciklas-Wright, H.; Still, C.D.; Rolston, D.D.K.; Jensen, G.L. Obesity as a risk factor for developing functional limitation among older adults: A conditional inference tree analysis. Obes. (Silver Spring Md.) 2017, 25, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.H.; Petersen, C.L.; Fanous, M.M.; Spangler, H.B.; Kahkoska, A.R.; Jimenez, D.; Batsis, J.A. The relationship between multimorbidity, obesity and functional impairment in older adults. J. Am. Geriatr. Soc. 2022, 70, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Shi, Y. Body weight status and onset of functional limitations in U.S. middle-aged and older adults. Disabil. Health J. 2015, 8, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Senthilkumar, S.R.; Said, H.M. Effect of chronic alcohol exposure on folate uptake by liver mitochondria. Am. J. Physiol. Cell Physiol. 2012, 302, C203–C209. [Google Scholar] [CrossRef]
- Eichner, E.R.; Hillman, R.S. Effect of alcohol on serum folate level. J. Clin. Investig. 1973, 52, 584–591. [Google Scholar] [CrossRef]
- Hanger, H.C.; Sainsbury, R.; Gilchrist, N.L.; Beard, M.E.; Duncan, J.M. A community study of vitamin B12 and folate levels in the elderly. J. Am. Geriatr. Soc. 1991, 39, 1155–1159. [Google Scholar] [CrossRef]
- Vinker, S.; Krantman, E.; Shani, M.; Nakar, S. Low clinical utility of folate determinations in primary care setting. Am. J. Manag. Care 2013, 19, e100–e105. [Google Scholar]
- Rosenberg, I.H.; Bowman, B.B.; Cooper, B.A.; Halsted, C.H.; Lindenbaum, J. Folate nutrition in the elderly. Am. J. Clin. Nutr. 1982, 36, 1060–1066. [Google Scholar] [CrossRef]
- Allen, L.H. Causes of vitamin B12 and folate deficiency. Food Nutr. Bull. 2008, 29, S20–S34; discussion S35–S37. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Ortega, R.M.; Redondo, R.; Andres, P.; Eguileor, I. Nutritional assessment of folate and cyanocobalamin status in a Spanish elderly group. Int. J. Vitam. Nutr. Res. Int. Z. Fur Vitam.—Und Ernahrungsforschung. J. Int. De Vitaminol. Et De Nutr. 1993, 63, 17–21. [Google Scholar]
- Groce, N.; Challenger, E.; Berman-Bieler, R.; Farkas, A.; Yilmaz, N.; Schultink, W.; Clark, D.; Kaplan, C.; Kerac, M. Malnutrition and disability: Unexplored opportunities for collaboration. Paediatr. Int. Child Health 2014, 34, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.K.; Leveille, S.G.; Yen, C.J.; Chai, H.M.; Chang, C.H.; Yeh, Y.C.; Yu, Y.H.; Bean, J.F. Exploring how peak leg power and usual gait speed are linked to late-life disability: Data from the National Health and Nutrition Examination Survey (NHANES), 1999-2002. Am. J. Phys. Med. Rehabil. 2006, 85, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.K.; Leveille, S.G.; Yu, Y.H.; Milberg, W.P. Cognitive function, habitual gait speed, and late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999–2002. Gerontology 2007, 53, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.P.; Mazzella, M.J.; Malin, A.J.; Hair, G.M.; Busgang, S.A.; Saland, J.M.; Curtin, P. Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in NHANES 2009-2014. Environ. Int. 2019, 131, 104993. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Ashurst, H.; Phillips, N.J.; Moat, S.J.; Bonham, J.R.; Martyn, C.N. Renal function, plasma homocysteine and carotid atherosclerosis in elderly people. Atherosclerosis 2001, 154, 141–146. [Google Scholar] [CrossRef]
- Garg, A.X.; Blake, P.G.; Clark, W.F.; Clase, C.M.; Haynes, R.B.; Moist, L.M. Association between renal insufficiency and malnutrition in older adults: Results from the NHANES III. Kidney Int. 2001, 60, 1867–1874. [Google Scholar] [CrossRef]

| Characteristics | With IADL Disability | Without IADL Disability | p Value | With GPA Disability | Without GPA Disability | p Value |
|---|---|---|---|---|---|---|
| Number of participants, n (%) | 1703 (29.11) | 4147 (70.89) | 3699 (63.23) | 2151 (36.77) | ||
| Age (year), n (%) a | <0.001 | <0.001 | ||||
| 60–69 | 764 (42.37) | 2269 (57.89) | 1717 (47.32) | 1316 (64.98) | ||
| 70–79 | 502 (31.94) | 1220 (28.81) | 1125 (31.79) | 597 (25.91) | ||
| ≥80 | 437 (25.69) | 658 (13.29) | 857 (20.89) | 238 (9.11) | ||
| Sex, n (%) a | <0.001 | <0.001 | ||||
| Female | 1002 (65.70) | 1944 (50.29) | 2054 (59.94) | 892 (44.74) | ||
| Male | 701 (34.30) | 2203 (49.71) | 1645 (40.06) | 1259 (55.26) | ||
| Race/ethnicity, n (%) a | <0.001 | 0.898 | ||||
| Mexican American | 218 (5.25) | 464 (3.77) | 422 (4.17) | 260 (4.16) | ||
| Other Hispanic | 192 (4.80) | 447 (3.61) | 400 (4.10) | 239 (3.63) | ||
| Non-Hispanic White | 741 (71.10) | 1833 (78.18) | 1732 (76.21) | 842 (76.45) | ||
| Non-Hispanic Black | 382 (10.64) | 899 (7.84) | 768 (8.57) | 513 (8.61) | ||
| Other races | 170 (8.21) | 504 (6.59) | 377 (6.95) | 297 (7.16) | ||
| Educational level, n (%) a | <0.001 | <0.001 | ||||
| Below high school | 593 (22.44) | 1039 (13.75) | 1124 (18.52) | 508 (11.78) | ||
| High school | 412 (27.71) | 934 (23.35) | 896 (25.94) | 450 (22.02) | ||
| Above high school | 693 (49.85) | 2170 (62.91) | 1671 (55.54) | 1192 (66.20) | ||
| Marital status, n (%) a | <0.001 | <0.001 | ||||
| Not living alone | 785 (52.76) | 2538 (66.79) | 1913 (58.33) | 1410 (71.29) | ||
| Living alone | 916 (47.24) | 1605 (33.21) | 1781 (41.67) | 740 (28.71) | ||
| Poverty–income ratio, n (%) a | <0.001 | <0.001 | ||||
| <1 | 402 (14.91) | 596 (7.43) | 731 (11.45) | 267 (5.89) | ||
| ≥1 | 1301 (85.09) | 3551 (92.57) | 2968 (88.55) | 1884 (94.11) | ||
| Physical activity, n (%) a | <0.001 | <0.001 | ||||
| Low | 1149 (63.24) | 1927 (41.81) | 2244 (54.80) | 832 (34.78) | ||
| High | 540 (36.76) | 2205 (58.19) | 1433 (45.20) | 1312 (65.22) | ||
| Body mass index, n (%) a | <0.001 | <0.001 | ||||
