H2S Donors with Cytoprotective Effects in Models of MI/R Injury and Chemotherapy-Induced Cardiotoxicity
Abstract
:1. Introduction
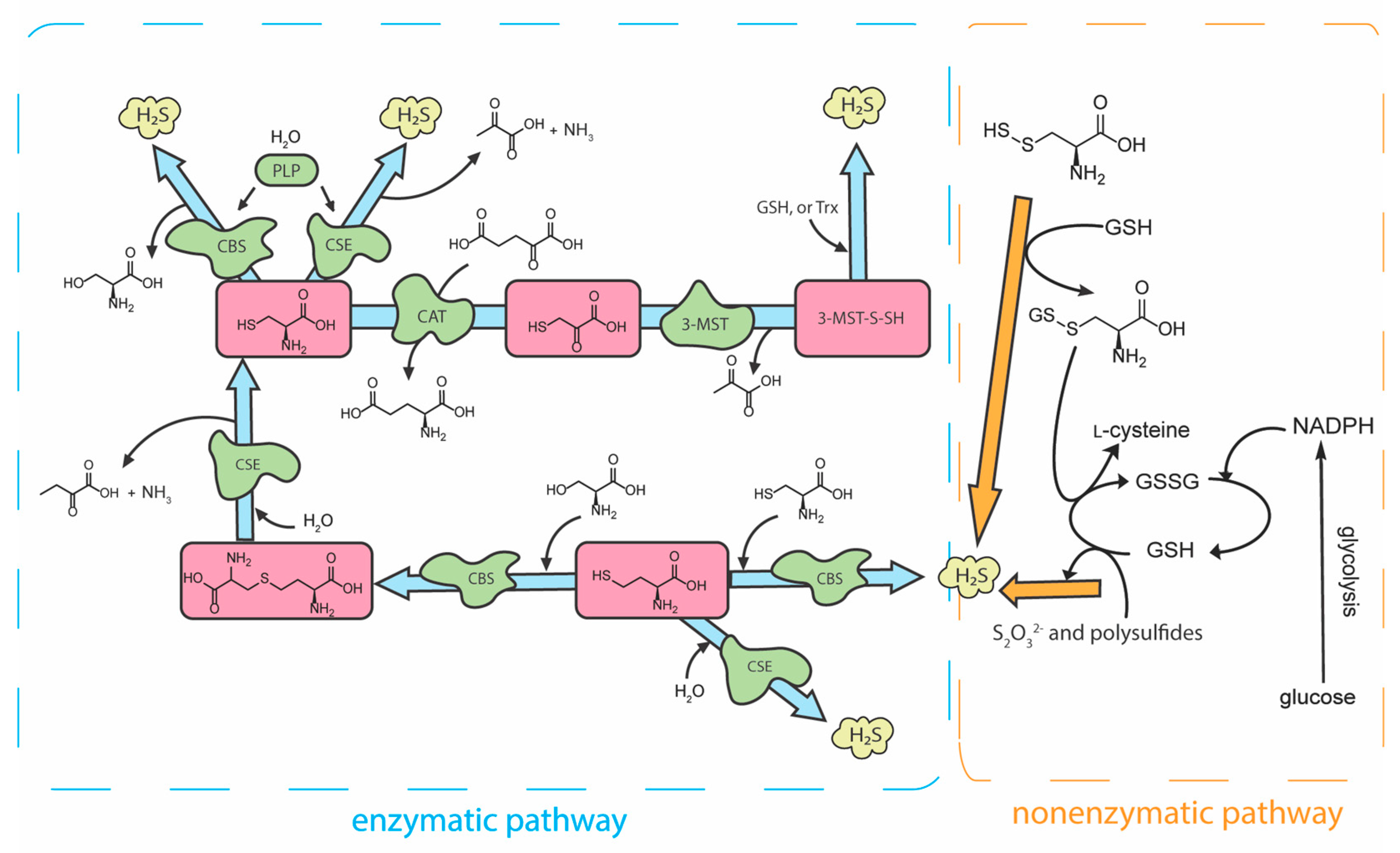
2. H2S Biosynthesis and Metabolism
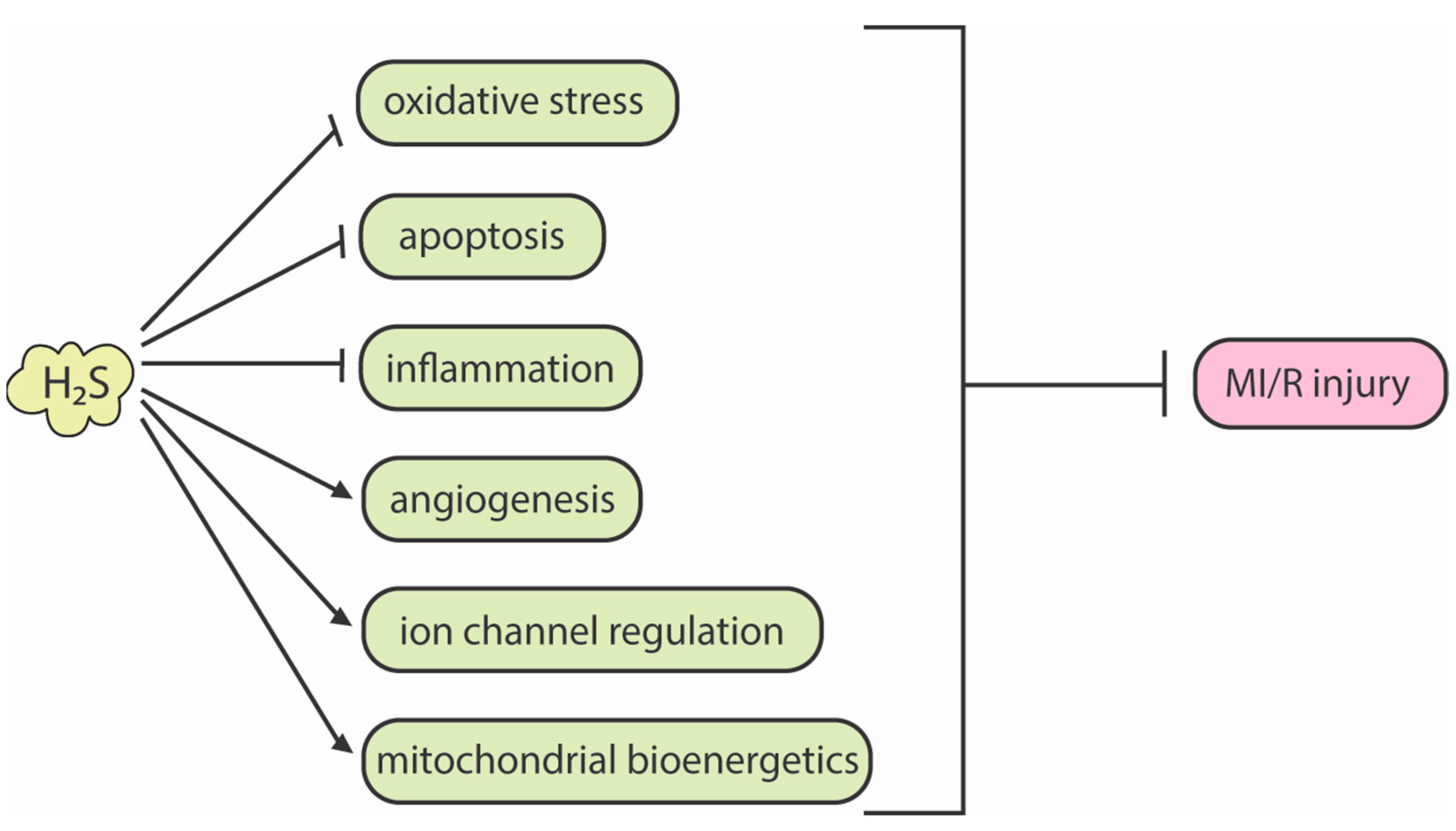
3. H2S Bioactivity and Its Attenuation of Myocardial Ischemia-Reperfusion Injury
4. H2S Donors That Protect against Myocardial Ischemia-Reperfusion Injury
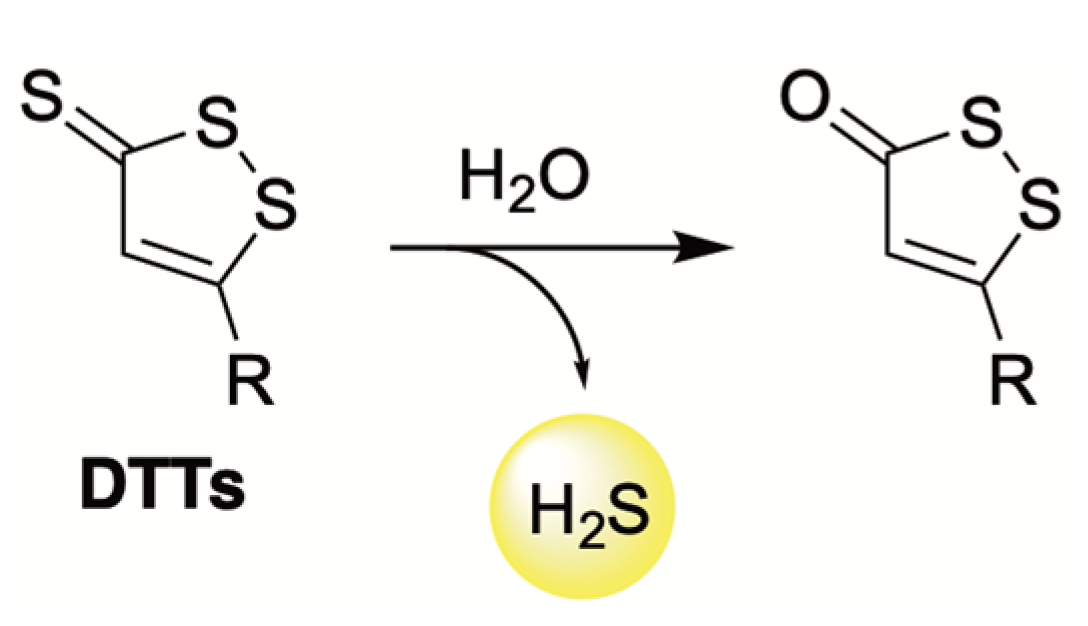
4.1. Hydrolysis-Triggered Donors
4.2. pH-Triggered Donors
4.3. Thiol-Triggered Donors
4.4. Enzyme-Triggered Donors
4.5. ROS-Triggered Donors
5. Chemotherapy-Induced Cardiotoxicity
6. H2S Conjugated Codrugs That Combat Anthracycline-Induced Cardiotoxicity
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beauchamp, R.O.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A.; Leber, P. A Critical Review of the Literature on Hydrogen Sulfide Toxicity. CRC Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef]
- Tomlin, S. Smelly Cats. Nature 1998, 396, 628. [Google Scholar] [CrossRef]
- Fiedler, N.; Kipen, H.; Ohman-Strickland, P.; Zhang, J.; Weisel, C.; Laumbach, R.; Kelly-McNeil, K.; Olejeme, K.; Lioy, P. Sensory and Cognitive Effects of Acute Exposure to Hydrogen Sulfide. Environ. Health Perspect. 2008, 116, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, K.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, H. Hydrogen Sulfide as a Neuromodulator. Mol. Neurobiol. 2002, 26, 013–020. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M. Hydrogen Sulfide as a Vasodilator. IUBMB Life Int. Union Biochem. Mol. Biol. Life 2005, 57, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Banerjee, R. Redox Biochemistry of Hydrogen Sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef] [Green Version]
- Wang, R. Two’s Company, Three’s a Crowd: Can H2S Be the Third Endogenous Gaseous Transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [Green Version]
- Wang, R. The Gasotransmitter Role of Hydrogen Sulfide. Antioxid. Redox Signal. 2003, 5, 493–501. [Google Scholar] [CrossRef]
- Wang, R. Shared Signaling Pathways among Gasotransmitters. Proc. Natl. Acad. Sci. USA 2012, 109, 8801–8802. [Google Scholar] [CrossRef] [Green Version]
- Papapetropoulos, A.; Foresti, R.; Ferdinandy, P. Pharmacology of the ‘Gasotransmitters’ NO, CO and H2S: Translational Opportunities. Br. J. Pharmacol. 2015, 172, 1395–1396. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.N.; Centelles, M.N.; Moore, K.P. Making and Working with Hydrogen Sulfide. Free Radic. Biol. Med. 2009, 47, 1346–1353. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Carrington, S.J.; Tantillo, D.J.; Harrison, J.G.; Ignarro, L.J.; Freeman, B.A.; Chen, A.; Wink, D.A. Small Molecule Signaling Agents: The Integrated Chemistry and Biochemistry of Nitrogen Oxides, Oxides of Carbon, Dioxygen, Hydrogen Sulfide, and Their Derived Species. Chem. Res. Toxicol. 2012, 25, 769–793. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Ding, L.; Xie, Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.-S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, H. Hydrogen Sulfide: Its Production, Release and Functions. Amino Acids 2011, 41, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Li, M.; Mao, J.; Zhu, Y. New Therapeutic Approaches Using Hydrogen Sulfide Donors in Inflammation and Immune Response. Antioxid. Redox Signal. 2021, 35, 341–356. [Google Scholar] [CrossRef]
- Whiteman, M.; Winyard, P.G. Hydrogen Sulfide and Inflammation: The Good, the Bad, the Ugly and the Promising. Expert Rev. Clin. Pharmacol. 2011, 4, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-X.; Liu, S.-J.; Tang, X.-L.; Duan, G.-L.; Ni, X.; Zhu, X.-Y.; Liu, Y.-J.; Wang, C.-N. H2S Attenuates LPS-Induced Acute Lung Injury by Reducing Oxidative/Nitrative Stress and Inflammation. Cell. Physiol. Biochem. 2016, 40, 1603–1612. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R.; et al. Hydrogen Sulfide Is an Endogenous Stimulator of Angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.W.; Kraus, J.P. Cystathionine β-Synthase: Structure, Function, Regulation, and Location of Homocystinuria-Causing Mutations. J. Biol. Chem. 2004, 279, 29871–29874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Yang, H.B.; Zhu, Y.Z. Role of Cystathionine γ -Lyase/Hydrogen Sulfide Pathway in Cardiovascular Disease: A Novel Therapeutic Strategy? Antioxid. Redox Signal. 2012, 17, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the Transsulfuration Pathway: Modulating the Transsulfuration Pathway in Disease. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabil, O.; Vitvitsky, V.; Xie, P.; Banerjee, R. The Quantitative Significance of the Transsulfuration Enzymes for H2S Production in Murine Tissues. Antioxid. Redox Signal. 2011, 15, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, Y.; Kimura, Y.; Kimura, H.; Niki, I. l-Cysteine Inhibits Insulin Release from the Pancreatic β-Cell. Diabetes 2006, 55, 1391–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.; Vatish, M.; Heptinstall, J.; Wang, R.; Carson, R.J. The Endogenous Production of Hydrogen Sulphide in Intrauterine Tissues. Reprod. Biol. Endocrinol. 2009, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosoki, R.; Matsuki, N.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Smooth Muscle Relaxant in Synergy with Nitric Oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Ito, T.; Kitamura, H.; Nishino, T. Tissue and Subcellular Distribution of Mercaptopyruvate Sulfurtransferase in the Rat: Confocal Laser Fluorescence and Immunoelectron Microscopic Studies Combined with Biochemical Analysis. Histochem. Cell Biol. 1998, 110, 243–250. [Google Scholar] [CrossRef]
