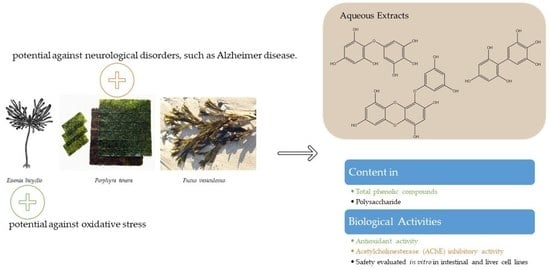
Edible Seaweeds Extracts: Characterization and Functional Properties for Health Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seaweeds
2.2. Chemical
2.3. Preparation of Seaweed Extracts
2.4. Extract Fractioning by Solid Phase Extraction (SPE)
2.5. Extracts and Fractions Characterization
2.5.1. Quantification of the Total Phenolic Content (TPC)
2.5.2. Quantification of the Total Proteins
2.5.3. Quantification of the Total Polysaccharides
2.6. Biological Activities
2.6.1. In Vitro Safety in Caco-2 and Hep-G2 Cells
2.6.2. Antioxidant Activity
2.6.3. AChE Inhibitory Activity
2.7. LC-HRMS/MS Extract Analysis
2.8. Data Analysis
3. Results
3.1. Extracts and Fractions Characterization
3.1.1. Quantification of the TPC
3.1.2. Quantification of Total Proteins
3.1.3. Quantification of Total Polysaccharides
3.2. Biological Activities
3.2.1. In Vitro Safety of Seaweed Extracts in Caco-2 and Hep-G2 Cells
3.2.2. Antioxidant Activity
3.2.3. AChE Inhibitory Activity
3.3. LC-HRMS/MS Extracts Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Zorita, S.; González-Arceo, M.; Trepiana, J.; Eseberri, I.; Fernández-Quintela, A.; Milton-Laskibar, I.; Aguirre, L.; González, M.; Portillo, M.P. Anti-Obesity Effects of Macroalgae. Nutrients 2020, 12, 2378. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef]
- Bizzaro, G.; Vatland, A.K.; Pampanin, D.M. The One-Health approach in seaweed food production. Environ. Int. 2022, 158, 106948. [Google Scholar] [CrossRef]
- André, R.; Guedes, L.; Melo, R.; Ascensão, L.; Pacheco, R.; Vaz, P.D.; Serralheiro, M.L. Effect of Food Preparations on In Vitro Bioactivities and Chemical Components of Fucus vesiculosus. Foods 2020, 9, 955. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.K.; Van, S.; Kim, S.T.; Kang, Y.H.; Park, S.R. Geographic Differentiation of Morphological Characteristics in the Brown Seaweed Sargassum thunbergii along the Korean Coast: A Response to Local Environmental Conditions. J. Mar. Sci. Eng. 2022, 10, 549. [Google Scholar] [CrossRef]
- Jung, H.A.; Roy, A.; Jung, J.H.; Choi, J.S. Evaluation of the inhibitory effects of eckol and dieckol isolated from edible brown alga Eisenia bicyclis on human monoamine oxidases A and B. Arch. Pharmacal Res. 2017, 40, 480–491. [Google Scholar] [CrossRef]
- Kim, H.J.; Dasagrandhi, C.; Kim, S.H.; Kim, B.G.; Eom, S.H.; Kim, Y.M. In Vitro Antibacterial Activity of Phlorotannins from Edible Brown Algae, Eisenia bicyclis Against Streptomycin-Resistant Listeria monocytogenes. Indian J. Microbiol. 2018, 58, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Kim, S.M.; Kang, S.W.; Jeon, S.I.; Um, B.H.; Jung, S.H. Edible seaweed, Eisenia bicyclis, protects retinal ganglion cells death caused by oxidative stress. Mar. Biotechnol. 2012, 14, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; Gaspar, M.M.; Ascensão, L.; Faísca, P.; Reis, C.P.; Pacheco, R. Nanoformulation of Seaweed Eisenia bicyclis in Albumin Nanoparticles Targeting Cardiovascular Diseases: In Vitro and In Vivo Evaluation. Mar. Drugs 2022, 20, 608. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Maheswari, V.; Babu, P.A.S. Phlorotannin and its Derivatives, a Potential Antiviral Molecule from Brown Seaweeds, an Overview. Russ. J. Mar. Biol. 2022, 48, 309–324. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [Green Version]
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. Sargassum Seaweed as a Source of Anti-Inflammatory Substances and the Potential Insight of the Tropical Species: A Review. Mar. Drugs 2019, 17, 590. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [Green Version]
- Balina, K.; Romagnoli, F.; Blumberga, D. Chemical Composition and Potential Use of Fucus Vesiculosus from Gulf of Riga. Energy Procedia 2016, 95, 43–49. [Google Scholar] [CrossRef] [Green Version]
