Metallothionein 3 Inhibits 3T3-L1 Adipocyte Differentiation via Reduction of Reactive Oxygen Species
Abstract
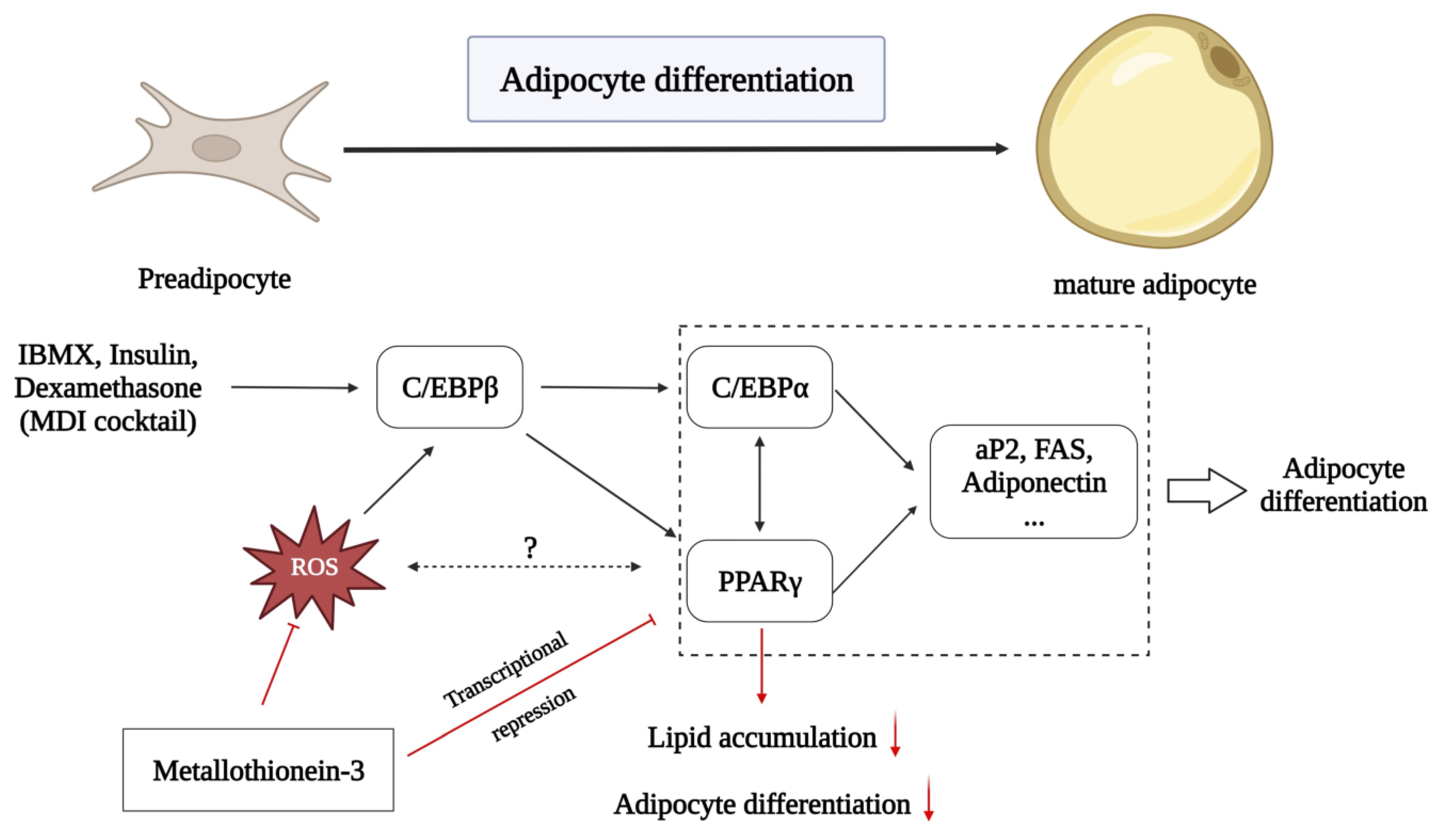
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. 3T3-L1 Adipocyte Differentiation
2.3. Plasmids and Transfection
2.4. Oil Red O Staining
2.5. Triglyceride (TG) Colorimetric Assay
2.6. Luciferase Reporter Assays
2.7. Immunoblotting
2.8. Immunoprecipitation
2.9. Reverse Transcription followed by Quantitative PCR (RT-qPCR)
2.10. Dihydroethidium (DHE) Staining
2.11. Statistical Analysis
3. Results
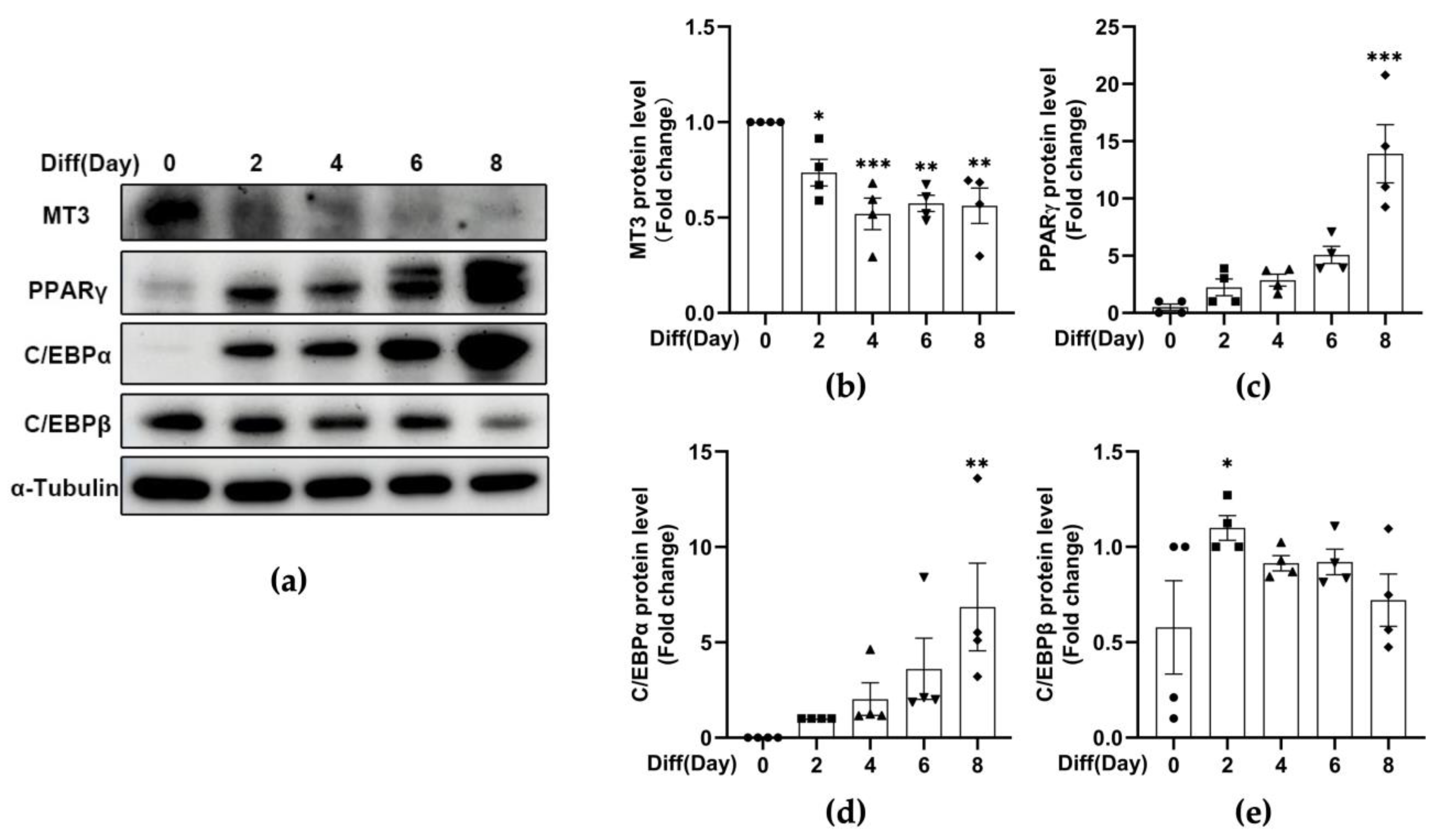
3.1. MT3 Is Significantly Downregulated during 3T3-L1 Adipocyte Differentiation
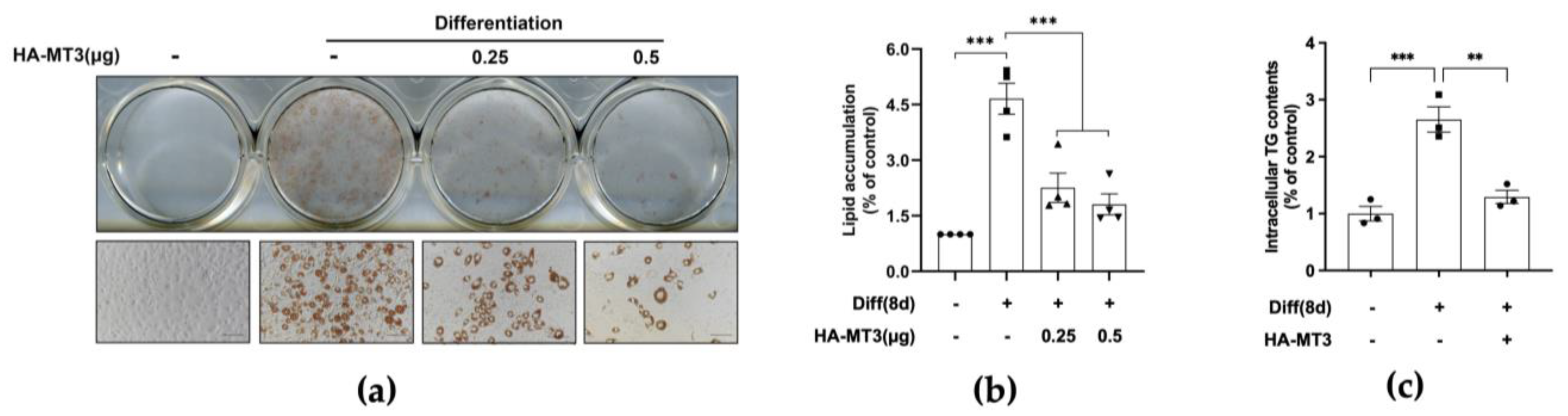
3.2. Mt3 Overexpression Inhibits Lipid Accumulation in 3T3-L1 Adipocytes
3.3. Mt3 Overexpression Suppresses the Protein Levels of Adipogenic Transcriptional Factors in 3T3-L1 Cells
3.4. Mt3 Overexpression Reduces Adipogenesis-Related Gene Expression in 3T3-L1 Cells
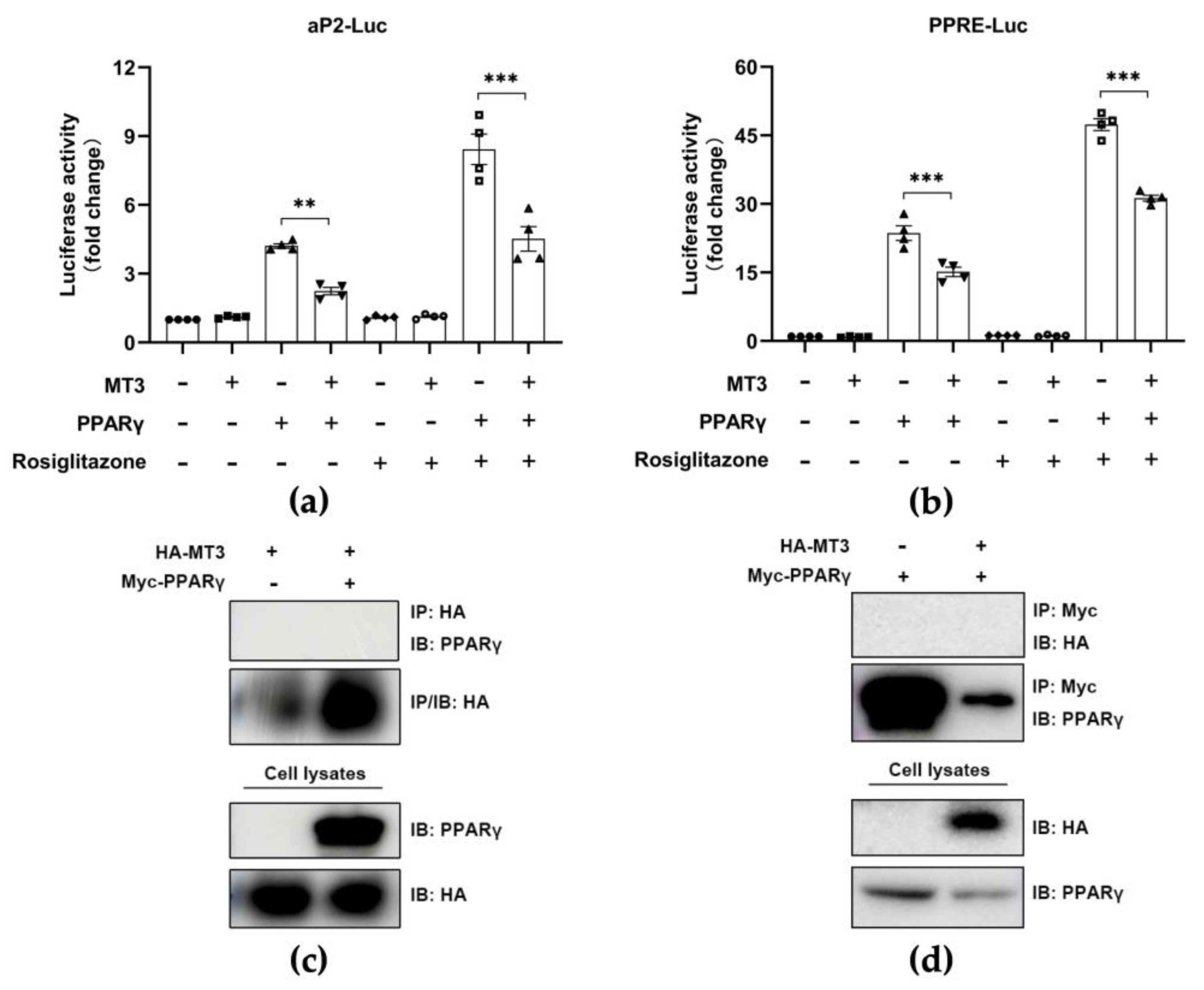
3.5. MT3 Indirectly Suppresses PPARγ Transcriptional Activity
3.6. MT3 Impedes ROS Production in the Early Stage of 3T3-L1 Adipocyte Differentiation
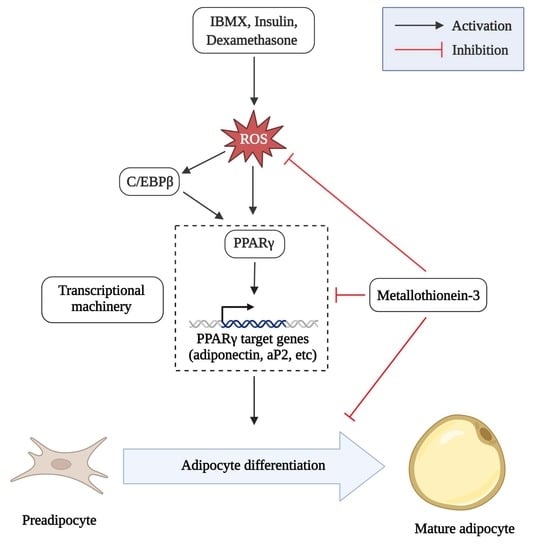
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havas, S.; Aronne, L.; Woodworth, K. The problem of obesity in the United States has become increasingly prominent and is now recognized as a critical target for public health intervention. Introduction. Am. J. Med. 2009, 122 (Suppl. S1), S1–S3. [Google Scholar]
- Lindemulder, S.J.; Stork, L.C.; Lu, X.; Hunger, S.; Neglia, J.P.; Kadan-Lottick, N.S. Reply to: Obesity is an important health problem in survivors of pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 2015, 62, 2057. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Azvolinsky, A. The Obesity-Cancer Link: A Growing Connection. J. Natl. Cancer Inst. 2016, 108, djw243. [Google Scholar] [CrossRef]