- Olson, K.R.; DeLeon, E.R.; Gao, Y.; Hurley, K.; Sadauskas, V.; Batz, C.; Stoy, G.F. Thiosulfate: A Readily Accessible Source of Hydrogen Sulfide in Oxygen Sensing. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 305, R592–R603. [Google Scholar] [CrossRef] [Green Version]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen Sulfide Chemical Biology: Pathophysiological Roles and Detection. Nitric Oxide 2013, 35, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Zuhra, K.; Augsburger, F.; Majtan, T.; Szabo, C. Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition. Biomolecules 2020, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.R.; Melideo, S.L.; Jorns, M.S. Human Sulfide:Quinone Oxidoreductase Catalyzes the First Step in Hydrogen Sulfide Metabolism and Produces a Sulfane Sulfur Metabolite. Biochemistry 2012, 51, 6804–6815. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.R.; Melideo, S.L.; Jorns, M.S. Role of Human Sulfide. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 554, pp. 255–270. ISBN 978-0-12-801512-4. [Google Scholar]
- Weisiger, R.A.; Pinkus, L.M.; Jakoby, W.B. Thiol S-Methyltransferase: Suggested Role in Detoxication of Intestinal Hydrogen Sulfide. Biochem. Pharmacol. 1980, 29, 2885–2887. [Google Scholar] [CrossRef]
- Cerda-Colón, J.F.; Silfa, E.; López-Garriga, J. Unusual Rocking Freedom of the Heme in the Hydrogen Sulfide-Binding Hemoglobin from Lucina pectinata. J. Am. Chem. Soc. 1998, 120, 9312–9317. [Google Scholar] [CrossRef]
- Insko, M.A.; Deckwerth, T.L.; Hill, P.; Toombs, C.F.; Szabo, C. Detection of Exhaled Hydrogen Sulphide Gas in Rats Exposed to Intravenous Sodium Sulphide. Br. J. Pharmacol. 2009, 157, 944–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishigami, M.; Hiraki, K.; Umemura, K.; Ogasawara, Y.; Ishii, K.; Kimura, H. A Source of Hydrogen Sulfide and a Mechanism of Its Release in the Brain. Antioxid. Redox Signal. 2009, 11, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Bartholomew, T.C.; Powell, G.M.; Dodgson, K.S.; Curtis, C.G. Oxidation of Sodium Sulphide by Rat Liver, Lungs and Kidney. Biochem. Pharmacol. 1980, 29, 2431–2437. [Google Scholar] [CrossRef]
- Módis, K.; Ju, Y.; Ahmad, A.; Untereiner, A.A.; Altaany, Z.; Wu, L.; Szabo, C.; Wang, R. S-Sulfhydration of ATP Synthase by Hydrogen Sulfide Stimulates Mitochondrial Bioenergetics. Pharmacol. Res. 2016, 113, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Szabo, C. Hydrogen Sulfide, an Endogenous Stimulator of Mitochondrial Function in Cancer Cells. Cells 2021, 10, 220. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen Sulfide (H2S) and Polysulfide (H2Sn) Signaling: The First 25 Years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R. Persulfidation (S-Sulfhydration) and H2S. In Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Moore, P.K., Whiteman, M., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2015; Volume 230, pp. 29–59. ISBN 978-3-319-18143-1. [Google Scholar]
- Kabil, O.; Motl, N.; Banerjee, R. H2S and Its Role in Redox Signaling. Biochim. Biophys. Acta BBA Proteins Proteomics 2014, 1844, 1355–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- di Masi, A.; Ascenzi, P. H2S: A “Double Face” Molecule in Health and Disease. BioFactors 2013, 39, 186–196. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S: A Novel Gasotransmitter That Signals by Sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen Sulfide Mediates the Vasoactivity of Garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [Green Version]
- Benedetti, F.; Curreli, S.; Krishnan, S.; Davinelli, S.; Cocchi, F.; Scapagnini, G.; Gallo, R.C.; Zella, D. Anti-Inflammatory Effects of H2S during Acute Bacterial Infection: A Review. J. Transl. Med. 2017, 15, 100. [Google Scholar] [CrossRef]
- Gemici, B.; Elsheikh, W.; Feitosa, K.B.; Costa, S.K.P.; Muscara, M.N.; Wallace, J.L. H2S-Releasing Drugs: Anti-Inflammatory, Cytoprotective and Chemopreventative Potential. Nitric Oxide 2015, 46, 25–31. [Google Scholar] [CrossRef]
- Gemici, B.; Wallace, J.L. Anti-Inflammatory and Cytoprotective Properties of Hydrogen Sulfide. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 555, pp. 169–193. ISBN 978-0-12-801511-7. [Google Scholar]
- Paganelli, F.; Mottola, G.; Fromonot, J.; Marlinge, M.; Deharo, P.; Guieu, R.; Ruf, J. Hyperhomocysteinemia and Cardiovascular Disease: Is the Adenosinergic System the Missing Link? Int. J. Mol. Sci. 2021, 22, 1690. [Google Scholar] [CrossRef]
- Fromonot, J.; Deharo, P.; Bruzzese, L.; Cuisset, T.; Quilici, J.; Bonatti, S.; Fenouillet, E.; Mottola, G.; Ruf, J.; Guieu, R. Adenosine Plasma Level Correlates with Homocysteine and Uric Acid Concentrations in Patients with Coronary Artery Disease. Can. J. Physiol. Pharmacol. 2016, 94, 272–277. [Google Scholar] [CrossRef]
- Szabó, G.; Veres, G.; Radovits, T.; Gerő, D.; Módis, K.; Miesel-Gröschel, C.; Horkay, F.; Karck, M.; Szabó, C. Cardioprotective Effects of Hydrogen Sulfide. Nitric Oxide 2011, 25, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Shen, Z.; Luo, S.; Guo, W.; Zhu, Y.Z. The Cardioprotective Effects of Hydrogen Sulfide in Heart Diseases: From Molecular Mechanisms to Therapeutic Potential. Oxid. Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, F.; Yin, J.; Wu, S.; Zhou, X. Protective Mechanisms of Hydrogen Sulfide in Myocardial Ischemia. J. Cell. Physiol. 2020, 235, 9059–9070. [Google Scholar] [CrossRef]