- André, R.; Pacheco, R.; Bourbon, M.; Serralheiro, M.L. Brown Algae Potential as a Functional Food against Hypercholesterolemia: Review. Foods 2021, 10, 234. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An Overview to the Health Benefits of Seaweeds Consumption. Mar. Drugs 2021, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a Source of Natural Antioxidants: Therapeutic Activity and Food Applications. J. Food Qual. 2021, 2021, 5753391. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I.; Ustyuzhanina, N.E.; Nifantiev, N.E. Fucoidans of Brown Algae: Comparison of Sulfated Polysaccharides from Fucus vesiculosus and Ascophyllum nodosum. Mar. Drugs 2022, 20, 638. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva, A.; Cruz, M.T.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins from Fucus vesiculosus: Modulation of Inflammatory Response by Blocking NF-κB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 6897. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Beaulieu, L. Insights into the Regulation of Algal Proteins and Bioactive Peptides Using Proteomic and Transcriptomic Approaches. Molecules 2019, 24, 1708. [Google Scholar] [CrossRef] [Green Version]
- Negara, B.F.; Sohn, J.H.; Kim, J.-S.; Choi, J.-S. Effects of Phlorotannins on Organisms: Focus on the Safety, Toxicity, and Availability of Phlorotannins. Foods 2021, 10, 452. [Google Scholar] [CrossRef]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins–bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef]
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal Polysaccharides as Therapeutic Agents for Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Watanabe, K.; Okuda, H. Effects of soluble sodium alginate on cholesterol excretion and glucose tolerance in rats. J. Ethnopharmacol. 1996, 54, 47–54. [Google Scholar] [CrossRef]
- Park, J.; Yeom, M.; Hahm, D.H. Fucoidan improves serum lipid levels and atherosclerosis through hepatic SREBP-2-mediated regulation. J. Pharm. Sci. 2016, 131, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichihara, T.; Wanibuchi, H.; Taniyama, T.; Okai, Y.; Yano, Y.; Otani, S.; Imaoka, S.; Funae, Y.; Fukushima, S. Inhibition of liver glutathione S-transferase placental form-positive foci development in the rat hepatocarcinogenesis by Porphyra tenera (Asakusa-nori). Cancer Lett. 1999, 141, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Drago, S.R.; de Medina, F.S.; Martínez-Augustin, O. Proteins and Carbohydrates from Red Seaweeds: Evidence for Beneficial Effects on Gut Function and Microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpena, M.; Garcia-Perez, P.; Garcia-Oliveira, P.; Chamorro, F.; Otero, P.; Lourenço-Lopes, C.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Biological properties and potential of compounds extracted from red seaweeds. Phytochem. Rev. 2022, 1, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Caleja, C.; Pereira, E.; Pereira, C.; Ćirić, A.; Soković, M.; Soria-Lopez, A.; Fraga-Corral, M.; Simal-Gandara, J.; Ferreira, I.; et al. Red Seaweeds as a Source of Nutrients and Bioactive Compounds: Optimization of the Extraction. Chemosensors 2021, 9, 132. [Google Scholar] [CrossRef]
- Ismail, M.M.; Alotaibi, B.S.; El-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Pereira, L.; Patel, N.B. Pioneering Role of Marine Macroalgae in Cosmeceuticals. Phycology 2022, 2, 172–203. [Google Scholar] [CrossRef]
- Yang, K.; Kim, S.-Y.; Park, J.-H.; Ahn, W.-G.; Jung, S.H.; Oh, D.; Park, H.C.; Choi, C. Topical Application of Phlorotannins from Brown Seaweed Mitigates Radiation Dermatitis in a Mouse Model. Mar. Drugs 2020, 18, 377. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef] [Green Version]
- Gotteland, M.; Riveros, K.; Gasaly, N.; Carcamo, C.; Magne, F.; Liabeuf, G.; Beattie, A.; Rosenfeld, S. The Pros and Cons of Using Algal Polysaccharides as Prebiotics. Front. Nutr. 2020, 7, 163. [Google Scholar] [CrossRef]
- Arantes, A.A.; Falé, P.L.; Costa, L.C.; Pacheco, R.; Ascensão, L.; Serralheiro, M.L. Inhibition of Hmg-Coa Redutase Activity and Cholesterol Permeation Through Caco-2 Cells by Caffeoylquinic Acids from Vernonia Condensata Leaves. Rev. Bras. Farmacogn. 2016, 26, 738–743. [Google Scholar] [CrossRef] [Green Version]
- Merck. Protein Determination Using 2-D Quant Kit. Available online: http://www.sigmaaldrich.com/PT/en/technical-documents/protocol/protein-biology/protein-quantitation/protein-determination-using-2-d-quant-kit (accessed on 20 December 2022).