- Argolo, D.F.; Iyengar, N.M.; Hudis, C.A. Obesity and Cancer: Concepts and Challenges. Indian J. Surg. Oncol. 2015, 6, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; McTernan, P.G.; Kumar, S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clin. Sci. 2005, 109, 243–256. [Google Scholar] [CrossRef]
- Obesity related to periodontal disease. J. Am. Dent. Assoc. 2000, 131, 729. [CrossRef] [PubMed]
- Koutnikova, H.; Auwerx, J. Regulation of adipocyte differentiation. Ann. Med. 2001, 33, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Cowherd, R.M.; Lyle, R.E.; McGehee, R.E. Molecular regulation of adipocyte differentiation. Semin. Cell Dev. Biol. 1999, 10, 3–10. [Google Scholar] [CrossRef]
- Tanaka, T.; Yoshida, N.; Kishimoto, T.; Akira, S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997, 16, 7432–7443. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef]
- Park, A.; Kim, W.K.; Bae, K.H. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 2014, 6, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Graves, R.A.; Budavari, A.I.; Spiegelman, B.M. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994, 8, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J.; Liu, X.S.; et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef]
- Albrecht, A.L.; Singh, R.K.; Somji, S.; Sens, M.A.; Sens, D.A.; Garrett, S.H. Basal and metal-induced expression of metallothionein isoform 1 and 2 genes in the RWPE-1 human prostate epithelial cell line. J. Appl. Toxicol. 2008, 28, 283–293. [Google Scholar] [CrossRef]
- Moleirinho, A.; Carneiro, J.; Matthiesen, R.; Silva, R.M.; Amorim, A.; Azevedo, L. Gains, losses and changes of function after gene duplication: Study of the metallothionein family. PLoS ONE 2011, 6, e18487. [Google Scholar] [CrossRef]
- Vasák, M.; Hasler, D.W. Metallothioneins: New functional and structural insights. Curr. Opin. Chem. Biol. 2000, 4, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D. The elusive function of metallothioneins. Proc. Natl. Acad. Sci. USA 1998, 95, 8428–8430. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. The function of zinc metallothionein: A link between cellular zinc and redox state. J. Nutr. 2000, 130 (Suppl. S5), 1455S–1458S. [Google Scholar] [CrossRef]