- Wen, Y.-D.; Wang, H.; Zhu, Y.-Z. The Drug Developments of Hydrogen Sulfide on Cardiovascular Disease. Oxid. Med. Cell. Longev. 2018, 2018, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Bhushan, S.; Yang, C.; Otsuka, H.; Stein, J.D.; Pacheco, A.; Peng, B.; Devarie-Baez, N.O.; Aguilar, H.C.; Lefer, D.J.; et al. Controllable Hydrogen Sulfide Donors and Their Activity against Myocardial Ischemia-Reperfusion Injury. ACS Chem. Biol. 2013, 8, 1283–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen Sulfide Mediates Cardioprotection Through Nrf2 Signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodha, N.R.; Clements, R.T.; Feng, J.; Liu, Y.; Bianchi, C.; Horvath, E.M.; Szabo, C.; Sellke, F.W. The Effects of Therapeutic Sulfide on Myocardial Apoptosis in Response to Ischemia–Reperfusion Injury. Eur. J. Cardiothorac. Surg. 2008, 33, 906–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osipov, R.M.; Robich, M.P.; Feng, J.; Liu, Y.; Clements, R.T.; Glazer, H.P.; Sodha, N.R.; Szabo, C.; Bianchi, C.; Sellke, F.W. Effect of Hydrogen Sulfide in a Porcine Model of Myocardial Ischemia-Reperfusion: Comparison of Different Administration Regimens and Characterization of the Cellular Mechanisms of Protection. J. Cardiovasc. Pharmacol. 2009, 54, 287–297. [Google Scholar] [CrossRef]
- Ji, Y.; Pang, Q.; Xu, G.; Wang, L.; Wang, J.; Zeng, Y. Exogenous Hydrogen Sulfide Postconditioning Protects Isolated Rat Hearts against Ischemia-Reperfusion Injury. Eur. J. Pharmacol. 2008, 587, 1–7. [Google Scholar] [CrossRef]
- Ahmed, N. Myocardial Ischemia. In Pathophysiology of Ischemia Reperfusion Injury and Use of Fingolimod in Cardioprotection; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–56. ISBN 978-0-12-818023-5. [Google Scholar]
- Crossman, D.C. The Pathophysiology of Myocardial Ischaemia. Heart 2004, 90, 576–580. [Google Scholar] [CrossRef] [Green Version]
- Heusch, G. Myocardial Ischaemia–Reperfusion Injury and Cardioprotection in Perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Parlakpinar, H.; Orum, M.; Sagir, M. Pathophysiology of Myocardial Ischemia Reperfusion Injury: A Review. Med. Sci. Int. Med. J. 2013, 2, 935. [Google Scholar] [CrossRef]
- Pei, H.; Yang, Y.; Zhao, H.; Li, X.; Yang, D.; Li, D.; Yang, Y. The Role of Mitochondrial Functional Proteins in ROS Production in Ischemic Heart Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Andreadou, I.; Schulz, R.; Papapetropoulos, A.; Turan, B.; Ytrehus, K.; Ferdinandy, P.; Daiber, A.; Di Lisa, F. The Role of Mitochondrial Reactive Oxygen Species, NO and H2S in Ischemia/Reperfusion Injury and Cardioprotection. J. Cell. Mol. Med. 2020, 24, 6510–6522. [Google Scholar] [CrossRef] [PubMed]
- Johansen, D.; Ytrehus, K.; Baxter, G.F. Exogenous Hydrogen Sulfide (H2S) Protects against Regional Myocardial Ischemia–Reperfusion Injury: Evidence for a Role of KATP Channels. Basic Res. Cardiol. 2006, 101, 53–60. [Google Scholar] [CrossRef]
- Sivarajah, A.; McDonald, M.C.; Thiemermann, C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock 2006, 26, 154–161. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen Sulfide Attenuates Myocardial Ischemia-Reperfusion Injury by Preservation of Mitochondrial Function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef] [Green Version]
- Moore, P.K.; Whiteman, M. (Eds.) Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide. In Handbook of Experimental Pharmacology, 1st ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-18144-8. [Google Scholar]
- Bora, P.; Chauhan, P.; Pardeshi, K.A.; Chakrapani, H. Small Molecule Generators of Biologically Reactive Sulfur Species. RSC Adv. 2018, 8, 27359–27374. [Google Scholar] [CrossRef] [Green Version]
- Hartle, M.D.; Pluth, M.D. A Practical Guide to Working with H2S at the Interface of Chemistry and Biology. Chem. Soc. Rev. 2016, 45, 6108–6117. [Google Scholar] [CrossRef] [Green Version]
- Levinn, C.M.; Cerda, M.M.; Pluth, M.D. Activatable Small-Molecule Hydrogen Sulfide Donors. Antioxid. Redox Signal. 2020, 32, 96–109. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A Review of Hydrogen Sulfide (H2S) Donors: Chemistry and Potential Therapeutic Applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hamsath, A.; Neill, D.L.; Wang, Y.; Yang, C.; Xian, M. Strategies for the Design of Donors and Precursors of Reactive Sulfur Species. Chem.-Eur. J. 2019, 25, 4005–4016. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.-H.; Moore, P.K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide–Releasing Molecule (GYY4137): New Insights Into the Biology of Hydrogen Sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, P.; Dymock, B.W.; Moore, P.K. GYY4137, a Novel Water-Soluble, H2S-Releasing Molecule. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 554, pp. 143–167. ISBN 978-0-12-801512-4. [Google Scholar]
- Alexander, B.E.; Coles, S.J.; Fox, B.C.; Khan, T.F.; Maliszewski, J.; Perry, A.; Pitak, M.B.; Whiteman, M.; Wood, M.E. Investigating the Generation of Hydrogen Sulfide from the Phosphonamidodithioate Slow-Release Donor GYY4137. MedChemComm 2015, 6, 1649–1655. [Google Scholar] [CrossRef]
- GYY4137 Protects against Myocardial Ischemia and Reperfusion Injury by Attenuating Oxidative Stress and Apoptosis in Rats. J. Biomed. Res. 2015, 29, 203–213. [CrossRef] [Green Version]
