The Impact of Plasma Membrane Ion Channels on Bone Remodeling in Response to Mechanical Stress, Oxidative Imbalance, and Acidosis
Abstract
:1. Introduction
2. The Role of Mechanical Stress, Oxidative Imbalance, and Acidosis in Bone Remodeling
2.1. Mechanical Stress
2.2. Oxidative Imbalance
2.3. Acidosis
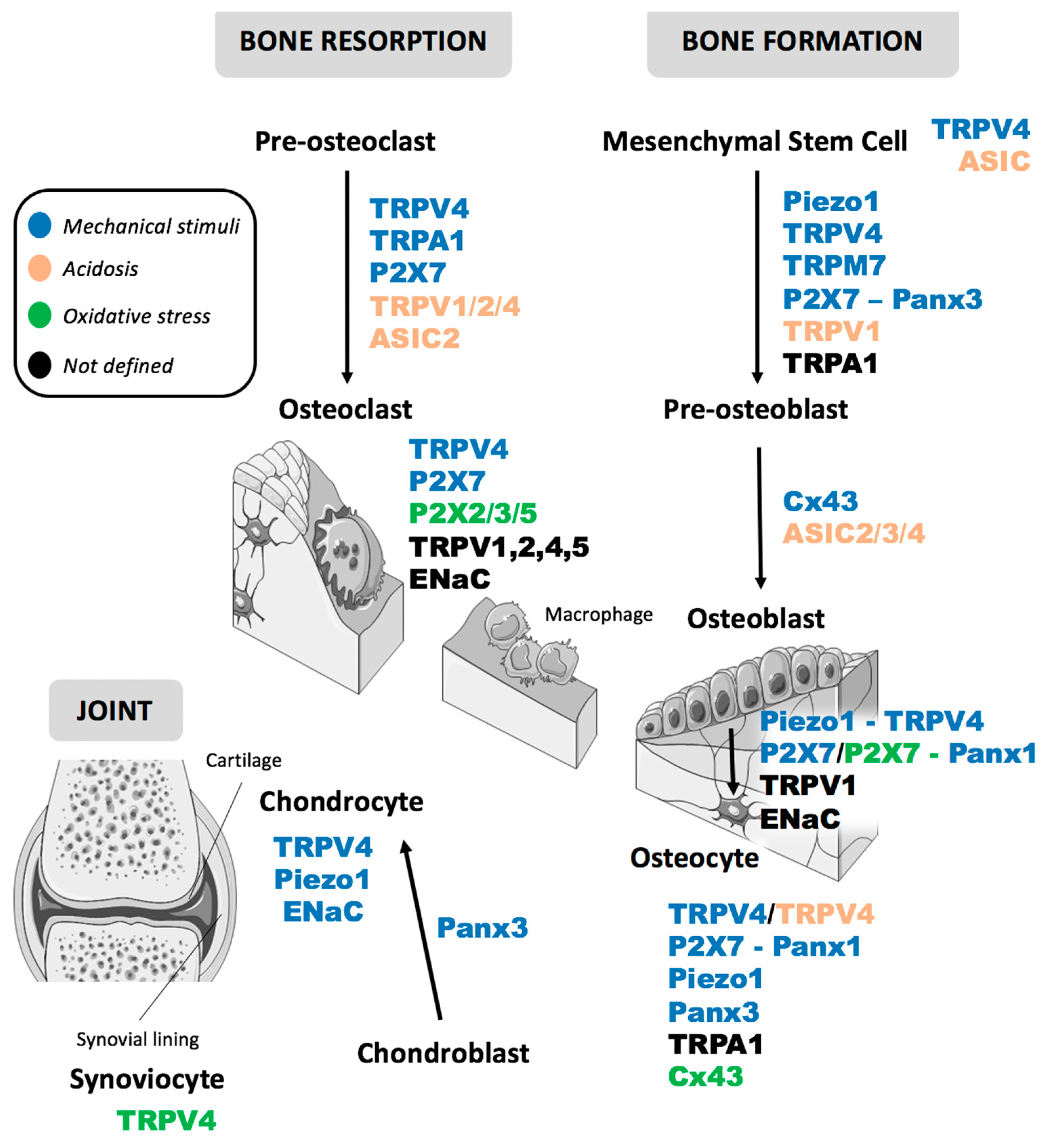
3. Ion Channels as Membrane Multi-Sensors and Transducers in Bone Remodeling: A Converging Machinery for Mechanical Stress, Extracellular Acidosis and Redox Balance
3.1. Piezo
3.2. TRP
3.2.1. TRPV4
3.2.2. TRPA1
3.2.3. TRPM7
3.3. ASIC/ENaC
3.3.1. ASIC
3.3.2. ENaC
3.4. P2X Purinergic Receptors
3.5. Connexins and Pannexins
3.5.1. Connexins
3.5.2. Pannexins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahrir, N.A.; Ooi, F.K. Physical Activity, Bone Remodelling and Bone Metabolism Markers. J. Exerc. Sports Orthop. 2018, 5, 1–4. [Google Scholar] [CrossRef]
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone remodeling: An operational process ensuring survival and bone mechanical competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef]
- Faienza, M.F.; Lassandro, G.; Chiarito, M.; Valente, F.; Ciaccia, L.; Giordano, P. How Physical Activity across the Lifespan Can Reduce the Impact of Bone Ageing: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 1862. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, G.; Ziemann, E.; Banfi, G. Physical Activity and Bone Health: What Is the Role of Immune System? A Narrative Review of the Third Way. Front. Endocrinol. 2019, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.; Elliott-Sale, K.; Sale, C. Exercise and bone health across the lifespan. Biogerontology 2017, 18, 931–946. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Xu, S.; Zhang, H. Regulation of bone health through physical exercise: Mechanisms and types. Front. Endocrinol. 2022, 13, 1029475. [Google Scholar] [CrossRef]
- Scott, A.; Khan, K.M.; Duronio, V.; Hart, D.A. Mechanotransduction in Human Bone: In Vitro Cellular Physiology that Underpins Bone Changes with Exercise. Sport. Med. 2008, 38, 139–160. [Google Scholar] [CrossRef]
- Allen, J.; Sun, Y.; Woods, J.A. Exercise and the Regulation of Inflammatory Responses. Prog. Mol. Biol. Transl. Sci. 2015, 135, 337–354. [Google Scholar] [CrossRef]
- Karsenty, G.; Olson, E.N. Bone and Muscle Endocrine Functions: Unexpected Paradigms of Inter-organ Communication. Cell 2016, 164, 1248–1256. [Google Scholar] [CrossRef] [Green Version]
- Confavreux, C.B.; Levine, R.L.; Karsenty, G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol. Cell. Endocrinol. 2009, 310, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Obri, A.; Khrimian, L.; Karsenty, G.; Oury, F. Osteocalcin in the brain: From embryonic development to age-related decline in cognition. Nat. Rev. Endocrinol. 2018, 14, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Rashdan, N.A.; Sim, A.M.; Cui, L.; Phadwal, K.; Roberts, F.; Carter, R.; Ozdemir, D.D.; Hohenstein, P.; Hung, J.; Kaczynski, J.; et al. Osteocalcin Regulates Arterial Calcification Via Altered Wnt Signaling and Glucose Metabolism. J. Bone Miner. Res. 2019, 35, 357–367. [Google Scholar] [CrossRef]
- Genova, T.; Petrillo, S.; Zicola, E.; Roato, I.; Ferracini, R.; Tolosano, E.; Altruda, F.; Carossa, S.; Mussano, F.; Munaron, L. The Crosstalk Between Osteodifferentiating Stem Cells and Endothelial Cells Promotes Angiogenesis and Bone Formation. Front. Physiol. 2019, 10, 1291. [Google Scholar] [CrossRef] [Green Version]
- Mussano, F.; Genova, T.; Petrillo, S.; Roato, I.; Ferracini, R.; Munaron, L. Osteogenic Differentiation Modulates the Cytokine, Chemokine, and Growth Factor Profile of ASCs and SHED. Int. J. Mol. Sci. 2018, 19, 1454. [Google Scholar] [CrossRef]
- Genova, T.; Munaron, L.; Carossa, S.; Mussano, F. Overcoming physical constraints in bone engineering: “The importance of being vascularized”. J. Biomater. Appl. 2016, 30, 940–951. [Google Scholar] [CrossRef] [Green Version]
- Petrillo, S.; Genova, T.; Chinigò, G.; Roato, I.; Scarpellino, G.; Kopecka, J.; Altruda, F.; Tolosano, E.; Riganti, C.; Mussano, F.; et al. Endothelial Cells Promote Osteogenesis by Establishing a Functional and Metabolic Coupling with Human Mesenchymal Stem Cells. Front. Physiol. 2022, 12, 813547. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Miyazaki, A.; Kawarabayashi, K.; Shono, M.; Akazawa, Y.; Hasegawa, T.; Ueda-Yamaguchi, K.; Kitamura, T.; Yoshizaki, K.; Fukumoto, S.; et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci. Rep. 2017, 7, 17696. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. eLife 2019, 8, e47454. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, B.; Fan, Y.; Liu, Y.; Feng, S.; Cong, Q.; Zhang, X.; Zhou, Y.; Yadav, P.S.; Lin, J.; et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. eLife 2020, 9, e52779. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. eLife 2019, 8, e49631. [Google Scholar] [CrossRef]