- Sharma, S.; Moon, C.S.; Khogali, A.; Haidous, A.; Chabenne, A.; Ojo, C.; Jelebinkov, M.; Kurdi, Y.; Ebadi, M. Biomarkers in Parkinson’s disease (recent update). Neurochem. Int. 2013, 63, 201–229. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Adam, P.; Křížková, S.; Heger, Z.; Babula, P.; Pekařík, V.; Vaculovičoá, M.; Gomes, C.M.; Kizek, R.; Adam, V. Metallothioneins in Prion- and Amyloid-Related Diseases. J. Alzheimers Dis. 2016, 51, 637–656. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.H.; Wood, A.M.; Newman, A.M.; Bremner, I.; Choo, K.H.; Michalska, A.E.; Duncan, J.S.; Trayhurn, P. Obesity and hyperleptinemia in metallothionein (-I and -II) null mice. Proc. Natl. Acad. Sci. USA 1998, 95, 358–363. [Google Scholar] [CrossRef]
- Sato, M.; Kawakami, T.; Kondoh, M.; Takiguchi, M.; Kadota, Y.; Himeno, S.; Suzuki, S. Development of high-fat-diet-induced obesity in female metallothionein-null mice. FASEB J. 2010, 24, 2375–2384. [Google Scholar] [CrossRef]
- Byun, H.R.; Kim, D.K.; Koh, J.Y. Obesity and downregulated hypothalamic leptin receptors in male metallothionein-3-null mice. Neurobiol. Dis. 2011, 44, 125–132. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Holmgren, A.; Larsson, N.G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the biological roles of reactive oxygen species. Cell. Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, B.R.; Hansen, J.M. Extracellular redox environments regulate adipocyte differentiation. Differentiation 2010, 80, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Fritch, L.; Burkart, A.; Bell, G.; Mendelson, K.; Leszyk, J.; Nicoloro, S.; Czech, M.; Corvera, S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol. Cell Biol. 2003, 23, 1085–1094. [Google Scholar] [CrossRef]
- Sekiya, M.; Hiraishi, A.; Touyama, M.; Sakamoto, K. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem. Biophys. Res. Commun. 2008, 375, 602–607. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lee, S.H.; Bahn, M.; Yeo, C.Y.; Lee, K.Y. Pin1 enhances adipocyte differentiation by positively regulating the transcriptional activity of PPARγ. Mol. Cell Endocrinol. 2016, 436, 150–158. [Google Scholar] [CrossRef]