- Qiu, Y.; Wu, Y.; Meng, M.; Luo, M.; Zhao, H.; Sun, H.; Gao, S. GYY4137 Protects against Myocardial Ischemia/Reperfusion Injury via Activation of the PHLPP-1/Akt/Nrf2 Signaling Pathway in Diabetic Mice. J. Surg. Res. 2018, 225, 29–39. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Whiteman, M.; Wood, M.E.; Torregrossa, R.; Baxter, G.F. Pharmacological Postconditioning against Myocardial Infarction with a Slow-Releasing Hydrogen Sulfide Donor, GYY4137. Pharmacol. Res. 2016, 111, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Robinson, H.; Wray, S. A New Slow Releasing, H2S Generating Compound, GYY4137 Relaxes Spontaneous and Oxytocin-Stimulated Contractions of Human and Rat Pregnant Myometrium. PLoS ONE 2012, 7, e46278. [Google Scholar] [CrossRef] [Green Version]
- Landis, P.S. The Chemistry of 1,2-Dithiole-3-Thiones. Chem. Rev. 1965, 65, 237–245. [Google Scholar] [CrossRef]
- Hamada, T.; Nakane, T.; Kimura, T.; Arisawa, K.; Yoneda, K.; Yamamoto, T.; Osaki, T. Treatment of Xerostomia with the Bile Secretion-Stimulating Drug Anethole Trithione: A Clinical Trial. Am. J. Med. Sci. 1999, 318, 146–151. [Google Scholar] [CrossRef]
- Perrino, E.; Cappelletti, G.; Tazzari, V.; Giavini, E.; Soldato, P.D.; Sparatore, A. New Sulfurated Derivatives of Valproic Acid with Enhanced Histone Deacetylase Inhibitory Activity. Bioorg. Med. Chem. Lett. 2008, 18, 1893–1897. [Google Scholar] [CrossRef]
- Tazzari, V.; Cappelletti, G.; Casagrande, M.; Perrino, E.; Renzi, L.; Del Soldato, P.; Sparatore, A. New Aryldithiolethione Derivatives as Potent Histone Deacetylase Inhibitors. Bioorg. Med. Chem. 2010, 18, 4187–4194. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Traber, K.E.; Packer, L. Inhibition of NF-ΚB Activation in Human T-Cell Lines by Anetholdithiolthione. Biochem. Biophys. Res. Commun. 1996, 218, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, S.D.; Jarrott, B.; Williams, S.J. Synthesis and Preliminary Pharmacological Evaluation of Aryl Dithiolethiones with Cyclooxygenase-2-Selective Inhibitory Activity and Hydrogen Sulfide-Releasing Properties. Aust. J. Chem. 2010, 63, 946. [Google Scholar] [CrossRef] [Green Version]
- Qandil, A. Prodrugs of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), More Than Meets the Eye: A Critical Review. Int. J. Mol. Sci. 2012, 13, 17244–17274. [Google Scholar] [CrossRef] [Green Version]
- Caliendo, G.; Cirino, G.; Santagada, V.; Wallace, J.L. Synthesis and Biological Effects of Hydrogen Sulfide (H2S): Development of H2S-Releasing Drugs as Pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286. [Google Scholar] [CrossRef]
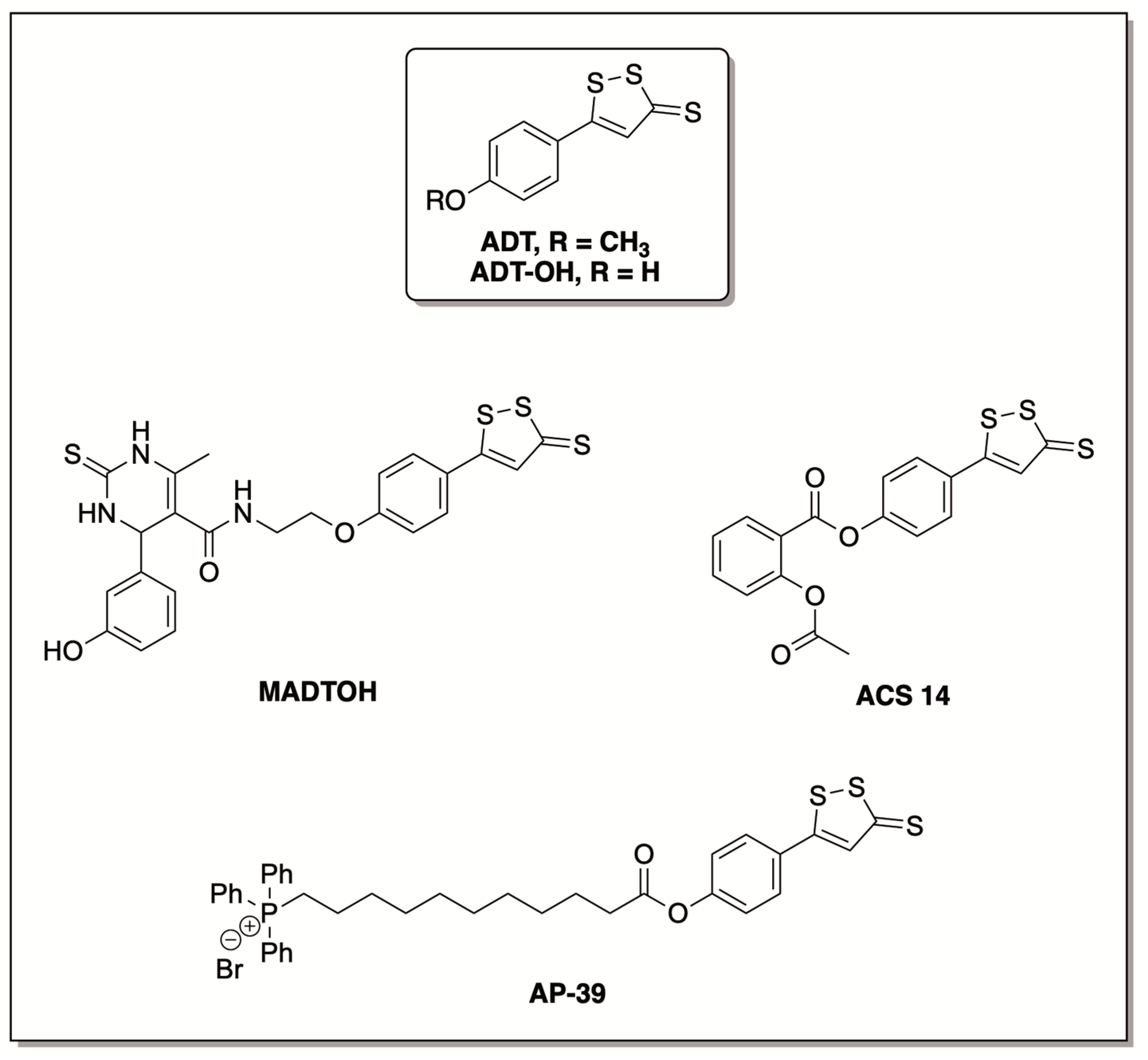
- Braga, T.C.; de Jesus, I.C.G.; Soares, K.V.; Guatimosim, S.; da Silva Neto, L.; da-Silva, C.J.; Modolo, L.V.; Menezes Filho, J.E.R.; Rhana, P.; Cruz, J.S.; et al. A Novel H2S Releasing-Monastrol Hybrid (MADTOH) Inhibits L-Type Calcium Channels. New J. Chem. 2021, 45, 671–678. [Google Scholar] [CrossRef]
- Majid, P.A.; De Jong, J. Acute Hemodynamic Effects of Nifedipine in Patients with Ischemic Heart Disease. Circulation 1982, 65, 1114–1118. [Google Scholar] [CrossRef] [Green Version]
- Sparatore, A.; Perrino, E.; Tazzari, V.; Giustarini, D.; Rossi, R.; Rossoni, G.; Erdman, K.; Schröder, H.; Soldato, P.D. Pharmacological Profile of a Novel H2S-Releasing Aspirin. Free Radic. Biol. Med. 2009, 46, 586–592. [Google Scholar] [CrossRef]
- Huang, Q.; Sparatore, A.; Del Soldato, P.; Wu, L.; Desai, K. Hydrogen Sulfide Releasing Aspirin, ACS14, Attenuates High Glucose-Induced Increased Methylglyoxal and Oxidative Stress in Cultured Vascular Smooth Muscle Cells. PLoS ONE 2014, 9, e97315. [Google Scholar] [CrossRef]
- Rossoni, G.; Manfredi, B.; Tazzari, V.; Sparatore, A.; Trivulzio, S.; Del Soldato, P.; Berti, F. Activity of a New Hydrogen Sulfide-Releasing Aspirin (ACS14) on Pathological Cardiovascular Alterations Induced by Glutathione Depletion in Rats. Eur. J. Pharmacol. 2010, 648, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Gerő, D.; Torregrossa, R.; Perry, A.; Waters, A.; Le-Trionnaire, S.; Whatmore, J.L.; Wood, M.; Whiteman, M. The Novel Mitochondria-Targeted Hydrogen Sulfide (H2S) Donors AP123 and AP39 Protect against Hyperglycemic Injury in Microvascular Endothelial Cells in Vitro. Pharmacol. Res. 2016, 113, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a Novel Mitochondria-Targeted Hydrogen Sulfide Donor, Stimulates Cellular Bioenergetics, Exerts Cytoprotective Effects and Protects against the Loss of Mitochondrial DNA Integrity in Oxidatively Stressed Endothelial Cells in Vitro. Nitric Oxide 2014, 41, 120–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, K.; Marutani, E.; Hirai, S.; Wood, M.E.; Whiteman, M.; Ichinose, F. Mitochondria-Targeted Hydrogen Sulfide Donor AP39 Improves Neurological Outcomes after Cardiac Arrest in Mice. Nitric Oxide 2015, 49, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Olah, G.; Szczesny, B.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a Mitochondrially Targeted Hydrogen Sulfide Donor, Exerts Protective Effects in Renal Epithelial Cells Subjected to Oxidative Stress in Vitro and in Acute Renal Injury in Vivo. Shock 2016, 45, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Karwi, Q.G.; Bornbaum, J.; Boengler, K.; Torregrossa, R.; Whiteman, M.; Wood, M.E.; Schulz, R.; Baxter, G.F. AP39, a Mitochondria-Targeting Hydrogen Sulfide (H2S) Donor, Protects against Myocardial Reperfusion Injury Independently of Salvage Kinase Signalling: Cardioprotection with AP39. Br. J. Pharmacol. 