- Bae, C.; Sachs, F.; Gottlieb, P.A. Protonation of the Human PIEZO1 Ion Channel Stabilizes Inactivation. J. Biol. Chem. 2015, 290, 5167–5173. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, M.A.; Johnson, G.P.; Stavenschi, E.; Riffault, M.; Labour, M.-N.; Hoey, D.A. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci. Rep. 2018, 8, 3824. [Google Scholar] [CrossRef]
- Alakpa, E.V.; Jayawarna, V.; Lampel, A.; Burgess, K.V.; West, C.C.; Bakker, S.C.; Roy, S.; Javid, N.; Fleming, S.; Lamprou, D.A.; et al. Tunable Supramolecular Hydrogels for Selection of Lineage-Guiding Metabolites in Stem Cell Cultures. Chem 2016, 1, 298–319. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.; Han, L.; Ambrogini, E.; Weinstein, R.S.; Manolagas, S.C. Glucocorticoids and Tumor Necrosis Factor α Increase Oxidative Stress and Suppress Wnt Protein Signaling in Osteoblasts. J. Biol. Chem. 2011, 286, 44326–44335. [Google Scholar] [CrossRef] [Green Version]
- Son, A.; Kang, N.; Kang, J.Y.; Kim, K.W.; Yang, Y.M.; Shin, D.M. TRPM3/TRPV4 regulates Ca2+-mediated RANKL/NFATc1 expression in osteoblasts. J. Mol. Endocrinol. 2018, 61, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, M.; Suzuki, H.; Hatano, N.; Nakano, S.; Muraki, Y.; Miyazawa, K.; Goto, S.; Muraki, K. PIEZO1 and TRPV4, which Are Distinct Mechano-Sensors in the Osteoblastic MC3T3-E1 Cells, Modify Cell-Proliferation. Int. J. Mol. Sci. 2019, 20, 4960. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Dai, X.; Wang, W. Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca2+–calcineurin–NFATc1 pathway. J. Cell. Physiol. 2019, 234, 6831–6841. [Google Scholar] [CrossRef]
- Masuyama, R.; Vriens, J.; Voets, T.; Karashima, Y.; Owsianik, G.; Vennekens, R.; Lieben, L.; Torrekens, S.; Moermans, K.; Bosch, A.V.; et al. TRPV4-Mediated Calcium Influx Regulates Terminal Differentiation of Osteoclasts. Cell Metab. 2008, 8, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Lieben, L.; Carmeliet, G. The Involvement of TRP Channels in Bone Homeostasis. Front. Endocrinol. 2012, 3, 99. [Google Scholar] [CrossRef] [Green Version]
- Lyons, J.S.; Joca, H.C.; Law, R.A.; Williams, K.M.; Kerr, J.P.; Shi, G.; Khairallah, R.J.; Martin, S.S.; Konstantopoulos, K.; Ward, C.W.; et al. Microtubules tune mechanotransduction through NOX2 and TRPV4 to decrease sclerostin abundance in osteocytes. Sci. Signal. 2017, 10, eaan5748. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.L.; Guevarra, M.D.; Nguyen, A.M.; Chua, M.C.; Wang, Y.; Jacobs, C.R. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia 2015, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Sun, Z.; Xu, X.; Lv, Z.; Li, J.; Wu, R.; Fei, Y.; Tan, G.; Liu, Z.; Liu, Y.; et al. Blocking TRPV4 Ameliorates Osteoarthritis by Inhibiting M1 Macrophage Polarization via the ROS/NLRP3. Int. J. Mol. Sci. 2022, 11, 2315. [Google Scholar] [CrossRef]
- Dutta, B.; Goswami, R.; Rahaman, S.O. TRPV4 Plays a Role in Matrix Stiffness-Induced Macrophage Polarization. Front. Immunol. 2020, 11, 570195. [Google Scholar] [CrossRef]
- Du, G.; Li, L.; Zhang, X.; Liu, J.; Hao, J.; Zhu, J.; Wu, H.; Chen, W.; Zhang, Q. Roles of TRPV4 and piezo channels in stretch-evoked Ca2+ response in chondrocytes. Exp. Biol. Med. 2019, 245, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Hui, Z.; Wei, W.; Yang, J.; Chen, Z.; Guo, B.; Xing, F.; Zhang, X.; Pan, L.; Xu, J. Hypotonic stress promotes ATP release, reactive oxygen species production and cell proliferation via TRPV4 activation in rheumatoid arthritis rat synovial fibroblasts. Biochem. Biophys. Res. Commun. 2017, 486, 108–115. [Google Scholar] [CrossRef]
- Mikami, R.; Mizutani, K.; Aoki, A.; Tamura, Y.; Aoki, K.; Izumi, Y. Low-level ultrahigh-frequency and ultrashort-pulse blue laser irradiation enhances osteoblast extracellular calcification by upregulating proliferation and differentiation via transient receptor potential vanilloid 1. Lasers Surg. Med. 2018, 50, 340–352. [Google Scholar] [CrossRef]
- He, L.-H.; Liu, M.; He, Y.; Xiao, E.; Zhao, L.; Zhang, T.; Yang, H.-Q.; Zhang, Y. TRPV1 deletion impaired fracture healing and inhibited osteoclast and osteoblast differentiation. Sci. Rep. 2017, 7, srep42385. [Google Scholar] [CrossRef] [Green Version]
- Kajiya, H.; Okamoto, F.; Nemoto, T.; Kimachi, K.; Toh-Goto, K.; Nakayana, S.; Okabe, K. RANKL-induced TRPV2 expression regulates osteoclastogenesis via calcium oscillations. Cell Calcium 2010, 48, 260–269. [Google Scholar] [CrossRef]
- Kato, K.; Morita, I. Acidosis environment promotes osteoclast formation by acting on the last phase of preosteoclast differentiation: A study to elucidate the action points of acidosis and search for putative target molecules. Eur. J. Pharmacol. 2011, 663, 27–39. [Google Scholar] [CrossRef]
