Entomotherapeutic Role of Periplaneta americana Extract in Alleviating Aluminum Oxide Nanoparticles-Induced Testicular Oxidative Impairment in Migratory Locusts (Locusta migratoria) as an Ecotoxicological Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Insects
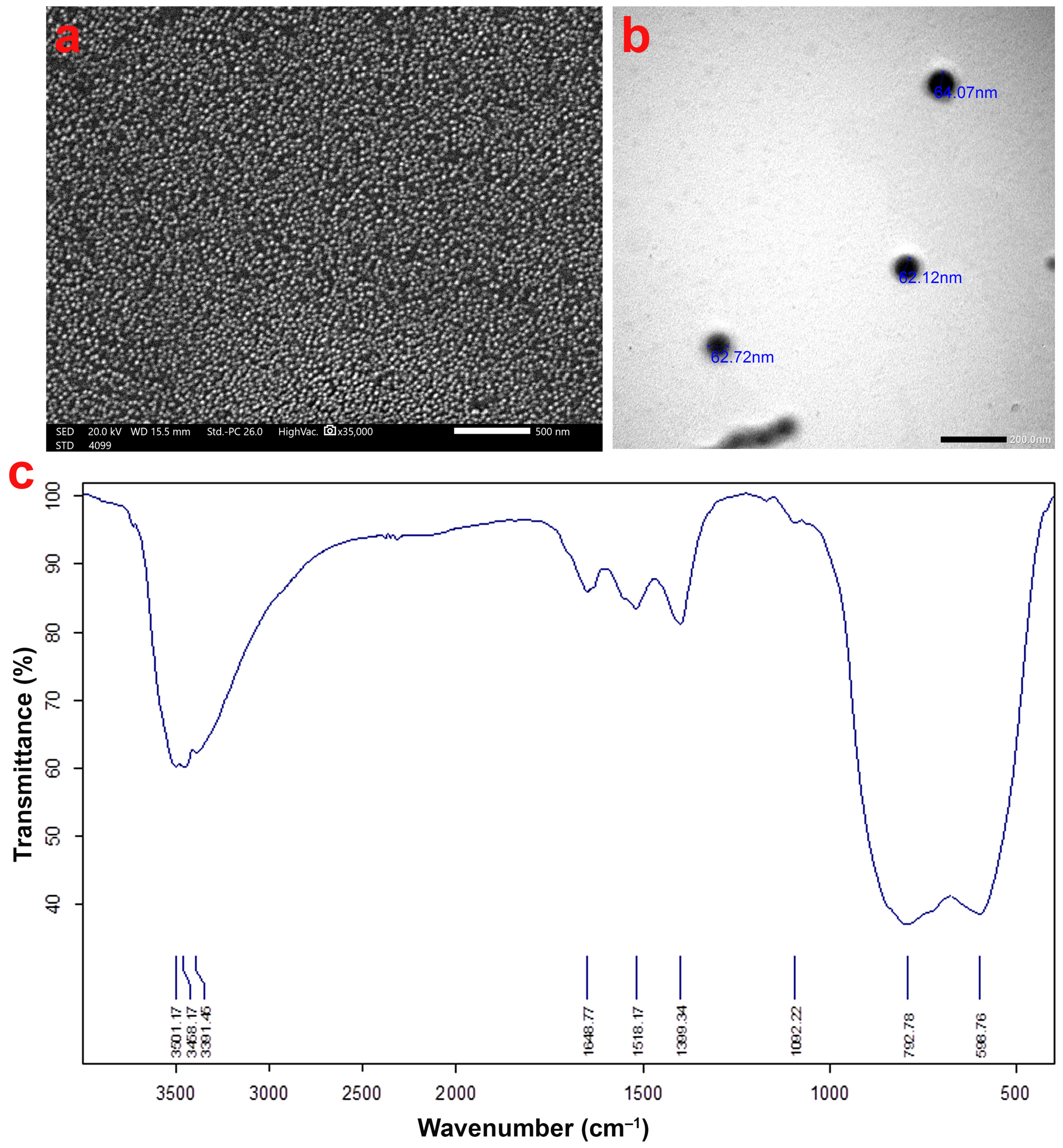
2.2. Al2O3 NPs Characterization
2.3. Preparation of Al2O3 NPs Suspension and PAE Solution
2.4. Experimental Design
2.5. Dissection Procedures
2.6. X-ray Detection of Al in the Testes of L. migratoria
2.7. Biochemical Evaluations
2.7.1. Determination of Protein Content in Hemolymph and Total Hemocyte Count
2.7.2. Biochemical Assays in Testicular Tissues
2.7.3. Molecular Docking and Computational Studies
2.7.4. Assessment of HSP70 and HSP90 mRNA Expressions
2.8. Cells Viability Assessment by Flow Cytometry
2.9. Assessment of DNA Damage
2.10. Scanning Electron Microscope (SEM) Analysis of Testes
2.11. Histological and Ultrastructural Analyses of Testicular Tissues
2.12. Statistical Analysis
3. Results
3.1. Characterization of Al2O3 NPs
3.2. Survival and Mortality Analyses of L. migratoria Male after Injection with Al2O3 NPs and PAE
3.3. Evaluation of Al Accumulated in Testicular Tissues of Locusts
3.4. Impact of Al2O3 NPs and Combinatorial Treatment of Al2O3 NPs and PAE on the Physiological Properties of L. migratoria
3.5. Evaluation of DNA Impairment by Comet Assay
3.6. Assessment of Cell Viability by Flow Cytometric Analysis
3.7. Molecular Docking Analysis
3.8. SEM Analysis
3.9. Histological Analysis
3.10. Ultrastructural Analysis
4. Discussion
4.1. Size and Bioaccumulation of Al2O3 in the Testicular Tissues
4.2. Impact of Alumina and PAE on Hemocytes
4.3. Al-induced Oxidative Stress in the Locust Testes
4.4. Apoptotic Analysis and DNA Damage of Testicular Tissues
4.5. Histological and Ultrastructural Analyses of Testicular Tissues
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keck, C.M.; Müller, R.H. Nanotoxicological classification system (NCS)—A guide for the risk-benefit assessment of nanoparticulate drug delivery systems. Eur. J. Pharm. Biopharm. 2013, 84, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- El-Kady, M.M.; Ansari, I.; Arora, C.; Rai, N.; Soni, S.; Verma, D.K.; Singh, P.; Mahmoud, A.E.D. Nanomaterials: A comprehensive review of applications, toxicity, impact, and fate to environment. J. Mol. Liq. 2023, 370, 121046. [Google Scholar] [CrossRef]
- El-Samad, L.M.; El-Ashram, S.; Hussein, H.K.; Abdul-Aziz, K.K.; Radwan, E.H.; Bakr, N.R.; El Wakil, A.; Augustyniak, M. Time-delayed effects of a single application of AgNPs on structure of testes and functions in Blaps polychresta Forskal, 1775 (Coleoptera: Tenebrionidae). Sci. Total Environ. 2022, 806, 150644. [Google Scholar] [CrossRef]