- Li, S.; Kim, M.J.; Lee, S.H.; Jin, L.; Cong, W.; Jeong, H.G.; Lee, K.Y. Metallothionein 3 Promotes Osteoblast Differentiation in C2C12 Cells via Reduction of Oxidative Stress. Int. J. Mol. Sci. 2021, 22, 4312. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Osteoblast-adipocyte lineage plasticity in tissue development, maintenance and pathology. Cell Mol. Life Sci. 2014, 71, 493–497. [Google Scholar] [CrossRef]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.M.; Griesel, B.A.; Gurley, J.M.; Szweda, L.I.; Olson, A.L. Glucose availability controls adipogenesis in mouse 3T3-L1 adipocytes via up-regulation of nicotinamide metabolism. J. Biol. Chem. 2017, 292, 18556–18564. [Google Scholar] [CrossRef] [PubMed]
- Berndt, J.; Kovacs, P.; Ruschke, K.; Klöting, N.; Fasshauer, M.; Schön, M.R.; Körner, A.; Stumvoll, M.; Blüher, M. Fatty acid synthase gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Diabetologia 2007, 50, 1472–1480. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, S.H.; Kim, S.Z.; Park, W.H. Antimycin A as a mitochondria damage agent induces an S phase arrest of the cell cycle in HeLa cells. Life Sci. 2008, 83, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Duncan, J.S.; Wood, A.M.; Beattie, J.H. Metallothionein gene expression and secretion in white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R2329–R2335. [Google Scholar] [CrossRef]
- Cancello, R.; Zulian, A.; Gentilini, D.; Mencarelli, M.; Della Barba, A.; Maffei, M.; Vitti, P.; Invitti, C.; Liuzzi, A.; Di Blasio, A.M. Permanence of molecular features of obesity in subcutaneous adipose tissue of ex-obese subjects. Int. J. Obes. 2013, 37, 867–873. [Google Scholar] [CrossRef]
- Haynes, V.; Connor, T.; Tchernof, A.; Vidal, H.; Dubois, S. Metallothionein 2a gene expression is increased in subcutaneous adipose tissue of type 2 diabetic patients. Mol. Genet. Metab. 2013, 108, 90–94. [Google Scholar] [CrossRef]