2017, 174, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Li, Z.; Organ, C.L.; Park, C.-M.; Yang, C.; Pacheco, A.; Wang, D.; Lefer, D.J.; Xian, M. PH-Controlled Hydrogen Sulfide Release for Myocardial Ischemia-Reperfusion Injury. J. Am. Chem. Soc. 2016, 138, 6336–6339. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Wang, Y.; Li, J.; Bai, Z.; Zhao, Q.; He, D.; Wang, Z.; Zhang, J.; Chen, Y. Toxicity, Bioactivity, Release of H2S in Vivo and Pharmaco-Kinetics of H2S-Donors with Thiophosphamide Structure. Eur. J. Med. Chem. 2019, 176, 456–475. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Organ, C.; Li, Z.; Bhushan, S.; Otsuka, H.; Pacheco, A.; Kang, J.; Aguilar, H.C.; Lefer, D.J.; et al. Design, Synthesis, and Cardioprotective Effects of N -Mercapto-Based Hydrogen Sulfide Donors. J. Med. Chem. 2015, 58, 7501–7511. [Google Scholar] [CrossRef]
- Yao, H.; Luo, S.; Liu, J.; Xie, S.; Liu, Y.; Xu, J.; Zhu, Z.; Xu, S. Controllable Thioester-Based Hydrogen Sulfide Slow-Releasing Donors as Cardioprotective Agents. Chem. Commun. 2019, 55, 6193–6196. [Google Scholar] [CrossRef]
- Khodade, V.S.; Pharoah, B.M.; Paolocci, N.; Toscano, J.P. Alkylamine-Substituted Perthiocarbamates: Dual Precursors to Hydropersulfide and Carbonyl Sulfide with Cardioprotective Actions. J. Am. Chem. Soc. 2020, 142, 4309–4316. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Marino, A.; Piano, I.; Brancaleone, V.; Tomita, K.; Di Cesare Mannelli, L.; Martelli, A.; Citi, V.; Breschi, M.C.; Levi, R.; et al. The Novel H2S-Donor 4-Carboxyphenyl Isothiocyanate Promotes Cardioprotective Effects against Ischemia/Reperfusion Injury through Activation of MitoK ATP Channels and Reduction of Oxidative Stress. Pharmacol. Res. 2016, 113, 290–299. [Google Scholar] [CrossRef]
- Citi, V.; Corvino, A.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Brogi, S.; Flori, L.; Gorica, E.; et al. Structure-Activity Relationships Study of Isothiocyanates for H2S Releasing Properties: 3-Pyridyl-Isothiocyanate as a New Promising Cardioprotective Agent. J. Adv. Res. 2021, 27, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Suarez, S.I.; Hankins, R.A.; Lukesh, J.C. Intramolecular Thiol- and Selenol-Assisted Delivery of Hydrogen Sulfide. Angew. Chem. Int. Ed. 2022, 61, e202210754. [Google Scholar] [CrossRef] [PubMed]
- Lougiakis, N.; Papapetropoulos, A.; Gikas, E.; Toumpas, S.; Efentakis, P.; Wedmann, R.; Zoga, A.; Zhou, Z.; Iliodromitis, E.K.; Skaltsounis, A.-L.; et al. Synthesis and Pharmacological Evaluation of Novel Adenine–Hydrogen Sulfide Slow Release Hybrids Designed as Multitarget Cardioprotective Agents. J. Med. Chem. 2016, 59, 1776–1790. [Google Scholar] [CrossRef]
- Ried, K.; Frank, O.R.; Stocks, N.P. Aged Garlic Extract Reduces Blood Pressure in Hypertensives: A Dose–Response Trial. Eur. J. Clin. Nutr. 2013, 67, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Jacob, C.; Anwar, A.; Burkholz, T. Perspective on Recent Developments on Sulfur-Containing Agents and Hydrogen Sulfide Signaling. Planta Med. 2008, 74, 1580–1592. [Google Scholar] [CrossRef] [Green Version]
- Kashfi, K.; Olson, K.R. Biology and Therapeutic Potential of Hydrogen Sulfide and Hydrogen Sulfide-Releasing Chimeras. Biochem. Pharmacol. 2013, 85, 689–703. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Hsu, A.; Moore, P.K. Actions and Interactions of Nitric Oxide, Carbon Monoxide and Hydrogen Sulphide in the Cardiovascular System and in Inflammation—A Tale of Three Gases! Pharmacol. Ther. 2009, 123, 386–400. [Google Scholar] [CrossRef]
- Nicholson, C.K.; Calvert, J.W. Hydrogen Sulfide and Ischemia–Reperfusion Injury. Pharmacol. Res. 2010, 62, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Olson, K.R. The Therapeutic Potential of Hydrogen Sulfide: Separating Hype from Hope. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R297–R312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen Sulfide in Biochemistry and Medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levinn, C.M.; Cerda, M.M.; Pluth, M.D. Development and Application of Carbonyl Sulfide-Based Donors for H2S Delivery. Acc. Chem. Res. 2019, 52, 2723–2731. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, X.; Lu, Y.; Liang, D.; Huang, D. Isothiocyanates as H2S Donors Triggered by Cysteine: Reaction Mechanism and Structure and Activity Relationship. Org. Lett. 2019, 21, 5977–5980. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zou, L.; Jin, Q.; Hou, J.; Ge, G.; Yang, L. Human Carboxylesterases: A Comprehensive Review. Acta Pharm. Sin. B 2018, 8, 699–712. [Google Scholar] [CrossRef]
- Chauhan, P.; Bora, P.; Ravikumar, G.; Jos, S.; Chakrapani, H. Esterase Activated Carbonyl Sulfide/Hydrogen Sulfide (H2S) Donors. Org. Lett. 2017, 19, 62–65. [Google Scholar] [CrossRef]
- Fosnacht, K.G.; Cerda, M.M.; Mullen, E.J.; Pigg, H.C.; Pluth, M.D. Esterase-Activated Perthiocarbonate Persulfide Donors Provide Insights into Persulfide Persistence and Stability. ACS Chem. Biol. 2022, 17, 331–339. [Google Scholar] [CrossRef]
- Shyaka, C.; Xian, M.; Park, C.-M. Esterase-Sensitive Trithiane-Based Hydrogen Sulfide Donors. Org. Biomol. Chem. 2019, 17, 9999–10003. [Google Scholar] [CrossRef]