- Yokel, R.A. Aluminum reproductive toxicity: A summary and interpretation of scientific reports. Crit. Rev. Toxicol. 2020, 50, 551–593. [Google Scholar] [CrossRef]
- El-Samad, L.M.; Hassan, M.A.; Bakr, N.R.; El-Ashram, S.; Radwan, E.H.; Abdul Aziz, K.K.; Hussein, H.K.; El Wakil, A. Insights into Ag-NPs-mediated pathophysiology and ultrastructural aberrations in ovarian tissues of darkling beetles. Sci. Rep. 2022, 12, 13899. [Google Scholar] [CrossRef]
- El-Samad, L.M.; Bakr, N.R.; El-Ashram, S.; Radwan, E.H.; Abdul Aziz, K.K.; Hussein, H.K.; El Wakil, A.; Hassan, M.A. Silver nanoparticles instigate physiological, genotoxicity, and ultrastructural anomalies in midgut tissues of beetles. Chem. -Biol. Interact. 2022, 367, 110166. [Google Scholar] [CrossRef]
- Danielsson, R.; Eriksson, H. Aluminium adjuvants in vaccines—A way to modulate the immune response. Semin. Cell Dev. Biol. 2021, 115, 3–9. [Google Scholar] [CrossRef]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. WIREs Nanomed. Nanobiotechnol. 2010, 2, 544–568. [Google Scholar] [CrossRef]
- De, A.; Ghosh, S.; Chakrabarti, M.; Ghosh, I.; Banerjee, R.; Mukherjee, A. Effect of low-dose exposure of aluminium oxide nanoparticles in Swiss albino mice: Histopathological changes and oxidative damage. Toxicol. Ind. Health 2020, 36, 567–579. [Google Scholar] [CrossRef]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef]
- Ema, M.; Okuda, H.; Gamo, M.; Honda, K. A review of reproductive and developmental toxicity of silver nanoparticles in laboratory animals. Reprod. Toxicol. 2017, 67, 149–164. [Google Scholar] [CrossRef]
- Benelli, G. Mode of action of nanoparticles against insects. Environ. Sci. Pollut. Res. 2018, 25, 12329–12341. [Google Scholar] [CrossRef]
- El-Samad, L.M.; El-Gerbed, M.S.; Hussein, H.S.; Flaven-Pouchon, J.; El Wakil, A.; Moussian, B. Imidacloprid-induced pathophysiological damage in the midgut of Locusta migratoria (Orthoptera: Acrididae) in the field. Environ. Sci. Pollut. Res. 2022, 29, 57644–57655. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Locusts: A Model to Investigate Human Disease and Sickness Behavior. ACS Pharmacol. Transl. Sci. 2020, 3, 1423–1424. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Zhang, B.-W.; Yang, J.; Zou, C.-S.; Li, T.; Zhang, G.-C.; Chen, G.-S. Detoxification, antioxidant, and digestive enzyme activities and gene expression analysis of Lymantria dispar larvae under carvacrol. J. Asia-Pac. Entomol. 2021, 24, 208–216. [Google Scholar] [CrossRef]