- Kawakami, T.; Takasaki, S.; Kadota, Y.; Fukuoka, D.; Sato, M.; Suzuki, S. Regulatory role of metallothionein-1/2 on development of sex differences in a high-fat diet-induced obesity. Life Sci. 2019, 226, 12–21. [Google Scholar] [CrossRef]
- Lindeque, J.Z.; Jansen van Rensburg, P.J.; Louw, R.; van der Westhuizen, F.H.; Florit, S.; Ramírez, L.; Giralt, M.; Hidalgo, J. Obesity and metabolomics: Metallothioneins protect against high-fat diet-induced consequences in metallothionein knockout mice. OMICS 2015, 19, 92–103. [Google Scholar] [CrossRef]
- Kadota, Y.; Toriuchi, Y.; Aki, Y.; Mizuno, Y.; Kawakami, T.; Nakaya, T.; Sato, M.; Suzuki, S. Metallothioneins regulate the adipogenic differentiation of 3T3-L1 cells via the insulin signaling pathway. PLoS ONE 2017, 12, e0176070. [Google Scholar] [CrossRef]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef] [PubMed]
- Howells, C.; West, A.K.; Chung, R.S. Neuronal growth-inhibitory factor (metallothionein-3): Evaluation of the biological function of growth-inhibitory factor in the injured and neurodegenerative brain. FEBS J. 2010, 277, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Gomi, F.; Masumizu, T.; Miura, Y. Growth inhibitory factor prevents neurite extension and the death of cortical neurons caused by high oxygen exposure through hydroxyl radical scavenging. J. Biol. Chem. 2002, 277, 32353–32359. [Google Scholar] [CrossRef] [PubMed]
- You, H.J.; Lee, K.J.; Jeong, H.G. Overexpression of human metallothionein-III prevents hydrogen peroxide-induced oxidative stress in human fibroblasts. FEBS Lett. 2002, 521, 175–179. [Google Scholar] [CrossRef]
- Kanda, Y.; Hinata, T.; Kang, S.W.; Watanabe, Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011, 89, 250–258. [Google Scholar] [CrossRef]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011, 14, 537–544. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.H.; Song, S.Y.; Kim, W.S.; Song, S.U.; Yi, T.; Jeon, M.S.; Chung, H.M.; Xia, Y.; Sung, J.H. Hypoxia induces adipocyte differentiation of adipose-derived stem cells by triggering reactive oxygen species generation. Cell Biol. Int. 2014, 38, 32–40. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, S.R.; Park, S.J.; Park, H.S.; Min, K.H.; Jin, S.M.; Lee, M.K.; Kim, U.H.; Lee, Y.C. Peroxisome proliferator activated receptor-gamma modulates reactive oxygen species generation and activation of nuclear factor-kappaB and hypoxia-inducible factor 1alpha in allergic airway disease of mice. J. Allergy Clin. Immunol. 2006, 118, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, J.; Li, D.; Zhang, X.; Mehta, J.L. Angiotensin II regulation of collagen type I expression in cardiac fibroblasts: Modulation by PPAR-gamma ligand pioglitazone. Hypertension 2004, 44, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Kollipara, R.K.; Singh, D.K.; Sudderth, J.; Hu, Z.; Nguyen, H.; Wang, S.; Humphries, C.G.; Carstens, R.; Huffman, K.E.; et al. Inhibition of cancer cell proliferation by PPARγ is mediated by a metabolic switch that increases reactive oxygen species levels. Cell Metab. 2014, 20, 650–661. [Google Scholar] [CrossRef]
- Bost, F.; Aouadi, M.; Caron, L.; Binetruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef]
- Hu, E.; Kim, J.B.; Sarraf, P.; Spiegelman, B.M. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 1996, 274, 2100–2103. [Google Scholar] [CrossRef]
- Kusuyama, J.; Ohnishi, T.; Bandow, K.; Amir, M.S.; Shima, K.; Semba, I.; Matsuguchi, T. Constitutive activation of p46JNK2 is indispensable for C/EBPdelta induction in the initial stage of adipogenic differentiation. Biochem. J. 2017, 474, 3421–3437. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Lisanti, M.P.; Scherer, P.E. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J. Biol. Chem. 1998, 273, 32111–32120. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.L.; Yang, C.C.; Lee, I.T.; Lin, C.C.; Chi, P.L.; Hsiao, L.D.; Yang, C.M. Lipopolysaccharide induces ICAM-1 expression via a c-Src/NADPH oxidase/ROS-dependent NF-kappaB pathway in human pulmonary alveolar epithelial cells. Am. J. Physiol. Lung. Cell Mol. Physiol. 2016, 310, L639–L657. [Google Scholar] [CrossRef]