- Steiger, A.K.; Marcatti, M.; Szabo, C.; Szczesny, B.; Pluth, M.D. Inhibition of Mitochondrial Bioenergetics by Esterase-Triggered COS/H2S Donors. ACS Chem. Biol. 2017, 12, 2117–2123. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Zheng, Y.; Yu, B.; Wang, S.; Yang, X.; Wang, B. Esterase-Sensitive Glutathione Persulfide Donor. Org. Lett. 2018, 20, 6364–6367. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; Ji, K.; Pan, Z.; Chittavong, V.; Wang, B. Esterase-Sensitive Prodrugs with Tunable Release Rates and Direct Generation of Hydrogen Sulfide. Angew. Chem. Int. Ed. 2016, 55, 4514–4518. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Yu, B.; Li, Z.; Yuan, Z.; Organ, C.L.; Trivedi, R.K.; Wang, S.; Lefer, D.J.; Wang, B. An Esterase-Sensitive Prodrug Approach for Controllable Delivery of Persulfide Species. Angew. Chem. 2017, 129, 11911–11915. [Google Scholar] [CrossRef]
- Liu, Q.; Ji, G.; Chu, Y.; Hao, T.; Qian, M.; Zhao, Q. Enzyme-Responsive Hybrid Prodrug of Nitric Oxide and Hydrogen Sulfide for Heart Failure Therapy. Chem. Commun. 2022, 58, 7396–7399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pluth, M.D. Hydrogen Sulfide Donors Activated by Reactive Oxygen Species. Angew. Chem. 2016, 128, 14858–14862. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Wang, Y.; Carrazzone, R.J.; Matson, J.B. A Persulfide Donor Responsive to Reactive Oxygen Species: Insights into Reactivity and Therapeutic Potential. Angew. Chem. Int. Ed. 2018, 57, 6324–6328. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Chauhan, P.; Manna, S.; Chakrapani, H. A Vinyl-Boronate Ester-Based Persulfide Donor Controllable by Hydrogen Peroxide, a Reactive Oxygen Species (ROS). Org. Lett. 2018, 20, 7916–7920. [Google Scholar] [CrossRef]
- Hankins, R.A.; Suarez, S.I.; Kalk, M.A.; Green, N.M.; Harty, M.N.; Lukesh, J.C. An Innovative Hydrogen Peroxide-Sensing Scaffold and Insight Towards Its Potential as an ROS-Activated Persulfide Donor. Angew. Chem. Int. Ed. 2020, 59, 22238–22245. [Google Scholar] [CrossRef]
- Zhu, C.; Suarez, S.I.; Lukesh, J.C. Illuminating and Alleviating Cellular Oxidative Stress with an ROS-Activated, H2S-Donating Theranostic. Tetrahedron Lett. 2021, 69, 152944. [Google Scholar] [CrossRef]
- Lippert, A.R.; Van de Bittner, G.C.; Chang, C.J. Boronate Oxidation as a Bioorthogonal Reaction Approach for Studying the Chemistry of Hydrogen Peroxide in Living Systems. Acc. Chem. Res. 2011, 44, 793–804. [Google Scholar] [CrossRef] [Green Version]
- Bezner, B.J.; Ryan, L.S.; Lippert, A.R. Reaction-Based Luminescent Probes for Reactive Sulfur, Oxygen, and Nitrogen Species: Analytical Techniques and Recent Progress. Anal. Chem. 2020, 92, 309–326. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Y.; Liu, X.; Chen, Y.; Duan, S.; Zhu, R.; Liu, Y.; Yin, L. Recent Advances on Reactive Oxygen Species-Responsive Delivery and Diagnosis System. Biomacromolecules 2019, 20, 2441–2463. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, X.; Fang, Y.; Shi, W.; Li, X.; Chen, W.; Xian, M.; Ma, H. Reactive Oxygen Species-Triggered off-on Fluorescence Donor for Imaging Hydrogen Sulfide Delivery in Living Cells. Chem. Sci. 2019, 10, 7690–7694. [Google Scholar] [CrossRef]
- Zhang, N.; Hu, P.; Wang, Y.; Tang, Q.; Zheng, Q.; Wang, Z.; He, Y. A Reactive Oxygen Species (ROS) Activated Hydrogen Sulfide (H2S) Donor with Self-Reporting Fluorescence. ACS Sens. 2020, 5, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Lu, Y.; Shi, L.; Huang, Y.; Zhang, Q.; Tan, J.; Hu, P.; Zhang, J.; Luo, G.; Zhang, N. A ROS-Responsive, Self-Immolative and Self-Reporting Hydrogen Sulfide Donor with Multiple Biological Activities for the Treatment of Myocardial Infarction. Bioact. Mater. 2022, 9, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Buzdar, A.U.; Marcus, C.; Blumenschein, G.R.; Smith, T.L. Early and Delayed Clinical Cardiotoxicity of Doxorubicin. Cancer 1985, 55, 2761–2765. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive Heart Failure in Patients Treated with Doxorubicin: A Retrospective Analysis of Three Trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Banchs, J.; Owusu-Agyemang, P.; Cata, J.P. Chemotherapy-Induced Cardiovascular Toxicity: Beyond Anthracyclines. Minerva Anestesiol. 2014, 80, 586–594. [Google Scholar] [PubMed]
- Lipshultz, S.E.; Franco, V.I.; Cochran, T.R. Cardiotoxicity in Childhood Cancer Survivors: A Problem with Long-Term Consequences in Need of Early Detection and Prevention: Cardiotoxicity in Childhood Cancer Survivors. Pediatr. Blood Cancer 2013, 60, 1395–1396. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on Cancer Treatments and Cardiovascular Toxicity Developed under the Auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- O’Hare, M.; Sharma, A.; Murphy, K.; Mookadam, F.; Lee, H. Cardio-Oncology Part I: Chemotherapy and Cardiovascular Toxicity. Expert Rev. Cardiovasc. Ther. 2015, 13, 511–518. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gewirtz, D. A Critical Evaluation of the Mechanisms of Action Proposed for the Antitumor Effects of the Anthracycline Antibiotics Adriamycin and Daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, J.H.; Davies, K.J. Redox Cycling of Anthracyclines by Cardiac Mitochondria. II. Formation of Superoxide Anion, Hydrogen Peroxide, and Hydroxyl Radical. J. Biol. Chem. 1986, 261, 3068–3074. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. New Advances in Molecular Mechanisms and the Prevention of Adriamycin Toxicity by Antioxidant Nutrients. Food Chem. Toxicol. 2010, 48, 1425–1438. [Google Scholar] [CrossRef]