- Abo-El-Sooud, K.; Abd-Elhakim, Y.M.; Hashem, M.M.M.; El-Metwally, A.E.; Hassan, B.A.; El-Nour, H.H.M. Ameliorative effects of quercetin against hepatic toxicity of oral sub-chronic co-exposure to aluminum oxide nanoparticles and lead-acetate in male rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. Online ahead of print. 2022. [Google Scholar]
- Ajiboye, B.O.; Oyinloye, B.E.; Ojo, O.A.; Lawal, O.E.; Jokomba, Y.A.; Balogun, B.A.; Adeoye, A.O.; Ajuwon, O.R. Effect of Flavonoid-Rich Extract From Dalbergiella welwitschii Leaf on Redox, Cholinergic, Monoaminergic, and Purinergic Dysfunction in Oxidative Testicular Injury: Ex Vivo and In Silico Studies. Bioinform. Biol. Insights 2022, 16, 11779322221115546. [Google Scholar] [CrossRef]
- Tonisi, S.; Okaiyeto, K.; Hoppe, H.; Mabinya, L.V.; Nwodo, U.U.; Okoh, A.I. Chemical constituents, antioxidant and cytotoxicity properties of Leonotis leonurus used in the folklore management of neurological disorders in the Eastern Cape, South Africa. 3 Biotech 2020, 10, 141. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, J.; Huang, F.; Yang, Y.; Zhang, X.; Yue, B. GC–MS analysis of chemical constituents and determination of the total antioxidant capacity of adult powder of Periplaneta americana. Entomol. Res. 2022, 52, 68–76. [Google Scholar] [CrossRef]
- Basseri, H.; Bakhtiyari, R.; Hashemi, S.J.; Baniardelani, M.; Shahraki, H.; Hosainpour, L. Antibacterial/Antifungal Activity of Extracted Chitosan from American Cockroach (Dictyoptera: Blattidae) and German Cockroach (Blattodea: Blattellidae). J. Med. Entomol. 2019, 56, 1208–1214. [Google Scholar] [CrossRef]
- Li, Y.; Cai, J.; Du, C.; Lin, Y.; Li, S.; Ma, A.; Qin, Y. Bioinformatic analysis and antiviral effect of Periplaneta americana defensins. Virus Res. 2022, 308, 198627. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Chen, X.; Chai, J.; Li, R.; Han, X.; Chen, X.; Liu, S.; Chen, M.; Xu, X. Antipyretic, anti-inflammatory and analgesic activities of Periplaneta americana extract and underlying mechanisms. Biomed. Pharmacother. 2020, 123, 109753. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, M. Why 4F: 1G fixative works for me. Microsc. Today 2010, 18, 50–53. [Google Scholar] [CrossRef]
- Bergin, D.; Reeves, E.P.; Renwick, J.; Wientjes, F.B.; Kavanagh, K. Superoxide Production in Galleria mellonella Hemocytes: Identification of Proteins Homologous to the NADPH Oxidase Complex of Human Neutrophils. Infect. Immun. 2005, 73, 4161–4170. [Google Scholar] [CrossRef]