- Idris, A.I.; Landao-Bassonga, E.; Ralston, S.H. The TRPV1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone 2010, 46, 1089–1099. [Google Scholar] [CrossRef]
- Goto, K.; Kajiya, H.; Nemoto, T.; Tsutsumi, T.; Tsuzuki, T.; Sato, H.; Okabe, K. Hyperocclusion Stimulates Osteoclastogenesis via CCL2 Expression. J. Dent. Res. 2011, 90, 793–798. [Google Scholar] [CrossRef]
- Tsutsumi, T.; Kajiya, H.; Fukawa, T.; Sasaki, M.; Nemoto, T.; Tsuzuki, T.; Takahashi, Y.; Fujii, S.; Maeda, H.; Okabe, K. The potential role of transient receptor potential type A1 as a mechanoreceptor in human periodontal ligament cells. Eur. J. Oral Sci. 2013, 121, 538–544. [Google Scholar] [CrossRef]
- Goralczyk, A.; Vijven, M.; Koch, M.; Badowski, C.; Yassin, M.S.; Toh, S.; Shabbir, A.; Franco-Obregón, A.; Raghunath, M. TRP channels in brown and white adipogenesis from human progenitors: New therapeutic targets and the caveats associated with the common antibiotic, streptomycin. FASEB J. 2017, 31, 3251–3266. [Google Scholar] [CrossRef] [Green Version]
- El Karim, I.; McCrudden, M.T.; Linden, G.J.; Abdullah, H.; Curtis, T.M.; McGahon, M.; About, I.; Irwin, C.; Lundy, F.T. TNF-α-induced p38MAPK activation regulates TRPA1 and TRPV4 activity in odontoblast-like cells. Am. J. Pathol. 2015, 185, 2994–3002. [Google Scholar] [CrossRef]
- Kringelbach, T.M.; Aslan, D.; Novak, I.; Ellegaard, M.; Syberg, S.; Andersen, C.K.; Kristiansen, K.A.; Vang, O.; Schwarz, P.; Jørgensen, N.R. Fine-tuned ATP signals are acute mediators in osteocyte mechanotransduction. Cell. Signal. 2015, 27, 2401–2409. [Google Scholar] [CrossRef]
- de Almeida, A.S.; Pereira, G.C.; Brum, E.D.S.; Silva, C.R.; Antoniazzi, C.T.D.D.; Ardisson-Araújo, D.; Oliveira, S.M.; Trevisan, G. Role of TRPA1 expressed in bone tissue and the antinociceptive effect of the TRPA1 antagonist repeated administration in a breast cancer pain model. Life Sci. 2021, 276, 119469. [Google Scholar] [CrossRef]
- Moparthi, L.; Zygmunt, P.M. Human TRPA1 is an inherently mechanosensitive bilayer-gated ion channel. Cell Calcium 2020, 91, 102255. [Google Scholar] [CrossRef]
- Vancauwenberghe, E.; Noyer, L.; Derouiche, S.; Lemonnier, L.; Gosset, P.; Sadofsky, L.R.; Mariot, P.; Warnier, M.; Bokhobza, A.; Slomianny, C.; et al. Activation of mutated TRPA1 ion channel by resveratrol in human prostate cancer associated fibroblasts (CAF). Mol. Carcinog. 2017, 56, 1851–1867. [Google Scholar] [CrossRef]
- Liu, Y.S.; Liu, Y.A.; Huang, C.J.; Yen, M.H.; Tseng, C.T.; Chien, S.; Lee, O.K. Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci. Rep. 2015, 5, 16522. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.; Mori, S.; Mizoguchi, T.; Arai, A.; Kajiya, H.; Okamoto, F.; Bartlett, J.D.; Matsushita, M.; Udagawa, N.; Okabe, K. Mesenchymal cell TRPM7 expression is required for bone formation via the regulation of chondrogenesis. Bone 2023, 166, 116579. [Google Scholar] [CrossRef]
- Abed, E.; Labelle, D.; Martineau, C.; Loghin, A.; Moreau, R. Expression of transient receptor potential (TRP) channels in human and murine osteoblast-like cells. Mol. Membr. Biol. 2009, 26, 146–158. [Google Scholar] [CrossRef]
- Kwon, M.; Baek, S.H.; Park, C.-K.; Chung, G.; Oh, S.B. Single-cell RT-PCR and immunocytochemical detection of mechanosensitive transient receptor potential channels in acutely isolated rat odontoblasts. Arch. Oral Biol. 2014, 59, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Vang, H.; Kim, J.H.; Lee, P.R.; Kang, Y.; Oh, S.B. TRPM7 Mediates Mechanosensitivity in Adult Rat Odontoblasts. J. Dent. Res. 2018, 97, 1039–1046. [Google Scholar] [CrossRef]
- Roy, B.; Das, T.; Mishra, D.; Maiti, T.K.; Chakraborty, S. Oscillatory shear stress induced calcium flickers in osteoblast cells. Integr. Biol. 2014, 6, 289–299. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Huang, T.-J.; Wu, M.-H.; Li, Y.-Y.; Lee, K.-D. High Expression of Acid-Sensing Ion Channel 2 (ASIC2) in Bone Cells in Osteoporotic Vertebral Fractures. BioMed Res. Int. 2019, 2019, 4714279. [Google Scholar] [CrossRef]
- Gilbert, H.T.J.; Mallikarjun, V.; Dobre, O.; Jackson, M.R.; Pedley, R.; Gilmore, A.P.; Richardson, S.M.; Swift, J. Nuclear decoupling is part of a rapid protein-level cellular response to high-intensity mechanical loading. Nat. Commun. 2019, 10, 4149. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Ding, J.; Wu, J.; Liu, T.; Liang, J.; Tang, Q.; Jiao, M. Resveratrol attenuates bone cancer pain through regulating the expression levels of ASIC3 and activating cell autophagy. Acta Biochim. Biophys. Sin. 2017, 49, 1008–1014. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, H.; Zhang, X.; Yang, G.; Lu, L.; Lu, X.; Wan, C.; Ijiri, K.; Ji, H.; Li, Q. Epithelial sodium channel enhanced osteogenesis via cGMP/PKGII/ENaC signaling in rat osteoblast. Mol. Biol. Rep. 2014, 41, 2161–2169. [Google Scholar] [CrossRef]