| <25 kg/m2 | 371 (21.73) | 1086 (24.08) | 785 (19.79) | 672 (29.77) | ||
| 25 to <30 kg/m2 | 491 (30.30) | 1579 (38.68) | 1208 (33.55) | 862 (41.57) | ||
| ≥30 kg/m2 | 769 (47.98) | 1445 (37.24) | 1608 (46.66) | 606 (28.67) | ||
| Smoking status, n (%) a | 899 (53.44) | 2008 (48.43) | 0.043 | 1931 (51.79) | 976 (46.24) | 0.007 |
| Alcohol consumption, n (%) a | 281 (20.23) | 554 (15.01) | 0.011 | 584 (17.52) | 251 (14.31) | 0.077 |
| Hypertension, n (%) a | 1448 (83.98) | 3241 (74.40) | <0.001 | 3058 (79.81) | 1631 (71.97) | <0.001 |
| Diabetes, n (%) a | 602 (27.50) | 1002 (19.88) | <0.001 | 1162 (24.95) | 442 (16.61) | <0.001 |
| Arthritis, n (%) a | 1151 (72.22) | 1753 (45.62) | <0.001 | 2286 (64.25) | 618 (32.57) | <0.001 |
| Stroke, n (%) a | 263 (13.09) | 218 (4.83) | <0.001 | 392 (9.07) | 89 (3.46) | <0.001 |
| Gout, n (%) a | 209 (12.12) | 340 (8.99) | 0.005 | 407 (10.72) | 142 (8.26) | 0.068 |
| Cancer, n (%) a | 373 (27.26) | 811 (24.29) | 0.154 | 827 (27.28) | 357 (21.25) | 0.001 |
| Congestive heart failure, n (%) a | 230 (10.59) | 202 (4.64) | <0.001 | 372 (7.93) | 60 (3.25) | <0.001 |
| Coronary heart disease, n (%) a | 245 (14.33) | 339 (9.10) | <0.001 | 443 (11.91) | 141 (8.02) | 0.007 |
| Angina, n (%) a | 145 (8.76) | 171 (4.32) | 0.002 | 260 (7.02) | 56 (2.86) | 0.002 |
| Asthma, n (%) a | 326 (20.44) | 489 (12.24) | <0.001 | 615 (16.82) | 200 (10.23) | <0.001 |
| Chronic bronchitis, n (%) a | 214 (14.60) | 215 (6.57) | <0.001 | 369 (11.54) | 60 (3.76) | <0.001 |
| Emphysema, n (%) a | 125 (8.67) | 109 (2.80) | <0.001 | 200 (5.93) | 34 (1.63) | <0.001 |
| Total energy intake (kcal/day), median (IQR) b | 1670.8 (835.5) | 1765 (858.5) | <0.001 | 1702.5 (832) | 1800.5 (877) | <0.001 |
| Total folate (nmol/L), median (IQR) b | 41.8 (40.7) | 45.4 (39.6) | 0.002 | 44.1 (41.1) | 45.1 (37.8) | 0.412 |
| 5-Methyltetrahydrofolate (nmol/L), median (IQR) b | 39.2 (38) | 43.1 (37.7) | <0.001 | 41.2 (38.9) | 42.6 (35.9) | 0.227 |
| Quartile of 5-Methyltetrahydrofolate | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| LEM | ||||
| Cases/Participants | 709/1469 | 596/1465 | 552/1456 | 584/1460 |
| Age-adjusted | Ref. | 0.72 (0.59–0.89) ** | 0.58 (0.46–0.73) ** | 0.62 (0.51–0.74) ** |
| Multivariate-adjusted | Ref. | 1.05 (0.78–1.43) | 0.80 (0.57–1.14) | 0.87 (0.65–1.16) |
| IADL | ||||
| Cases/Participants | 494/1469 | 437/1465 | 373/1456 | 399/1460 |
| Age-adjusted | Ref. | 0.77 (0.59–0.99) * | 0.60 (0.45–0.79) ** | 0.56 (0.42–0.74) ** |
| Multivariate-adjusted | Ref. | 0.92 (0.63–1.33) | 0.74 (0.52–1.04) | 0.65 (0.46–0.91) * |
| ADL | ||||
| Cases/Participants | 448/1469 | 400/1465 | 350/1456 | 354/1460 |
| Age-adjusted | Ref. | 0.79 (0.58–1.06) | 0.71 (0.53–0.95) * | 0.70 (0.55–0.90) ** |
| Multivariate-adjusted | Ref. | 1.00 (0.69–1.45) | 0.90 (0.61–1.32) | 0.87 (0.62–1.21) |