- Knorr, D.Y.; Hartung, D.; Schneider, K.; Hintz, L.; Pies, H.S.; Heinrich, R. Locust Hemolymph Conveys Erythropoietin-Like Cytoprotection via Activation of the Cytokine Receptor CRLF3. Front. Physiol. 2021, 12, 648245. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Thompson, H.M. Esterases as Markers of Exposure to Organophosphates and Carbamates. Ecotoxicology 1999, 8, 369–384. [Google Scholar] [CrossRef]
- Carmagnol, F.; Sinet, P.-M.; Rapin, J.; Jerome, H. Glutathione-S-transferase of human red blood cells; assay, values in normal subjects and in two pathological circumstances: Hyperbilirubinemia and impaired renal function. Clin. Chim. Acta 1981, 117, 209–217. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Wang, H.-S.; Zhou, C.-S.; Guo, W.; Kang, L. Thermoperiodic acclimations enhance cold hardiness of the eggs of the migratory locust. Cryobiology 2006, 53, 206–217. [Google Scholar] [CrossRef]
- Wang, H.-S.; Wang, X.-H.; Zhou, C.-S.; Huang, L.-H.; Zhang, S.-F.; Guo, W.; Kang, L. cDNA cloning of heat shock proteins and their expression in the two phases of the migratory locust. Insect Mol. Biol. 2007, 16, 207–219. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.-C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- García-Medina, S.; Galar-Martínez, M.; Cano-Viveros, S.; Ruiz-Lara, K.; Gómez-Oliván, L.M.; Islas-Flores, H.; Gasca-Pérez, E.; Pérez-Pastén-Borja, R.; Arredondo-Tamayo, B.; Hernández-Varela, J.; et al. Bioaccumulation and oxidative stress caused by aluminium nanoparticles and the integrated biomarker responses in the common carp (Cyprinus carpio). Chemosphere 2022, 288, 132462. [Google Scholar] [CrossRef]
- Koodalingam, A.; Dayanidhi, M.K. Studies on biochemical and synergistic effects of immunosuppressive concentration of imidacloprid with Beauveria bassiana and Metarhizium anisopliae for enhancement of virulence against vector mosquito Culex quinquefasciatus. Pestic. Biochem. Physiol. 2021, 176, 104882. [Google Scholar] [CrossRef]
- Eskin, A.; Ekremoglu, M.; Altinkaynak, C.; Özdemir, N. Effects of organic-inorganic hybrid nanoflowers’ framework on hemocytes and enzymatic responses of the model organism, Galleria mellonella (Lepidoptera: Pyralidae). Int. J. Trop. Insect Sci. 2022, 42, 333–344. [Google Scholar] [CrossRef]
- Tunçsoy, B.; Sugeçti, S.; Büyükgüzel, E.; Özalp, P.; Büyükgüzel, K. Effects of Copper Oxide Nanoparticles on Immune and Metabolic Parameters of Galleria mellonella L. Bull. Environ. Contam. Toxicol. 2021, 107, 412–420. [Google Scholar] [CrossRef]
- Mir, A.H.; Qamar, A.; Qadir, I.; Naqvi, A.H.; Begum, R. Accumulation and trafficking of zinc oxide nanoparticles in an invertebrate model, Bombyx mori, with insights on their effects on immuno-competent cells. Sci. Rep. 2020, 10, 1617. [Google Scholar] [CrossRef]