- Kizer, N.; Guo, X.-L.; Hruska, K. Reconstitution of stretch-activated cation channels by expression of the α-subunit of the epithelial sodium channel cloned from osteoblasts. Proc. Natl. Acad. Sci. USA 1997, 94, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Killick, R.; Richardson, G. Isolation of chicken alpha ENaC splice variants from a cochlear cDNA library. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1997, 1350, 33–37. [Google Scholar] [CrossRef]
- Mobasheri, A.; Shakibaei, M.; Canessa, C.; Martín-Vasallo, P. Enigmatic Roles of the Epithelial Sodium Channel (ENaC) in Articular Chondrocytes and Osteoblasts: Mechanotransduction, Sodium Transport or Extracellular Sodium Sensing? In Mechanosensitivity Cells Tissues; Academia: Moscow, Russia, 2005. Available online: https://www.ncbi.nlm.nih.gov/books/NBK7513/ (accessed on 27 February 2023).
- Hu, S.-Y.; Jin, X.-D.; Zhang, H.; Chen, J.; Yang, G.-Z.; Wang, X.-D.; Tang, L.; Lu, X.-Y.; Lu, L.; Li, Q.-N. Role of epithelial sodium channel in rat osteoclast differentiation and bone resorption. Nan Fang Yi Ke Da Xue Xue Bao = J. South. Med Univ. 2016, 36, 1148–1152. [Google Scholar]
- Ilatovskaya, D.V.; Pavlov, T.S.; Levchenko, V.; Staruschenko, A. ROS production as a common mechanism of ENaC regulation by EGF, insulin, and IGF-1. Am. J. Physiol. Cell Physiol. 2013, 304, C102. [Google Scholar] [CrossRef] [Green Version]
- Bratengeier, C.; Bakker, A.D.; Fahlgren, A. Mechanical loading releases osteoclastogenesis-modulating factors through stimulation of the P2X7 receptor in hematopoietic progenitor cells. J. Cell. Physiol. 2018, 234, 13057–13067. [Google Scholar] [CrossRef]
- Genetos, D.C.; Karin, N.J.; Geist, D.J.; Donahue, H.J.; Duncan, R.L. Purinergic signaling is required for fluid shear stress-induced NF-κB translocation in osteoblasts. Exp. Cell. Res. 2011, 317, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panupinthu, N.; Zhao, L.; Possmayer, F.; Ke, H.Z.; Sims, S.M.; Dixon, S.J. P2X7 Nucleotide Receptors Mediate Blebbing in Osteoblasts through a Pathway Involving Lysophosphatidic Acid. J. Biol. Chem. 2007, 282, 3403–3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kariya, T.; Tanabe, N.; Shionome, C.; Manaka, S.; Kawato, T.; Zhao, N.; Maeno, M.; Suzuki, N.; Shimizu, N. Tension Force-Induced ATP Promotes Osteogenesis Through P2X7 Receptor in Osteoblasts. J. Cell. Biochem. 2014, 116, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Agrawal, A.; Jørgensen, N.R.; Gartland, A. P2X7 receptor regulates osteoclast function and bone loss in a mouse model of osteoporosis. Sci. Rep. 2018, 8, 3507. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Walsh, M.C.; Takegahara, N.; Middleton, S.A.; Shin, H.-I.; Kim, J.; Choi, Y. The purinergic receptor P2X5 regulates inflammasome activity and hyper-multinucleation of murine osteoclasts. Sci. Rep. 2017, 7, 196. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, N.R. Role of the purinergic P2X receptors in osteoclast pathophysiology. Curr. Opin. Pharmacol. 2019, 47, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.M.; Islam, S.; Suadicani, S.O.; Spray, D.C. Connexin43 and Pannexin1 Channels in Osteoblasts: Who Is the “Hemichannel”? J. Membr. Biol. 2012, 245, 401–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kar, R.; Riquelme, M.A.; Werner, S.; Jiang, J.X. Connexin 43 Channels Protect Osteocytes Against Oxidative Stress-Induced Cell Death. J. Bone Miner. Res. 2013, 28, 1611–1621. [Google Scholar] [CrossRef] [Green Version]
- Hua, R.; Zhang, J.; Riquelme, M.A.; Jiang, J.X. Connexin Gap Junctions and Hemichannels Link Oxidative Stress to Skeletal Physiology and Pathology. Curr. Osteoporos. Rep. 2021, 19, 66–74. [Google Scholar] [CrossRef]
- Schalper, K.A.; Sánchez, H.A.; Lee, S.C.; Altenberg, G.A.; Nathanson, M.H.; Sáez, J.C. Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. Am. J. Physiol. Physiol. 2010, 299, C1504–C1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seref-Ferlengez, Z.; Maung, S.; Schaffler, M.B.; Spray, D.C.; Suadicani, S.O.; Thi, M.M. P2X7R-Panx1 Complex Impairs Bone Mechanosignaling under High Glucose Levels Associated with Type-1 Diabetes. PLoS ONE 2016, 11, e0155107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seref-Ferlengez, Z.; Urban-Maldonado, M.; Sun, H.B.; Schaffler, M.B.; Suadicani, S.O.; Thi, M.M. Role of pannexin 1 channels in load-induced skeletal response. Ann. N. Y. Acad. Sci. 2019, 1442, 79–90. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Schappe, M.S.; Desai, B.N.; Bayliss, D.A. Revisiting multimodal activation and channel properties of Pannexin 1. J. Gen. Physiol. 