| LSA | ||||
| Cases/Participants | 421/1469 | 361/1465 | 329/1456 | 319/1460 |
| Age-adjusted | Ref. | 0.75 (0.57–1.00) | 0.69 (0.53–0.90) ** | 0.59 (0.43–0.82) ** |
| Multivariate-adjusted | Ref. | 0.93 (0.64–1.35) | 0.87 (0.60–1.25) | 0.74 (0.48–1.14) |
| GPA | ||||
| Cases/Participants | 979/1469 | 918/1465 | 881/1456 | 921/1460 |
| Age-adjusted | Ref. | 0.72 (0.58–0.89) ** | 0.64 (0.50–0.81) ** | 0.64 (0.50–0.82) ** |
| Multivariate-adjusted | Ref. | 0.77 (0.56–1.05) | 0.66 (0.44–0.99) * | 0.70 (0.50–0.96) * |
| Quartile of 5-Methyltetrahydrofolate | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| LEM | ||||
| Cases/Participants | 425/737 | 341/742 | 310/732 | 326/735 |
| Age-adjusted | Ref. | 0.75 (0.54–1.03) | 0.58 (0.42–0.80) ** | 0.49 (0.37–0.65) ** |
| Multivariate-adjusted | Ref. | 0.97 (0.58–1.62) | 0.78 (0.45–1.36) | 0.63 (0.40–1.00) |
| IADL | ||||
| Cases/Participants | 313/737 | 249/742 | 205/732 | 235/735 |
| Age-adjusted | Ref. | 0.62 (0.47–0.81) ** | 0.44 (0.31–0.61) ** | 0.45 (0.33–0.63) ** |
| Multivariate-adjusted | Ref. | 0.73 (0.47–1.14) | 0.49 (0.32–0.74) ** | 0.52 (0.35–0.79) ** |
| ADL | ||||
| Cases/Participants | 253/737 | 208/742 | 175/732 | 184/735 |
| Age-adjusted | Ref. | 0.70 (0.47–1.03) | 0.61 (0.42–0.88) ** | 0.68 (0.46–0.99) * |
| Multivariate-adjusted | Ref. | 0.87 (0.52–1.45) | 0.64 (0.37–1.10) | 0.79 (0.44–1.42) |
| LSA | ||||
| Cases/Participants | 245/737 | 202/742 | 173/732 | 178/735 |
| Age-adjusted | Ref. | 0.67 (0.47–0.95) * | 0.60 (0.42–0.87) ** | 0.56 (0.39–0.81) ** |
| Multivariate-adjusted | Ref. | 0.76 (0.47–1.25) | 0.68 (0.39–1.19) | 0.68 (0.41–1.14) |
| GPA | ||||
| Cases/Participants | 575/737 | 507/742 | 479/732 | 493/735 |
| Age-adjusted | Ref. | 0.50 (0.35–0.73) ** | 0.49 (0.35–0.69) ** | 0.43 (0.29–0.62) ** |
| Multivariate-adjusted | Ref. | 0.53 (0.32–0.88) * | 0.56 (0.33–0.96) * | 0.51 (0.32–0.79) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, L.; Zhang, T.; Zhang, L.; Zhang, D. Associations between Serum Folate Concentrations and Functional Disability in Older Adults. Antioxidants 2023, 12, 619. https://doi.org/10.3390/antiox12030619
Ji L, Zhang T, Zhang L, Zhang D. Associations between Serum Folate Concentrations and Functional Disability in Older Adults. Antioxidants. 2023; 12(3):619. https://doi.org/10.3390/antiox12030619
Chicago/Turabian StyleJi, Lujun, Tianhao Zhang, Liming Zhang, and Dongfeng Zhang. 2023. "Associations between Serum Folate Concentrations and Functional Disability in Older Adults" Antioxidants 12, no. 3: 619. https://doi.org/10.3390/antiox12030619
APA StyleJi, L., Zhang, T., Zhang, L., & Zhang, D. (2023). Associations between Serum Folate Concentrations and Functional Disability in Older Adults. Antioxidants, 12(3), 619. https://doi.org/10.3390/antiox12030619