- Erbaş, E.D.; Altuntaş, H. Effects of Juglone on the Antioxidant Metabolism in the Larval Hemolymph of the Greater Wax Moth Galleria mellonella L.(Lepidoptera: Pyralidae). Karadeniz Fen Bilim. Derg. 2021, 11, 18–28. [Google Scholar] [CrossRef]
- El-Moaty, Z.A.; Kheirallah, D.A.; Elgendy, D.A. Impact of cement dust on some biological parameters of Trachyderma hispida (Coleoptera: Tenebrionidae) inhabiting the vicinity of a cement factory, Mariout region, Alexandria, Egypt. J. Entomol. Zool. Stud. 2016, 4, 797–805. [Google Scholar]
- EL-Samad, L.M.; Mokhamer, E.; Osman, W.; Ali, A.; Shonouda, M.L. The ground beetle, Blaps polycresta (Coleoptera: Tenebrionidae) as bioindicator of heavy metals soil pollution. J Adv Biol 2015, 7, 1153–1160. [Google Scholar]
- Kheirallah, D.A.; El-Samad, L.M. Biochemical Changes Induced by Gamma Irradiation in the Ground Beetle Blaps polycresta. J. Adv. Biol. 2016, 9, 1937–1947. [Google Scholar]
- El-Gendy, A.H.; Augustyniak, M.; Toto, N.A.; Al Farraj, S.; El-Samad, L.M. Oxidative stress parameters, DNA damage and expression of HSP70 and MT in midgut of Trachyderma hispida (Forskål, 1775) (Coleoptera: Tenebrionidae) from a textile industry area. Environ. Pollut. 2020, 267, 115661. [Google Scholar] [CrossRef] [PubMed]
- Kheirallah, D.A.M.; Ali, A.M.; Osman, S.E.; Shouman, A.M. Nickel oxide nanoparticles induce genotoxicity and cellular alterations in the ground beetle Blaps polycresta (Coleoptera: Tenebrionidae). Toxicol. Ind. Health 2021, 37, 408–430. [Google Scholar] [CrossRef]
- Karpeta-Kaczmarek, J.; Dziewięcka, M.; Augustyniak, M.; Rost-Roszkowska, M. Effects of short-term exposure of Acheta domesticus to nanodiamonds in food: DNA damage but no histological alteration in tissues. Carbon 2016, 110, 458–468. [Google Scholar] [CrossRef]
- Dziewięcka, M.; Karpeta-Kaczmarek, J.; Augustyniak, M.; Majchrzycki, Ł.; Augustyniak-Jabłokow, M.A. Evaluation of in vivo graphene oxide toxicity for Acheta domesticus in relation to nanomaterial purity and time passed from the exposure. J. Hazard. Mater. 2016, 305, 30–40. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Roberts, B.R. Glutathione peroxidase 4: A new player in neurodegeneration? Mol. Psychiatry 2017, 22, 328–335. [Google Scholar] [CrossRef]
- Liang, X.; Wang, S.; Wang, L.; Ceylan, A.F.; Ren, J.; Zhang, Y. Mitophagy inhibitor liensinine suppresses doxorubicin-induced cardiotoxicity through inhibition of Drp1-mediated maladaptive mitochondrial fission. Pharmacol. Res. 2020, 157, 104846. [Google Scholar] [CrossRef]