2017, 150, 19–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtenbach, S.; Prochnow, N.; Kurtenbach, S.; Klooster, J.; Zoidl, C.; Dermietzel, R.; Kamermans, M.; Zoidl, G. Pannexin1 Channel Proteins in the Zebrafish Retina Have Shared and Unique Properties. PLoS ONE 2013, 8, e77722. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, M.; Yamada, Y. The Role of Pannexin 3 in Bone Biology. J. Dent. Res. 2017, 96, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, M.; Iwamoto, T.; Nakamura, T.; Doyle, A.; Fukumoto, S.; Yamada, Y. Pannexin 3 functions as an ER Ca2+ channel, hemichannel, and gap junction to promote osteoblast differentiation. J. Cell Biol. 2011, 193, 1257–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, T.; Nakamura, T.; Doyle, A.; Ishikawa, M.; de Vega, S.; Fukumoto, S.; Yamada, Y. Pannexin 3 Regulates Intracellular ATP/cAMP Levels and Promotes Chondrocyte Differentiation. J. Biol. Chem. 2010, 285, 18948–18958. [Google Scholar] [CrossRef] [Green Version]
- Bond, S.R.; Lau, A.; Penuela, S.; Sampaio, A.V.; Underhill, T.M.; Laird, D.W.; Naus, C.C. Pannexin 3 is a novel target for Runx2, expressed by osteoblasts and mature growth plate chondrocytes. J. Bone Miner. Res. 2011, 26, 2911–2922. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Stavenschi, E.; Labour, M.-N.; Hoey, D.A. Oscillatory fluid flow induces the osteogenic lineage commitment of mesenchymal stem cells: The effect of shear stress magnitude, frequency, and duration. J. Biomech. 2017, 55, 99–106. [Google Scholar] [CrossRef]
- Das, R.K.; Gocheva, V.; Hammink, R.; Zouani, O.F.; Rowan, A.E. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 2015, 15, 318–325. [Google Scholar] [CrossRef]
- Fang, H.; Deng, Z.; Liu, J.; Chen, S.; Deng, Z.; Li, W. The Mechanism of Bone Remodeling After Bone Aging. Clin. Interv. Aging 2022, 17, 405–415. [Google Scholar] [CrossRef]
- Reis, J.; Ramos, A. In Sickness and in Health: The Oxygen Reactive Species and the Bone. Front. Bioeng. Biotechnol. 2021, 9, 745911. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.H.; Mori, D.; Kobayashi, H.; Mori, Y.; Nakamoto, H.; Okada, K.; Taniguchi, Y.; Sugita, S.; Yano, F.; Chung, U.-I.; et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1–NF-κB pathway. Nat. Commun. 2019, 10, 1442. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strickland, M.; Yacoubi-Loueslati, B.; Bouhaouala-Zahar, B.; Pender, S.L.F.; Larbi, A. Relationships Between Ion Channels, Mitochondrial Functions and Inflammation in Human Aging. Front. Physiol. 2019, 10, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shum, L.C.; White, N.S.; Nadtochiy, S.M.; Bentley, K.L.D.M.; Brookes, P.S.; Jonason, J.H.; Eliseev, R.A. Cyclophilin D Knock-Out Mice Show Enhanced Resistance to Osteoporosis and to Metabolic Changes Observed in Aging Bone. PLoS ONE 2016, 11, e0155709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and Where to Find Them: A Meta-Analytic Approach to Investigate Their Chemistry, Biosynthesis, Distribution, and Effect on Human Health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef]
- Đudarić, L.; Fužinac-Smojver, A.; Muhvić, D.; Giacometti, J. The role of polyphenols on bone metabolism in osteoporosis. Food Res. Int. 2015, 77, 290–298. [Google Scholar] [CrossRef]
- Torre, E. Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, L.-M.; Guo, C.; Han, J.-F. Resveratrol promotes osteoblastic differentiation in a rat model of postmenopausal osteoporosis by regulating autophagy. Nutr. Metab. 2020, 17, 29. [Google Scholar] [CrossRef] [Green Version]
- Ornstrup, M.J.; Harsløf, T.; Kjær, T.N.; Langdahl, B.L.; Pedersen, S.B. Resveratrol Increases Bone Mineral Density and Bone Alkaline Phosphatase in Obese Men: A Randomized Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2014, 99, 4720–4729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieger, N.S.; Chen, L.; Becker, J.; Chan, M.R.; Bushinsky, D.A. Deletion of the proton receptor OGR1 in mouse osteoclasts impairs metabolic acidosis-induced bone resorption. Kidney Int. 2020, 99, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Mailhot, G.; Birnbaum, M.J.; MacKay, C.A.; Mason-Savas, A.; Odgren, P.R. Expression of and Role for Ovarian Cancer G-protein-coupled Receptor 1 (OGR1) during Osteoclastogenesis. J. Biol. Chem. 2006, 281, 23598–23605. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Lai, Q.; Li, Y.; Xu, C.; Tang, X.; Ci, J.; Sun, S.; Xu, B.; Li, Y. Acidic pH environment induces autophagy in osteoblasts. Sci. Rep. 2017, 7, 46161. [Google Scholar] [CrossRef]
- Kato, K.; Morita, I. Kato Promotion of osteoclast differentiation and activation in spite of impeded osteoblast-lineage differentiation under acidosis: Effects of acidosis on bone metabolism. Biosci. Trends 2013, 7, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Pla, A.F.; Munaron, L. Functional properties of ion channels and transporters in tumour vascularization. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130103. [Google Scholar] [CrossRef] [Green Version]