- Doroshow, J.H.; Locker, G.Y.; Myers, C.E. Enzymatic Defenses of the Mouse Heart Against Reactive Oxygen Metabolites. J. Clin. Investig. 1980, 65, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-Induced Cardiomyopathy: From Molecular Mechanisms to Therapeutic Strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Upadhayay, S.; Sharma, N.; Mantha, A.K.; Dhiman, M. Anti-Cancer Drug Doxorubicin Induced Cardiotoxicity: Understanding The Mechanisms Involved In Ros Generation Resulting In Mitochondrial Dysfunction. Rasayan J. Chem. 2020, 13, 1042–1053. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-Induced Cardiotoxicity: An Update on the Molecular Mechanism and Novel Therapeutic Strategies for Effective Management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Du, S.; Huang, Y.; Jin, H.; Wang, T. Protective Mechanism of Hydrogen Sulfide against Chemotherapy-Induced Cardiotoxicity. Front. Pharmacol. 2018, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.-W.; Liang, C.; Jin, H.-F.; Tang, X.-Y.; Han, W.; Chai, L.-J.; Zhang, C.-Y.; Geng, B.; Tang, C.-S.; Du, J.-B. Hydrogen Sulfide Regulates Cardiac Function and Structure in Adriamycin-Induced Cardiomyopathy. Circ. J. 2009, 73, 741–749. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Lin, J.; Xu, W.; Shen, N.; Mo, L.; Zhang, C.; Feng, J. Hydrogen Sulfide Attenuates Doxorubicin-Induced Cardiotoxicity by Inhibition of the P38 MAPK Pathway in H9c2 Cells. Int. J. Mol. Med. 2013, 31, 644–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-Y.; Yang, C.-T.; Zheng, D.-D.; Mo, L.-Q.; Lan, A.-P.; Yang, Z.-L.; Hu, F.; Chen, P.-X.; Liao, X.-X.; Feng, J.-Q. Hydrogen Sulfide Protects H9c2 Cells against Doxorubicin-Induced Cardiotoxicity through Inhibition of Endoplasmic Reticulum Stress. Mol. Cell. Biochem. 2012, 363, 419–426. [Google Scholar] [CrossRef]
- Liu, M.-H.; Lin, X.-L.; Zhang, Y.; He, J.; Tan, T.-P.; Wu, S.-J.; Liu, J.; Tian, W.; Chen, L.; Yu, S.; et al. Hydrogen Sulfide Attenuates Doxorubicin-Induced Cardiotoxicity by Inhibiting Reactive Oxygen Species-Activated Extracellular Signal-Regulated Kinase 1/2 in H9c2 Cardiac Myocytes. Mol. Med. Rep. 2015, 12, 6841–6848. [Google Scholar] [CrossRef] [Green Version]
- Waz, S.; Heeba, G.H.; Hassanin, S.O.; Abdel-latif, R.G. Nephroprotective Effect of Exogenous Hydrogen Sulfide Donor against Cyclophosphamide-Induced Toxicity Is Mediated by Nrf2/HO-1/NF-ΚB Signaling Pathway. Life Sci. 2021, 264, 118630. [Google Scholar] [CrossRef]
- Azarbarz, N.; Shafiei Seifabadi, Z.; Moaiedi, M.Z.; Mansouri, E. Assessment of the Effect of Sodium Hydrogen Sulfide (Hydrogen Sulfide Donor) on Cisplatin-Induced Testicular Toxicity in Rats. Environ. Sci. Pollut. Res. 2020, 27, 8119–8128. [Google Scholar] [CrossRef]
- Chegaev, K.; Rolando, B.; Cortese, D.; Gazzano, E.; Buondonno, I.; Lazzarato, L.; Fanelli, M.; Hattinger, C.M.; Serra, M.; Riganti, C.; et al. H2S-Donating Doxorubicins May Overcome Cardiotoxicity and Multidrug Resistance. J. Med. Chem. 2016, 59, 4881–4889. [Google Scholar] [CrossRef]
- Persidis, A. Cancer Multidrug Resistance. Nat. Biotechnol. 1999, 17, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Luceri, C.; De Angioletti, M.; Chegaev, K.; D’Ambrosio, M.; Riganti, C.; Gazzano, E.; Saponara, S.; Longini, M.; Luceri, F.; et al. New NO- and H2S-Releasing Doxorubicins as Targeted Therapy against Chemoresistance in Castration-Resistant Prostate Cancer: In Vitro and in Vivo Evaluations. Invest. New Drugs 2018, 36, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Frosini, M.; Chiaino, E.; Fusi, F.; Gamberucci, A.; Gorelli, B.; Chegaev, K.; Riganti, C.; Saponara, S. Sdox, a H2S Releasing Anthracycline, with a Safer Profile than Doxorubicin toward Vasculature. Vascul. Pharmacol. 2022, 143, 106969. [Google Scholar] [CrossRef]
- Gazzano, E.; Buondonno, I.; Marengo, A.; Rolando, B.; Chegaev, K.; Kopecka, J.; Saponara, S.; Sorge, M.; Hattinger, C.M.; Gasco, A.; et al. Hyaluronated Liposomes Containing H2S-Releasing Doxorubicin Are Effective against P-Glycoprotein-Positive/Doxorubicin-Resistant Osteosarcoma Cells and Xenografts. Cancer Lett. 2019, 456, 29–39. [Google Scholar] [CrossRef]
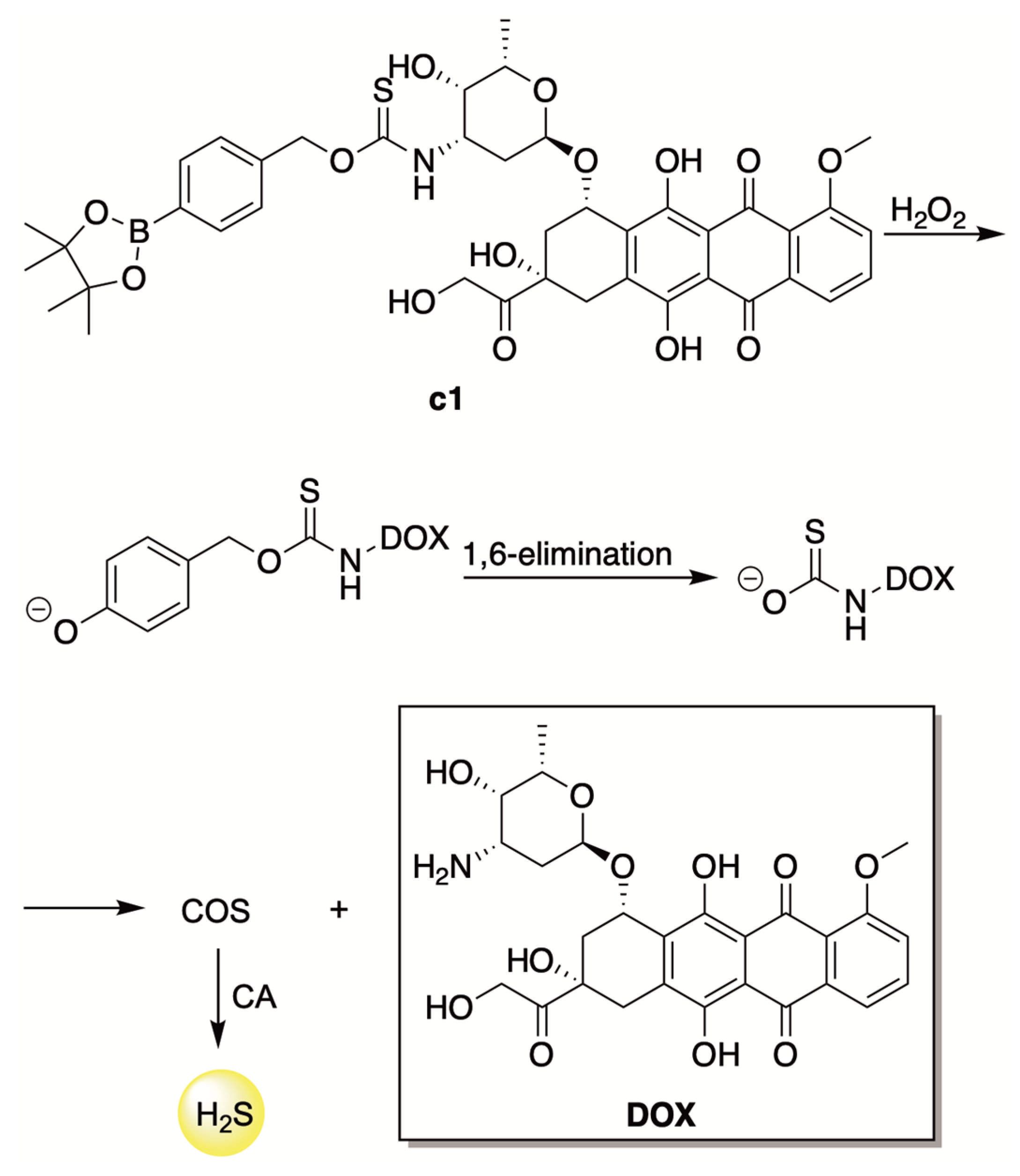
- Hu, Q.; Yammani, R.D.; Brown-Harding, H.; Soto-Pantoja, D.R.; Poole, L.B.; Lukesh, J.C. Mitigation of Doxorubicin-Induced Cardiotoxicity with an H2O2-Activated, H2S-Donating Hybrid Prodrug. Redox Biol. 2022, 53, 102338. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xie, S.; Ma, L.; Chen, Y.; Lu, W. 10-Boronic Acid Substituted Camptothecin as Prodrug of SN-38. Eur. J. Med. Chem. 2016, 116, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Balakrishnan, K.; Gandhi, V.; Peng, X. Hydrogen Peroxide Inducible DNA Cross-Linking Agents: Targeted Anticancer Prodrugs. J. Am. Chem. Soc. 2011, 133, 19278–19281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, Y.; Obianom, O.N.; Kuser, M.; Li, Y.; Shu, Y.; Xue, F. Enhanced Tumor Selectivity of 5-Fluorouracil Using a Reactive Oxygen Species-Activated Prodrug Approach. ACS Med. Chem. Lett. 2019, 10, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Skarbek, C.; Serra, S.; Maslah, H.; Rascol, E.; Labruère, R. Arylboronate Prodrugs of Doxorubicin as Promising Chemotherapy for Pancreatic Cancer. Bioorganic Chem. 2019, 91, 103158. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Azami, N.; Hamzehlou, S.; Farahani, M.V.; Hushmandi, K.; Ashrafizadeh, M.; et al. Nrf2 Signaling Pathway in Chemoprotection and Doxorubicin Resistance: Potential Application in Drug Discovery. Antioxidants 2021, 10, 349. [Google Scholar] [CrossRef]