- Dziewięcka, M.; Karpeta-Kaczmarek, J.; Augustyniak, M.; Rost-Roszkowska, M. Short-term in vivo exposure to graphene oxide can cause damage to the gut and testis. J. Hazard. Mater. 2017, 328, 80–89. [Google Scholar] [CrossRef]
- Flasz, B.; Dziewięcka, M.; Kędziorski, A.; Tarnawska, M.; Augustyniak, J.; Augustyniak, M. Multigenerational selection towards longevity changes the protective role of vitamin C against graphene oxide-induced oxidative stress in house crickets. Environ. Pollut. 2021, 290, 117996. [Google Scholar] [CrossRef]
- El-Samad, L.M.; El-Gendy, A.H.; Abdel-Moneim, A.M.; El-Ashram, S.; Augustyniak, M. CuO NPs-induced damage to testes and deregulation of the antioxidant system in wild terrestrial organism Blaps sulcata (Coleoptera: Tenebrionidae). Environ. Nanotechnol. Monit. Manag. 2022, 18, 100751. [Google Scholar] [CrossRef]
- Wang, R.; Song, B.; Wu, J.; Zhang, Y.; Chen, A.; Shao, L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018, 13, 8487–8506. [Google Scholar] [CrossRef]
- Cheraghi, E.; Golkar, A.; Roshanaei, K.; Alani, B. Aluminium-Induced Oxidative Stress, Apoptosis and Alterations in Testicular Tissue and Sperm Quality in Wistar Rats: Ameliorative Effects of Curcumin. Int. J. Fertil. Steril. 2017, 11, 166–175. [Google Scholar]
- Akinola, B.K.; Olawuyi, T.S.; Ukwenya, V.O.; Daniel, L.D.; Faleye, B.C. Protective effects of aloe vera gel (aloe baberdensis Miller) on aluminum chloride-induced reproductive toxicity in male Wistar rats. JBRA Assist. Reprod. 2021, 25, 193–201. [Google Scholar] [CrossRef]
- Zeng, C.; Liao, Q.; Hu, Y.; Shen, Y.; Geng, F.; Chen, L. The Role of Periplaneta americana (Blattodea: Blattidae) in Modern Versus Traditional Chinese Medicine. J. Med. Entomol. 2019, 56, 1522–1526. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Li, J.; Zhang, C.; Gao, F.; Ma, X.; Zhang, J.; Fu, C.; Geng, F. A feasible biocompatible hydrogel film embedding Periplaneta americana extract for acute wound healing. Int. J. Pharm. 2019, 571, 118707. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Pang, L.; Li, J.-J.; Zhang, C.; Li, J.-X.; Zhang, X.; Mao, T.; Wu, D.-T.; Ma, X.-Y.; Geng, F.-N.; et al. Characterization and diabetic wound healing benefits of protein-polysaccharide complexes isolated from an animal ethno-medicine Periplaneta americana L. Int. J. Biol. Macromol. 2022, 195, 466–474. [Google Scholar] [CrossRef] [PubMed]












| Control Group | Group Treated with Al2O3 NPs | Group Treated with PAE + Al2O3 NPs | ||||
|---|---|---|---|---|---|---|
| Element | Mass (%) | Atom (%) | Mass (%) | Atom (%) | Mass (%) | Atom (%) |
| C | 55.47 ± 0.30 | 62.00 ± 0.33 | 43.97 ± 0.29 | 50.12 ± 0.33 | 44.86 ± 0.29 | 51.10 ± 0.33 |