- Scarpellino, G.; Munaron, L.; Cantelmo, A.R.; Pla, A.F. Calcium-Permeable Channels in Tumor Vascularization: Peculiar Sensors of Microenvironmental Chemical and Physical Cues. Rev. Physiol. Biochem. Pharmacol. 2020, 182, 111–137. [Google Scholar] [CrossRef]
- Becchetti, A.; Munaron, L.; Arcangeli, A. The role of ion channels and transporters in cell proliferation and cancer. Front. Physiol. 2013, 4, 312. [Google Scholar] [CrossRef] [Green Version]
- Munaron, L. Systems biology of ion channels and transporters in tumor angiogenesis: An omics view. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2647–2656. [Google Scholar] [CrossRef] [Green Version]
- Scarpellino, G.; Genova, T.; Quarta, E.; Distasi, C.; Dionisi, M.; Pla, A.F.; Munaron, L. P2X Purinergic Receptors Are Multisensory Detectors for Micro-Environmental Stimuli That Control Migration of Tumoral Endothelium. Cancers 2022, 14, 2743. [Google Scholar] [CrossRef]
- Zheng, M.; Kim, D.-Y.; Sung, J.-H. Ion channels and transporters in adipose-derived stem cells. J. Pharm. Investig. 2018, 49, 287–294. [Google Scholar] [CrossRef]
- Pillozzi, S.; Becchetti, A. Ion Channels in Hematopoietic and Mesenchymal Stem Cells. Stem Cells Int. 2012, 2012, 217910. [Google Scholar] [CrossRef] [Green Version]
- An, H.; Jiao, D.; Guo, S.; Chen, Y.; Zhan, Y. Ion Channels and Bone Homeostasis Imbalance. Biomed. J. Sci. Tech. Res. 2019, 16, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Jin, P.; Jan, L.Y.; Jan, Y.-N. Mechanosensitive Ion Channels: Structural Features Relevant to Mechanotransduction Mechanisms. Annu. Rev. Neurosci. 2020, 43, 207–229. [Google Scholar] [CrossRef] [Green Version]
- Botello-Smith, W.M.; Jiang, W.; Zhang, H.; Ozkan, A.D.; Lin, Y.-C.; Pham, C.N.; Lacroix, J.J.; Luo, Y. A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat. Commun. 2019, 10, 4503. [Google Scholar] [CrossRef] [Green Version]
- Cinar, E.; Zhou, S.; DeCourcey, J.; Wang, Y.; Waugh, R.E.; Wan, J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc. Natl. Acad. Sci. USA 2015, 112, 11783–11788. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, Y.-X.; Li, J. The role of mechanosensor Piezo1 in bone homeostasis and mechanobiology. Dev. Biol. 2023, 493, 80–88. [Google Scholar] [CrossRef]
- Kuntze, A.; Goetsch, O.; Fels, B.; Najder, K.; Unger, A.; Wilhelmi, M.; Sargin, S.; Schimmelpfennig, S.; Neumann, I.; Schwab, A.; et al. Protonation of Piezo1 Impairs Cell-Matrix Interactions of Pancreatic Stellate Cells. Front. Physiol. 2020, 11, 89. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, S.; Liu, H.; Ru, K.; Jia, Y.; Wu, Z.; Liang, S.; Khan, Z.; Chen, Z.; Qian, A.; et al. Piezo Channels: Awesome Mechanosensitive Structures in Cellular Mechanotransduction and Their Role in Bone. Int. J. Mol. Sci. 2021, 22, 6429. [Google Scholar] [CrossRef]
- Qin, L.; He, T.; Chen, S.; Yang, D.; Yi, W.; Cao, H.; Xiao, G. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021, 9, 44. [Google Scholar] [CrossRef]
- Hendrickx, G.; Fischer, V.; Liedert, A.; von Kroge, S.; Haffner-Luntzer, M.; Brylka, L.; Pawlus, E.; Schweizer, M.; Yorgan, T.; Baranowsky, A.; et al. Piezo 1 Inactivation in Chondrocytes Impairs Trabecular Bone Formation. J. Bone Miner. Res. 2020, 36, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, L.; Lv, L.; Hu, S.; Tariq, A.; Wang, W.; Dang, X. Fluid shear stress induces Runx-2 expression via upregulation of PIEZO1 in MC3T3-E1 cells. Cell Biol. Int. 2020, 44, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, F.; Hayashi, M.; Mouri, Y.; Nakamura, S.; Adachi, T.; Nakashima, T. Mechanotransduction via the Piezo1-Akt pathway underlies Sost suppression in osteocytes. Biochem. Biophys. Res. Commun. 2019, 521, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef] [Green Version]
- Djamgoz, M.B.; Pchelintseva, E. Mechanosensitive Ion Channels and Stem Cell Differentiation. Bioelectricity 2021, 3, 249–254. [Google Scholar] [CrossRef]
- Shen, Y.; Pan, Y.; Guo, S.; Sun, L.; Zhang, C.; Wang, L. The roles of mechanosensitive ion channels and associated downstream MAPK signaling pathways in PDLC mechanotransduction. Mol. Med. Rep. 2020, 21, 2113–2122. [Google Scholar] [CrossRef] [Green Version]
- Samanta, A.; Hughes, T.E.; Moiseenkova-Bell, V.Y. Transient receptor potential (TRP) channels. Subcell. Biochem. 2018, 87, 141–165. [Google Scholar] [CrossRef]
- Guo, J.; Shan, C.; Xu, J.; Li, M.; Zhao, J.; Cheng, W. New Insights into TRP Ion Channels in Stem Cells. Int. J. Mol. Sci. 2022, 23, 7766. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Wang, L.; Liu, Z.; Yi, Q.; Geng, B.; Chen, X.; Yu, D.; Xia, Y. The mechanosensory and mechanotransductive processes mediated by ion channels and the impact on bone metabolism: A systematic review. Arch. Biochem. Biophys. 2021, 711, 109020. [Google Scholar] [CrossRef]