- Nordgren, K.K.S.; Wallace, K.B. Disruption of the Keap1/Nrf2-Antioxidant Response System After Chronic Doxorubicin Exposure In Vivo. Cardiovasc. Toxicol. 2020, 20, 557–570. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Rajendran, P.; Veeraraghavan, V.P.; Hanieh, H. Cardiac Protective Effect of Kirenol against Doxorubicin-Induced Cardiac Hypertrophy in H9c2 Cells through Nrf2 Signaling via PI3K/AKT Pathways. Int. J. Mol. Sci. 2021, 22, 3269. [Google Scholar] [CrossRef]
- Gu, J.; Huang, H.; Liu, C.; Jiang, B.; Li, M.; Liu, L.; Zhang, S. Pinocembrin Inhibited Cardiomyocyte Pyroptosis against Doxorubicin-Induced Cardiac Dysfunction via Regulating Nrf2/Sirt3 Signaling Pathway. Int. Immunopharmacol. 2021, 95, 107533. [Google Scholar] [CrossRef]
- Pan, T.-T.; Feng, Z.-N.; Lee, S.W.; Moore, P.K.; Bian, J.-S. Endogenous Hydrogen Sulfide Contributes to the Cardioprotection by Metabolic Inhibition Preconditioning in the Rat Ventricular Myocytes. J. Mol. Cell. Cardiol. 2006, 40, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Mazza, R.; Pasqua, T.; Cerra, M.C.; Angelone, T.; Gattuso, A. Akt/ENOS Signaling and PLN S-Sulfhydration Are Involved in H2S-Dependent Cardiac Effects in Frog and Rat. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 305, R443–R451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Chen, X.; Pan, T.-T.; Neo, K.L.; Lee, S.W.; Khin, E.S.W.; Moore, P.K.; Bian, J.-S. Cardioprotection Induced by Hydrogen Sulfide Preconditioning Involves Activation of ERK and PI3K/Akt Pathways. Pflüg. Arch.—Eur. J. Physiol. 2007, 455, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-H.; Zhang, Y.; He, J.; Tan, T.-P.; Wu, S.-J.; Guo, D.-M.; He, H.; Peng, J.; Tang, Z.-H.; Jiang, Z.-S. Hydrogen Sulfide Protects H9c2 Cardiac Cells against Doxorubicin-Induced Cytotoxicity through the PI3K/Akt/FoxO3a Pathway. Int. J. Mol. Med. 2016, 37, 1661–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, R.; Wu, K.; Chen, J.; Mo, L.; Hua, X.; Zheng, D.; Chen, P.; Chen, G.; Xu, W.; Feng, J. Exogenous Hydrogen Sulfide Protects against Doxorubicin-Induced Inflammation and Cytotoxicity by Inhibiting P38MAPK/NFκB Pathway in H9c2 Cardiac Cells. Cell. Physiol. Biochem. 2013, 32, 1668–1680. [Google Scholar] [CrossRef]
- Xu, W.; Chen, J.; Lin, J.; Liu, D.; Mo, L.; Pan, W.; Feng, J.; Wu, W.; Zheng, D. Exogenous H2S Protects H9c2 Cardiac Cells against High Glucose-Induced Injury and Inflammation by Inhibiting the Activation of the NF-ΚB and IL-1β Pathways. Int. J. Mol. Med. 2015, 35, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Yarmohammadi, F.; Hayes, A.W.; Karimi, G. The Cardioprotective Effects of Hydrogen Sulfide by Targeting Endoplasmic Reticulum Stress and the Nrf2 Signaling Pathway: A Review. BioFactors 2021, 47, 701–712. [Google Scholar] [CrossRef]
- Wu, D.; Gu, Y.; Zhu, D. Cardioprotective Effects of Hydrogen Sulfide in Attenuating Myocardial Ischemia-reperfusion Injury (Review). Mol. Med. Rep. 2021, 24, 875. [Google Scholar] [CrossRef]

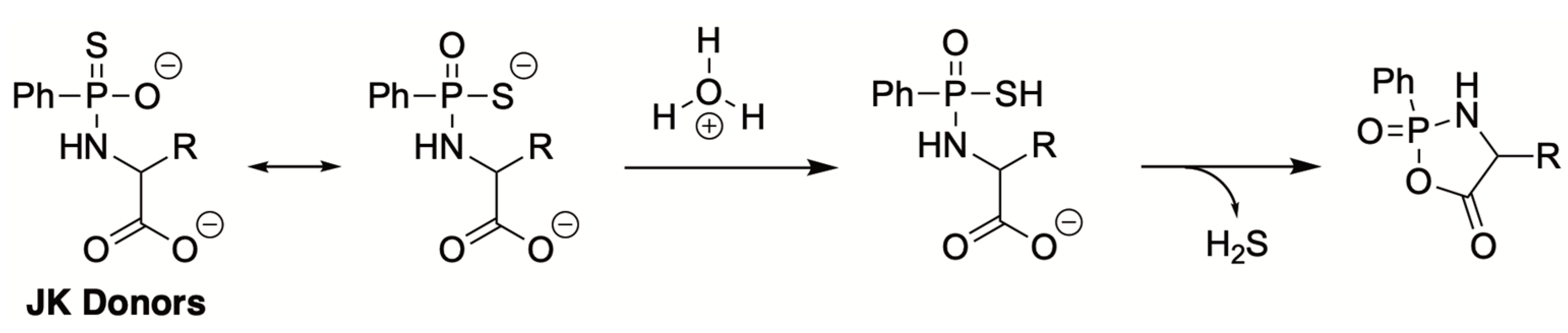


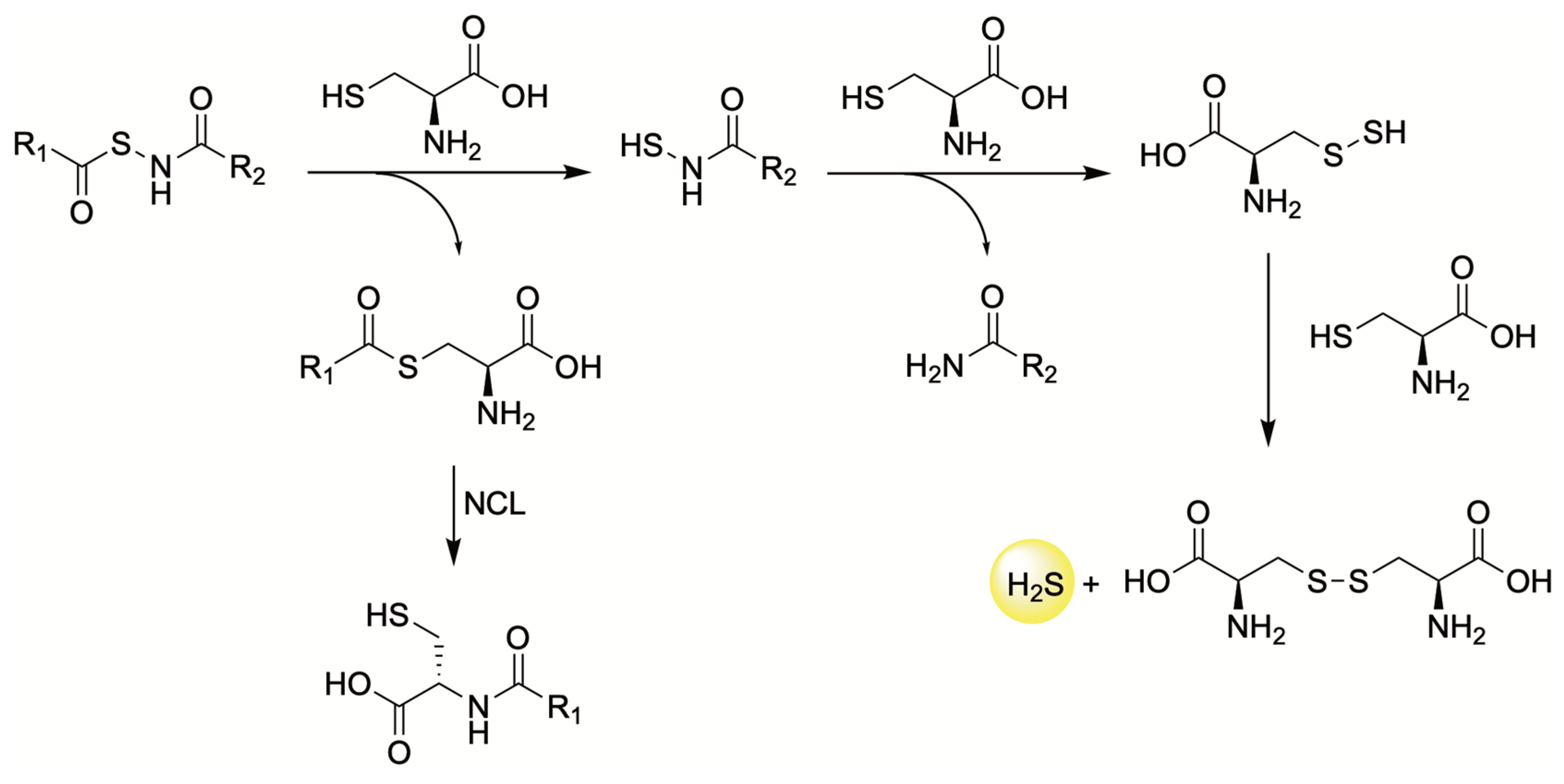
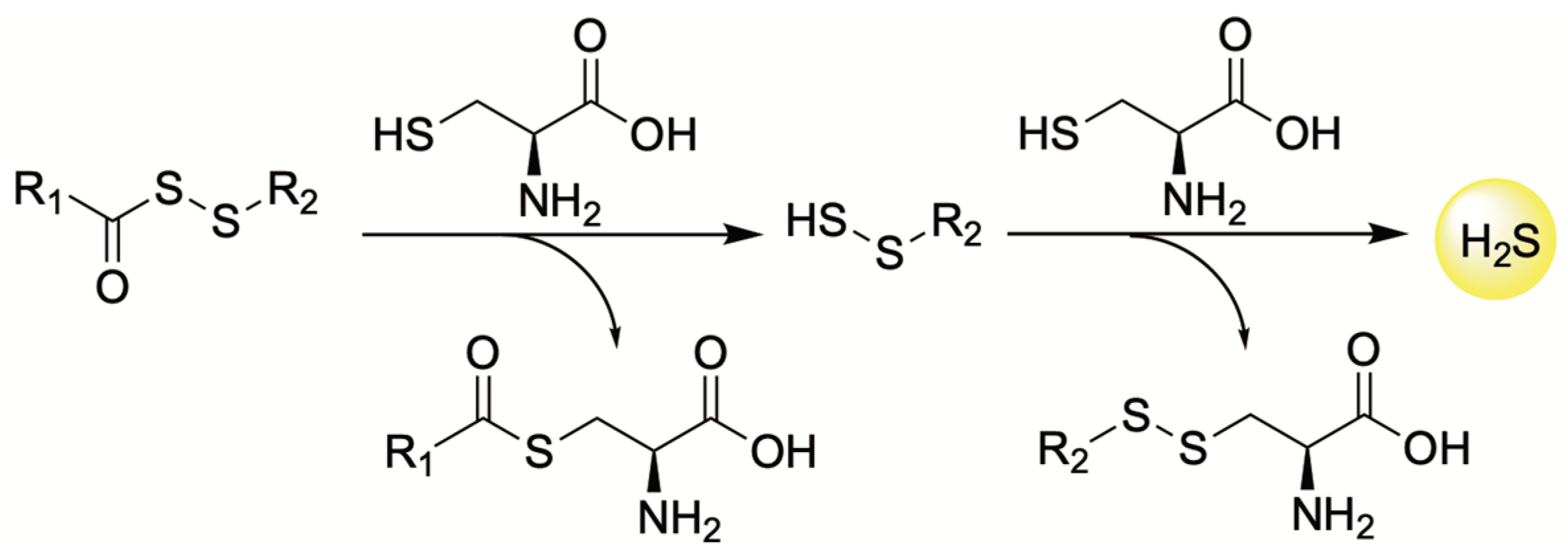
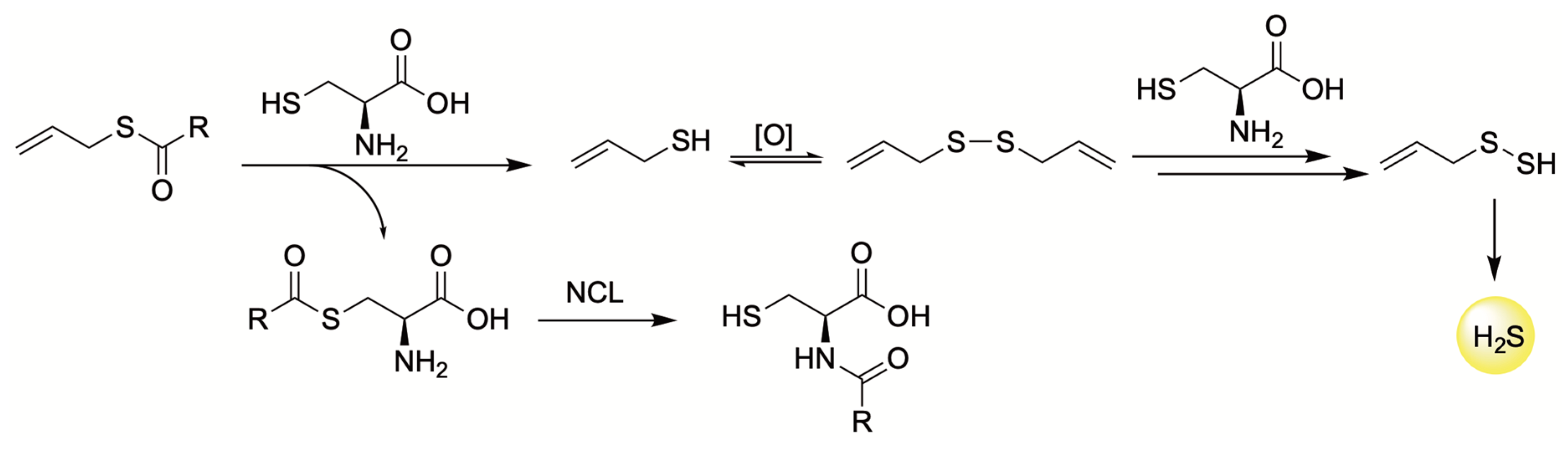

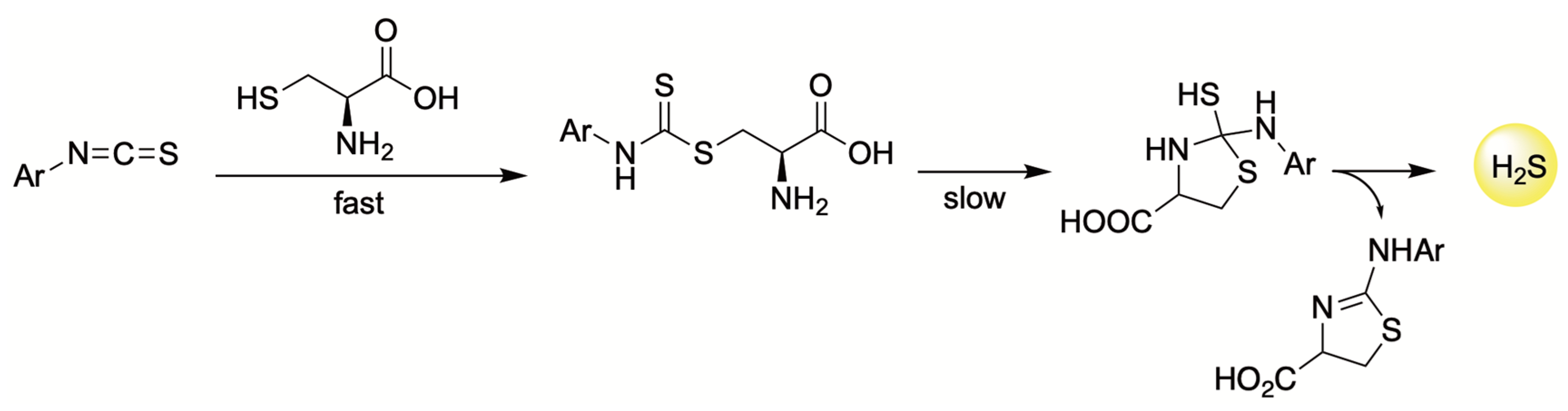
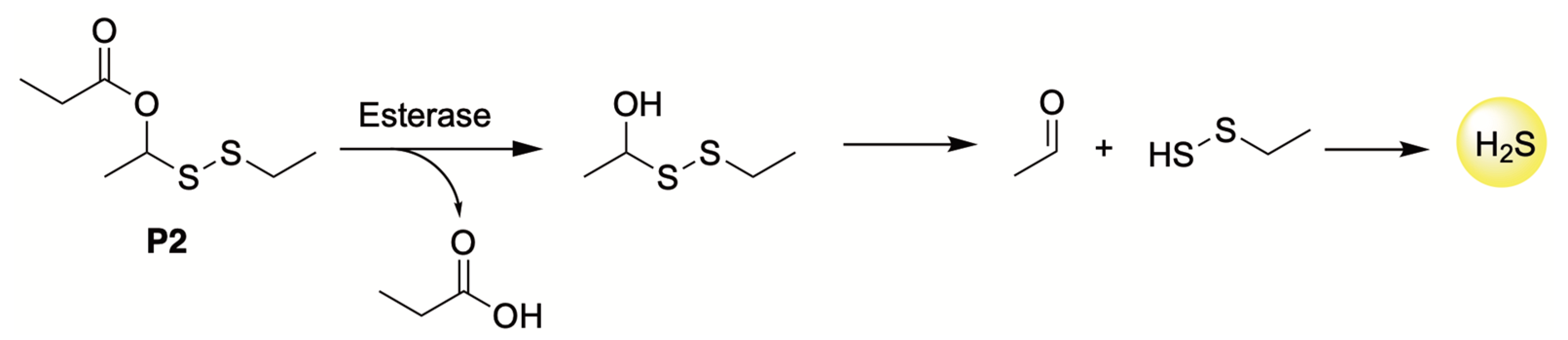
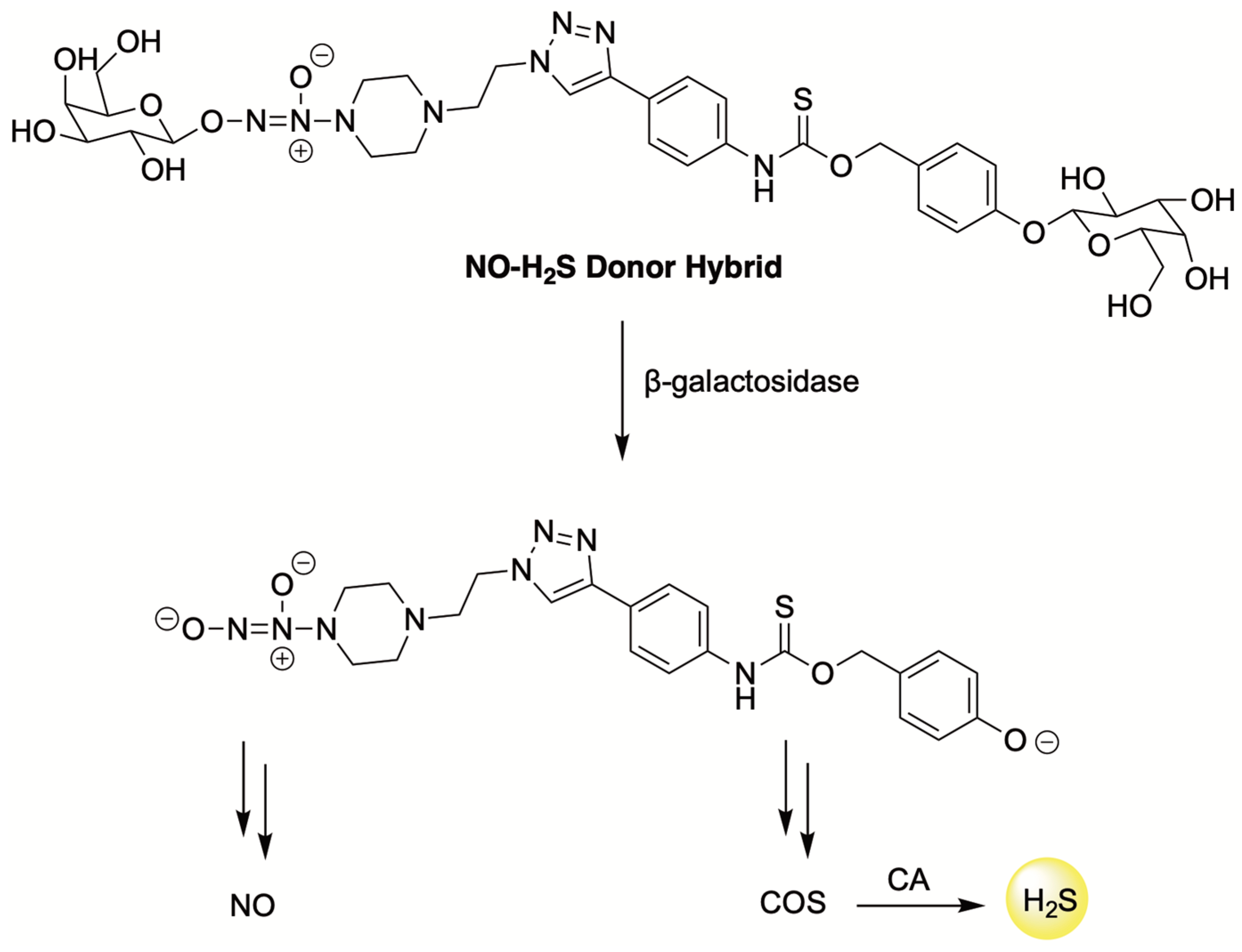



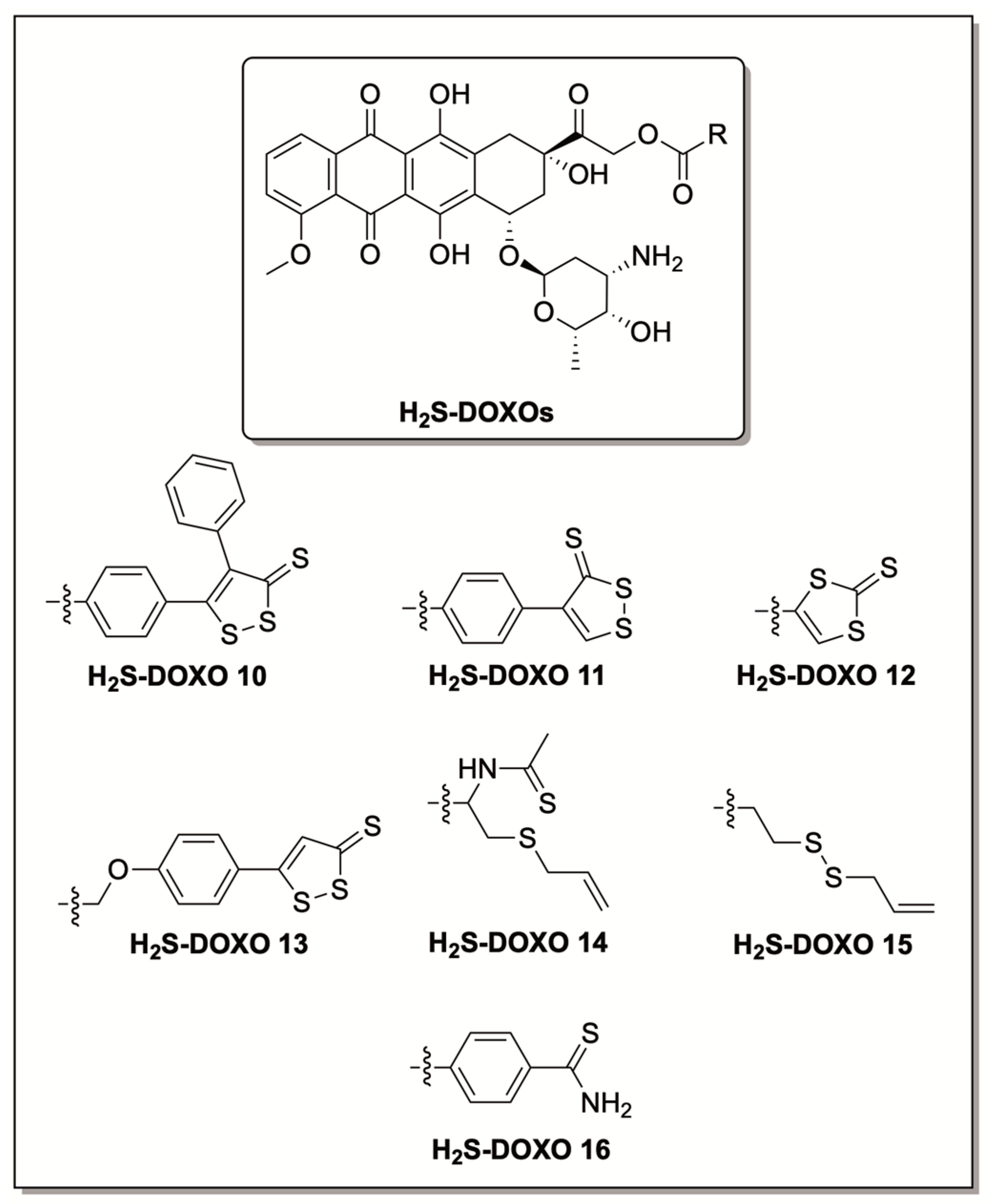
| H2S Donor | Release Mechanism | Preclinical Studies |
|---|---|---|
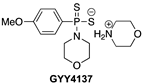 | Hydrolysis-Triggered | In vivo rat and diabetic mice models of MI/R injury |
 | Hydrolysis-Triggered | Rat isolated perfused heart model of MI/R injury |
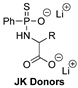 | pH-Triggered | H9c2 cardiomyocyte model of H/R injury and in vivo murine model of MI/R injury |
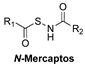 | Thiol-Triggered | In vivo murine model of MI/R injury |
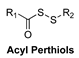 | Thiol-Triggered | In vivo murine model of MI/R injury |
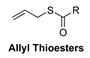 | Thiol-Triggered | In vivo murine model of MI/R injury |
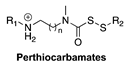 | Thiol-Triggered | Mouse isolated perfused heart model of MI/R injury |
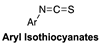 | Thiol-Triggered | Rat isolated perfused heart model and in vivo murine model of MI/R Injury |
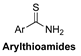 | Thiol-Triggered | In vivo rabbit model of MI/R injury |
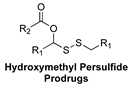 | Enzyme-Triggered | in vivo murine model of MI/R injury |
 | ROS-Triggered | H9c2 cardiomyocyte model of H/R injury and in vivo murine model of MI/R injury |
| H2S Hybrid | Release Mechanism | Preclinical Studies |
|---|---|---|
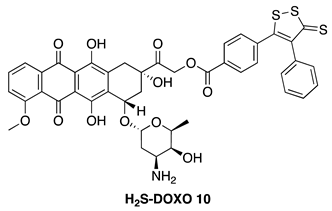 | Hydrolysis-Triggered | H9c2 cardiomyocytes, U-2OS osteosarcoma cells, DU-145 prostate cancer cells, and in vivo DOX-resistant mouse models |
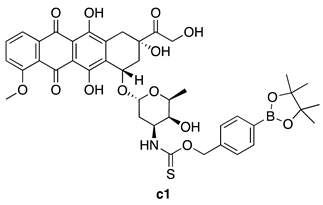 | ROS-Triggered | H9c2 cardiomyocytes and 4T1 breast cancer cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Lukesh, J.C., III. H2S Donors with Cytoprotective Effects in Models of MI/R Injury and Chemotherapy-Induced Cardiotoxicity. Antioxidants 2023, 12, 650. https://doi.org/10.3390/antiox12030650
Hu Q, Lukesh JC III. H2S Donors with Cytoprotective Effects in Models of MI/R Injury and Chemotherapy-Induced Cardiotoxicity. Antioxidants. 2023; 12(3):650. https://doi.org/10.3390/antiox12030650
Chicago/Turabian StyleHu, Qiwei, and John C. Lukesh, III. 2023. "H2S Donors with Cytoprotective Effects in Models of MI/R Injury and Chemotherapy-Induced Cardiotoxicity" Antioxidants 12, no. 3: 650. https://doi.org/10.3390/antiox12030650