| N | 9.63 ± 0.56 | 9.23 ± 0.54 | 18.83 ± 0.66 | 18.40 ± 0.64 | 20.93 ± 0.66 | 20.45 ± 0.65 |
| O | 33.45 ± 0.61 | 28.07 ± 0.52 | 36.26 ± 0.70 | 31.03 ± 0.60 | 32.11 ± 0.65 | 27.46 ± 0.56 |
| Na | 0.46 ± 0.06 | 0.27 ± 0.03 | 0.13 ± 0.05 | 0.08 ± 0.03 | 0.46 ± 0.06 | 0.28 ± 0.03 |
| P | 0.63 ± 0.04 | 0.27 ± 0.02 | 0.24 ± 0.04 | 0.11 ± 0.02 | 0.74 ± 0.05 | 0.33 ± 0.02 |
| S | 0.36 ± 0.03 | 0.15 ± 0.01 | 0.33 ± 0.04 | 0.14 ± 0.02 | 0.83 ± 0.05 | 0.35 ± 0.02 |
| Al | --- | --- | 0.25 ± 0.04 | 0.13 ± 0.02 | 0.07 ± 0.03 | 0.03 ± 0.01 |
| Ligand | 1AR5 | 8CAT | ||
|---|---|---|---|---|
| Ebinding (kcal/mol) | EIntermol. (kcal/mol) | Ebinding (kcal/mol) | EIntermol. (kcal/mol) | |
| MHI | −5.79 | −6.09 | −6.03 | −6.93 |
| MTTD | −4.66 | −8.24 | 16.14 | 12.56 |
| MTP | 171.76 | 168.78 | 470.36 | 467.37 |
| PAE | −3.79 | −4.68 | −4.12 | −5.02 |
| OAM | −3.73 | −8.56 | 20.11 | 15.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arafat, E.A.; El-Sayed, D.S.; Hussein, H.K.; Flaven-Pouchon, J.; Moussian, B.; El-Samad, L.M.; El Wakil, A.; Hassan, M.A. Entomotherapeutic Role of Periplaneta americana Extract in Alleviating Aluminum Oxide Nanoparticles-Induced Testicular Oxidative Impairment in Migratory Locusts (Locusta migratoria) as an Ecotoxicological Model. Antioxidants 2023, 12, 653. https://doi.org/10.3390/antiox12030653
Arafat EA, El-Sayed DS, Hussein HK, Flaven-Pouchon J, Moussian B, El-Samad LM, El Wakil A, Hassan MA. Entomotherapeutic Role of Periplaneta americana Extract in Alleviating Aluminum Oxide Nanoparticles-Induced Testicular Oxidative Impairment in Migratory Locusts (Locusta migratoria) as an Ecotoxicological Model. Antioxidants. 2023; 12(3):653. https://doi.org/10.3390/antiox12030653
Chicago/Turabian StyleArafat, Esraa A., Doaa S. El-Sayed, Hussein K. Hussein, Justin Flaven-Pouchon, Bernard Moussian, Lamia M. El-Samad, Abeer El Wakil, and Mohamed A. Hassan. 2023. "Entomotherapeutic Role of Periplaneta americana Extract in Alleviating Aluminum Oxide Nanoparticles-Induced Testicular Oxidative Impairment in Migratory Locusts (Locusta migratoria) as an Ecotoxicological Model" Antioxidants 12, no. 3: 653. https://doi.org/10.3390/antiox12030653
APA StyleArafat, E. A., El-Sayed, D. S., Hussein, H. K., Flaven-Pouchon, J., Moussian, B., El-Samad, L. M., El Wakil, A., & Hassan, M. A. (2023). Entomotherapeutic Role of Periplaneta americana Extract in Alleviating Aluminum Oxide Nanoparticles-Induced Testicular Oxidative Impairment in Migratory Locusts (Locusta migratoria) as an Ecotoxicological Model. Antioxidants, 12(3), 653. https://doi.org/10.3390/antiox12030653