- Liu, N.; Lu, W.; Dai, X.; Qu, X.; Zhu, C. The role of TRPV channels in osteoporosis. Mol. Biol. Rep. 2022, 49, 577–585. [Google Scholar] [CrossRef]
- Pla, A.F.; Ong, H.L.; Cheng, K.T.; Brossa, A.; Bussolati, B.; Lockwich, T.; Paria, B.; Munaron, L.; Ambudkar, I.S. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene 2011, 31, 200–212. [Google Scholar] [CrossRef] [Green Version]
- Pla, A.F.; Genova, T.; Pupo, E.; Tomatis, C.; Genazzani, A.; Zaninetti, R.; Munaron, L. Multiple Roles of Protein Kinase A in Arachidonic Acid–Mediated Ca2+ Entry and Tumor-Derived Human Endothelial Cell Migration. Mol. Cancer Res. 2010, 8, 1466–1476. [Google Scholar] [CrossRef] [Green Version]
- Pla, A.F.; Grange, C.; Antoniotti, S.; Tomatis, C.; Merlino, A.; Bussolati, B.; Munaron, L. Arachidonic Acid–Induced Ca2+ Entry Is Involved in Early Steps of Tumor Angiogenesis. Mol. Cancer Res. 2008, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Hope, J.; Greenlee, J.; King, M.R. Mechanosensitive Ion Channels: TRPV4 and P2X7 in Disseminating Cancer Cells. Cancer J. 2018, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Meng, N.; Wang, X.; Chen, W.; Zhang, Q. TRPV4 and PIEZO Channels Mediate the Mechanosensing of Chondrocytes to the Biomechanical Microenvironment. Membranes 2022, 12, 237. [Google Scholar] [CrossRef]
- van der Eerden, B.; Oei, L.; Roschger, P.; Fratzl-Zelman, N.; Hoenderop, J.; van Schoor, N.; Pettersson-Kymmer, U.; Schreuders-Koedam, M.; Uitterlinden, A.; Hofman, A.; et al. TRPV4 deficiency causes sexual dimorphism in bone metabolism and osteoporotic fracture risk. Bone 2013, 57, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Swain, S.M.; Liddle, R.A. Piezo1 acts upstream of TRPV4 to induce pathological changes in endothelial cells due to shear stress. J. Biol. Chem. 2021, 296, 100171. [Google Scholar] [CrossRef] [PubMed]
- O’Conor, C.J.; Leddy, H.A.; Benefield, H.C.; Liedtke, W.B.; Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. USA 2014, 111, 1316–1321. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Bellini, G.; Torella, M.; Tortora, C.; Manzo, I.; Giordano, C.; Guida, F.; Luongo, L.; Papale, F.; Rosso, F.; et al. The genetic ablation or pharmacological inhibition of TRPV1 signalling is beneficial for the restoration of quiescent osteoclast activity in ovariectomized mice. Br. J. Pharmacol. 2014, 171, 2621–2630. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Tortora, C.; Punzo, F.; Bellini, G.; Argenziano, M.; Di Paola, A.; Torella, M.; Perrotta, S. The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma. Int. J. Mol. Sci. 2019, 20, 1919. [Google Scholar] [CrossRef] [Green Version]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef] [Green Version]
- Brierley, S.M.; Castro, J.; Harrington, A.; Hughes, P.; Page, A.; Rychkov, G.; Blackshaw, A. TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J. Physiol. 2011, 589, 3575–3593. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient Receptor Potential A1 Is a Sensory Receptor for Multiple Products of Oxidative Stress. J. Neurosci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Mori, Y. TRP Channels as Sensors and Signal Integrators of Redox Status Changes. Front. Pharmacol. 2011, 2, 58. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Kuwaki, T.; Kiyonaka, S.; Numata, T.; Kozai, D.; Mizuno, Y.; Yamamoto, S.; Naito, S.; Knevels, E.; Carmeliet, P.; et al. TRPA1 underlies a sensing mechanism for O2. Nat. Chem. Biol. 2011, 7, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Mizuno, Y.; Kozai, D.; Yamamoto, S.; Kiyonaka, S.; Shibata, T.; Uchida, K.; Mori, Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels 2008, 2, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Ogata, K.; Tsumuraya, T.; Oka, K.; Shin, M.; Okamoto, F.; Kajiya, H.; Katagiri, C.; Ozaki, M.; Matsushita, M.; Okabe, K. The crucial role of the TRPM7 kinase domain in the early stage of amelogenesis. Sci. Rep. 2017, 7, 18099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanukoglu, I. ASIC and ENaC type sodium channels: Conformational states and the structures of the ion selectivity filters. FEBS J. 2016, 284, 525–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingueglia, E.; Deval, E.; Lazdunski, M. FMRFamide-gated sodium channel and ASIC channels: A new class of ionotropic receptors for FMRFamide and related peptides. Peptides 2006, 27, 1138–1152. [Google Scholar] [CrossRef]