- Kuo, C.W.; Shen, C.J.; Tung, Y.T.; Chen, H.L.; Chen, Y.H.; Chang, W.H.; Cheng, K.C.; Yang, S.H.; Chen, C.M. Extracellular superoxide dismutase ameliorates streptozotocin-induced rat diabetic nephropathy via inhibiting the ROS/ERK1/2 signaling. Life Sci. 2015, 135, 77–86. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Lahair, M.M.; Franklin, R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal. Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Sakaue, H.; Ogawa, W.; Matsumoto, M.; Kuroda, S.; Takata, M.; Sugimoto, T.; Spiegelman, B.M.; Kasuga, M. Posttranscriptional control of adipocyte differentiation through activation of phosphoinositide 3-kinase. J. Biol. Chem. 1998, 273, 28945–28952. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Kim, E.K.; Tucker, D.F.; Kim, C.D.; Birnbaum, M.J.; Bae, S.S. Isoform-specific regulation of adipocyte differentiation by Akt/protein kinase Balpha. Biochem. Biophys. Res. Commun. 2008, 371, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Annie-Mathew, A.S.; Prem-Santhosh, S.; Jayasuriya, R.; Ganesh, G.; Ramkumar, K.M.; Sarada, D.V.L. The pivotal role of Nrf2 activators in adipocyte biology. Pharmacol. Res. 2021, 173, 105853. [Google Scholar] [CrossRef]




| Antibody | Order# | Company | Dilution |
|---|---|---|---|
| MT3 | LS-C295361 | LifeSpan BioSciences, Seattle, WA, USA | 1:400 |
| PPARγ | D69 | Cell Signaling Technology, Danvers, MA, USA | 1:1000 |
| C/EBPα | SC-61 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | 1:1000 |
| C/EBPβ | SC-150 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | 1:1000 |
| adiponectin | C45B10 | Cell Signaling Technology, Danvers, MA, USA | 1:1000 |
| α-Tubulin | sc-53646 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | 1:1000 |
| HA | 12CA5 | Roche Applied Science, Basel, Switzerland | 1:1000 |
| Myc | 9E10 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | 1:1000 |
| Gene | Accession Number | Forward Primer (5′–>3′) | Reverse Primer (5′–>3′) |
|---|---|---|---|