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Falé, P.L. Antioxidant and Anti-Acetylcholinesterase Activity of Commercially Available Medicinal Infusions after In Vitro Gastrointestinal Digestion. J. Med. Plants Res. 2013, 7, 1370–1378. [Google Scholar]
- European Medicines Agency. Ich Guideline Q3 on Impurites: Guideline for Residual Solvents; European Medicines Agency: Amsterdam, The Netherlands, 2022; p. 11.
- O’ Connor, J.; Meaney, S.; Williams, G.A.; Hayes, M. Extraction of Protein from Four Different Seaweeds Using Three Different Physical Pre-Treatment Strategies. Molecules 2020, 25, 2005. [Google Scholar] [CrossRef]
- Ermakova, S.; Men’shova, R.; Vishchuk, O.; Kim, S.-M.; Um, B.-H.; Isakov, V.; Zvyagintseva, T. Water-soluble polysaccharides from the brown alga Eisenia bicyclis: Structural characteristics and antitumor activity. Algal Res. 2013, 2, 51–58. [Google Scholar] [CrossRef]
- Bhatia, S.; Rathee, P.; Sharma, K.; Chaugule, B.B.; Kar, N.; Bera, T. Immuno-modulation effect of sulphated polysaccharide (porphyran) from Porphyra vietnamensis. Int. J. Biol. Macromol. 2013, 57, 50–56. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef] [PubMed]
- Angelis, I.D.; Turco, L. Caco-2 cells as a model for intestinal absorption. Curr. Protoc. Toxicol. 2011, 47, 20.6.1–20.6.15. [Google Scholar] [CrossRef]
- Oonsivilai, R.; Mario, G. Antioxidant activity and Cytotoxicity of Rang Chuet (Thunbergia laurifolia Lindl.) Extracts. J. Food Agro-Ind. 2008, 1, 116–128. [Google Scholar]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B. Synergy-directed fractionation of botanical medicines: A case study with goldenseal (Hydrastis canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Pires, S.M.G.; Silva, S.; Costa, F.; Braga, S.S.; Pinto, D.C.G.A.; Silva, A.M.S.; Cardoso, S.M. Overview of Phlorotannins’ Constituents in Fucales. Mar. Drugs 2022, 20, 754. [Google Scholar] [CrossRef] [PubMed]
- Chouh, A.; Nouadri, T.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins of the Brown Algae Sargassum vulgare from the Mediterranean Sea Coast. Antioxidants 2022, 11, 1055. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; André, R.; Ressaissi, A.; Duarte, B.; Melo, R.; Serralheiro, M.L. Influence of Gender and Age of Brown Seaweed (Fucus vesiculosus) on Biochemical Activities of Its Aqueous Extracts. Foods 2022, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Yadav, D.K.; Subedi, L.; Venkatesan, R.; Venkanna, A.; Afzal, S.; Lee, E.; Yoo, J.; Ji, E.; Kim, S.Y.; et al. Identification of novel acetylcholinesterase inhibitors designed by pharmacophore-based virtual screening, molecular docking and bioassay. Sci. Rep. 2018, 8, 14921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Lopes, D.; Rey, F.; Leal, M.C.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Bioactivities of Lipid Extracts and Complex Lipids from Seaweeds: Current Knowledge and Future Prospects. Mar. Drugs 2021, 19, 686. [Google Scholar] [CrossRef]
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible Seaweeds: A Potential Novel Source of Bioactive Metabolites and Nutraceuticals With Human Health Benefits. Front. Mar. Sci. 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2007, 20, 705–711. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.C.; Rocha, J.B.; Morsch, V.M.; Neis, R.T.; Schetinger, M.R. Antidepressants inhibit human acetylcholinesterase and butyrylcholinesterase activity. Biochim. Biophys. Acta 2002, 1587, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharucha, A.E.; Low, P.; Camilleri, M.; Veil, E.; Burton, D.; Kudva, Y.; Shah, P.; Gehrking, T.; Zinsmeister, A.R. A randomised controlled study of the effect of cholinesterase inhibition on colon function in patients with diabetes mellitus and constipation. Gut 2013, 62, 708–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossberg, G.T. Cholinesterase inhibitors for the treatment of Alzheimer’s disease: Getting on and staying on. Curr. Res. Clin. Exp. 2003, 64, 216–235. [Google Scholar] [CrossRef] [Green Version]