| Mt1 | NM_013602.3 | AAGAGTGAGTTGGGACACCTT | CGAGACAATACAATG GCCTCC |
| Mt2 | NM_008630.2 | ATGCAAATGTACTTCCTGCAAGA | CTGGGAGCACTTCGCACAG |
| Mt3 | NM_013603.2 | TGCACCTGCTCGGACAAAT | CTTGGCACACTTCTCACATC |
| Pparg | NM_011146.4 | GCCCTTTGGTGACTTTATGGA | GCAGCAGGTTGCTTGGATG |
| Cebpa | NM_007678.3 | CAAGAACAGCAACGAGTACCG | GTCACTGGTCAACTCCAGCAC |
| Cebpb | NM_001287738.1 | ACCGGGTTTCGGGACTTGA | GTTGCGTCAGTCCCGTGTCCA |
| Fasn | NM_007988.3 | GCTATGCAGATGGCTGTCTCTCCCAG | GCAGCGCTGTTTACATTCCTCCCAGG |
| Glut4 | NM_001359114.1 | GTAACTTCATTGTCGGCATGG | AGCTGAGATCTGGTCAAACG |
| Adiponectin | NM_009605.5 | CATCCCAGGACATCCTGGCCACAATG | GGCCCTTCAGCTCCTGTCATTCCAAC |
| Fabp4 | NM_024406.4 | AAGGTGAAGAGCATCATAACCCT | TCACGCCTTTCATAACACATTCC |
| β-actin | NM_007393.5 | GCAGGAGTACGATGAGTCCG | ACGCAGCTCAGTAACAGTCC |
| Catalase | NM_009804.2 | CACACCTACACGCAGGCCGG | CTGCGCTCCGGAGTGGGAGA |
| Gpx1 | NM_008160.6 | AGTCCACCGTGTATGCCTTC | GAGAAGCGACATTCAATG |
| Sod1 | NM_011434.2 | GTGTGCGTGCTGAAGGGCGA | GACGTGGAACCCATGCTGGCC |
| Sod2 | NM_013671.3 | GTGGTGGAGAACCCAAAGGA | AACCTTGGACTCCCACAGACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Lee, S.H.; Piao, M.; Kim, H.S.; Lee, K.Y. Metallothionein 3 Inhibits 3T3-L1 Adipocyte Differentiation via Reduction of Reactive Oxygen Species. Antioxidants 2023, 12, 640. https://doi.org/10.3390/antiox12030640
Li Y, Lee SH, Piao M, Kim HS, Lee KY. Metallothionein 3 Inhibits 3T3-L1 Adipocyte Differentiation via Reduction of Reactive Oxygen Species. Antioxidants. 2023; 12(3):640. https://doi.org/10.3390/antiox12030640
Chicago/Turabian StyleLi, Yuankuan, Sung Ho Lee, Meiyu Piao, Hyung Sik Kim, and Kwang Youl Lee. 2023. "Metallothionein 3 Inhibits 3T3-L1 Adipocyte Differentiation via Reduction of Reactive Oxygen Species" Antioxidants 12, no. 3: 640. https://doi.org/10.3390/antiox12030640
APA StyleLi, Y., Lee, S. H., Piao, M., Kim, H. S., & Lee, K. Y. (2023). Metallothionein 3 Inhibits 3T3-L1 Adipocyte Differentiation via Reduction of Reactive Oxygen Species. Antioxidants, 12(3), 640. https://doi.org/10.3390/antiox12030640