- Darreh-Shori, T.; Soininen, H. Effects of cholinesterase inhibitors on the activities and protein levels of cholinesterases in the cerebrospinal fluid of patients with Alzheimer’s disease: A review of recent clinical studies. Curr. Alzheimer. Res. 2010, 7, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jivad, N.; Rabiei, Z. A review study on medicinal plants used in the treatment of learning and memory impairments. Asian Pac. J. Trop. Biomed. 2014, 4, 780–789. [Google Scholar] [CrossRef] [Green Version]
- Polidori, M.C. Preventive benefits of natural nutrition and lifestyle counseling against Alzheimer’s disease onset. J. Alzheimers Dis. 2014, 42 (Suppl. 4), S475–S482. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Olasehinde, T.A.; Ademosun, A.O. Essential oil from lemon peels inhibit key enzymes linked to neurodegenerative conditions and pro-oxidant induced lipid peroxidation. J. Oleo Sci. 2014, 63, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar] [PubMed]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Witherick, J.; Wilkins, A.; Scolding, N.; Kemp, K. Mechanisms of oxidative damage in multiple sclerosis and a cell therapy approach to treatment. Autoimmune Dis. 2010, 2011, 164608. [Google Scholar] [CrossRef] [PubMed]
- Vasanthi, P.; Nalini, G.; Rajasekhar, G. Status of oxidative stress in rheumatoid arthritis. Int. J. Rheum. Dis. 2009, 12, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Larregieu, C.A.; Benet, L.Z. Drug discovery and regulatory considerations for improving in silico and in vitro predictions that use Caco-2 as a surrogate for human intestinal permeability measurements. AAPS J. 2013, 15, 483–497. [Google Scholar] [CrossRef] [Green Version]





| Aramé | Nori | Fucus | Aramé MeOH | Nori MeOH | Fucus MeOH | Aramé H2O | Nori H2O | Fucus H2O |
|---|---|---|---|---|---|---|---|---|
| 65.1 ± 2.7 a * | 2.0 ± 0.4 b | 20.8 ± 0.1 c | 17.4 ± 1.0 c,d | 2.0 ± 1.6 b | 7.8 ± 1.0 d | 9.8 ± 1.4 d | 4.0 ± 0.9 b | 7.6 ± 0.04 d |
| Rt (min) | [M-H]− m/z | MS Fragmentation | Molecular Formula | Extracts | Tentative Identification | ||
|---|---|---|---|---|---|---|---|
| Aramé | Nori | Fucus | |||||
| 2 | 191.0196 | 57.03 (100%); 87.00 (86.0%); 111.00 (53%) | C6H8O7 | Citric acid [8] | |||
| 551.1821 | 505.18 (100%); 59.01 (59.5%);179.06 (34.8%); 71.01 (15.6%); 101.02 (10.4%) | C19H36O18 | Glycidil compound [55] | ||||
| 5.9 | 575.0126 | 495.06 (81.6%); 233.05 (59.9%); 139.00 (13.9%); 261.00 (13.5%); 125.02 (10.2%); 111 (10.1%) | C30H24O12 | Eckol derivative | |||
| 7.3 | 596.0338 | 261.00(100%); 369.02 (36%); 139.00 (33.2%); 125.02 (27.4%); 111.00 (18.9%); 556.06 (13.3%) | C30H28O13 | Eckol derivative | |||
| 13.2 | 265.1482 | 96.96 (100%); 79.96 (32.8%) | C12H10O7 | Fuhalol derivative | |||
| 14 | 311.1691 | 183.01 (47.6%); 79.96 (28.6%); 119.05 (27.8%) | C19H20O4 | Phloroglucinol derivative (Fucol type) | |||
| 15.1 | 325.1841 | 183.01 (42.6%); 119.05 (32.6%);79.96 (29%) | C19H18O5 | Phloroglucinol derivative (Fucol type) | |||
| 16.3 | 339.2002 | 183.01 (34%); 119.05 (23.5%); 79.96 (18.9%) | C19H16O6 | Phloroglucinol derivative (Fucol type) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, M.; Duarte, A.P.; Pinto, S.; Botelho, H.M.; Reis, C.P.; Serralheiro, M.L.; Pacheco, R. Edible Seaweeds Extracts: Characterization and Functional Properties for Health Conditions. Antioxidants 2023, 12, 684. https://doi.org/10.3390/antiox12030684
Coelho M, Duarte AP, Pinto S, Botelho HM, Reis CP, Serralheiro ML, Pacheco R. Edible Seaweeds Extracts: Characterization and Functional Properties for Health Conditions. Antioxidants. 2023; 12(3):684. https://doi.org/10.3390/antiox12030684
Chicago/Turabian StyleCoelho, Mariana, Ana Patrícia Duarte, Sofia Pinto, Hugo M. Botelho, Catarina Pinto Reis, Maria Luísa Serralheiro, and Rita Pacheco. 2023. "Edible Seaweeds Extracts: Characterization and Functional Properties for Health Conditions" Antioxidants 12, no. 3: 684. https://doi.org/10.3390/antiox12030684