- Xie, J.; Price, M.P.; Wemmie, J.A.; Askwith, C.C.; Welsh, M.J. ASIC3 and ASIC1 Mediate FMRFamide-Related Peptide Enhancement of H+-Gated Currents in Cultured Dorsal Root Ganglion Neurons. J. Neurophysiol. 2003, 89, 2459–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Ma, X.; Sabharwal, R.; Snitsarev, V.; Morgan, D.; Rahmouni, K.; Drummond, H.A.; Whiteis, C.A.; Costa, V.; Price, M.; et al. The Ion Channel ASIC2 Is Required for Baroreceptor and Autonomic Control of the Circulation. Neuron 2009, 64, 885–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Añoveros, J.; Samad, T.A.; Žuvela-Jelaska, L.; Woolf, C.J.; Corey, D.P. Transport and Localization of the DEG/ENaC Ion Channel BNaC1α to Peripheral Mechanosensory Terminals of Dorsal Root Ganglia Neurons. J. Neurosci. 2001, 21, 2678–2686. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-H.; Cheng, Y.-R.; Banks, R.W.; Min, M.-Y.; Bewick, G.S.; Chen, C.-C. Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors. Nat. Commun. 2016, 7, 11460. [Google Scholar] [CrossRef] [Green Version]
- Ruan, N.; Tribble, J.; Peterson, A.; Jiang, Q.; Wang, J.; Chu, X.-P. Acid-Sensing Ion Channels and Mechanosensation. Int. J. Mol. Sci. 2021, 22, 4810. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Liu, T.; Hu, W.; Qiu, C. Resveratrol inhibits the activity of acid-sensing ion channels in male rat dorsal root ganglion neurons. J. Neurosci. Res. 2022, 100, 1755–1764. [Google Scholar] [CrossRef]
- Barrett-Jolley, R.; Lewis, R.; Fallman, R.; Mobasheri, A. The emerging chondrocyte channelome. Front. Physiol. 2010, 1, 135. [Google Scholar] [CrossRef] [Green Version]
- Awayda, M.S.; Ismailov, I.I.; Berdiev, B.K.; Benos, D.J. A cloned renal epithelial Na+ channel protein displays stretch activation in planar lipid bilayers. Am. J. Physiol. Content 1995, 268, C1450–C1459. [Google Scholar] [CrossRef]
- Kellenberger, S.; Grutter, T. Architectural and Functional Similarities between Trimeric ATP-Gated P2X Receptors and Acid-Sensing Ion Channels. J. Mol. Biol. 2015, 427, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Illes, P.; Müller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2020, 178, 489–514. [Google Scholar] [CrossRef]
- Acuña-Castillo, C.; Coddou, C.; Bull, P.; Brito, J.; Huidobro-Toro, J.P. Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2X7 purinergic receptor. J. Neurochem. 2006, 101, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Coddou, C.; Stojilkovic, S.S.; Huidobro-Toro, J.P. Allosteric modulation of ATP-gated P2X receptor channels. Rev. Neurosci. 2011, 22, 335–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Liu, D.; Ke, H.Z.; Duncan, R.L.; Turner, C.H. The P2X7 Nucleotide Receptor Mediates Skeletal Mechanotransduction. J. Biol. Chem. 2005, 280, 42952–42959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, K.J.; Cabahug-Zuckerman, P.; Boorman-Padgett, J.F.; Basta-Pljakic, J.; Louie, J.; Stephen, S.; Spray, D.C.; Thi, M.M.; Seref-Ferlengez, Z.; Majeska, R.J.; et al. Estrogen depletion on In vivo osteocyte calcium signaling responses to mechanical loading. Bone 2021, 152, 116072. [Google Scholar] [CrossRef]
- Korcok, J.; Raimundo, L.N.; Ke, H.Z.; Sims, S.M.; Dixon, S.J. Extracellular nucleotides act through P2X7 receptors to activate NF-kappaB in osteoclasts. J. Bone Miner. Res. 2004, 19, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Husted, L.B.; Harsløf, T.; Stenkjær, L.; Carstens, M.; Jørgensen, N.R.; Langdahl, B.L. Functional polymorphisms in the P2X7 receptor gene are associated with osteoporosis. Osteoporos. Int. 2012, 24, 949–959. [Google Scholar] [CrossRef]
- Wesselius, A.; Bours, M.J.L.; Henriksen, Z.; Syberg, S.; Petersen, S.; Schwarz, P.; Jørgensen, N.R.; van Helden, S.; Dagnelie, P.C. Association of P2X7 receptor polymorphisms with bone mineral density and osteoporosis risk in a cohort of Dutch fracture patients. Osteoporos. Int. 2012, 24, 1235–1246. [Google Scholar] [CrossRef] [Green Version]
- Begandt, D.; E Good, M.; Keller, A.S.; DeLalio, L.J.; Rowley, C.; Isakson, B.E.; Figueroa, X.F. Pannexin channel and connexin hemichannel expression in vascular function and inflammation. BMC Cell Biol. 2017, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- Grek, C.L.; Rhett, J.M.; Bruce, J.S.; Abt, M.A.; Ghatnekar, G.S.; Yeh, E.S. Targeting connexin 43 with α-connexin carboxyl-terminal (ACT1) peptide enhances the activity of the targeted inhibitors, tamoxifen and lapatinib, in breast cancer: Clinical implication for ACT1. BMC Cancer 2015, 15, 296. [Google Scholar] [CrossRef] [Green Version]
- Sagar, G.V.; Larson, D. Carbenoxolone inhibits junctional transfer and upregulates connexin43 expression by a protein kinase A-dependent pathway. J. Cell. Biochem. 2006, 98, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Van Campenhout, R.; Gomes, A.R.; De Groof, T.W.; Muyldermans, S.; Devoogdt, N.; Vinken, M. Mechanisms Underlying Connexin Hemichannel Activation in Disease. Int. J. Mol. Sci. 2021, 22, 3503. [Google Scholar] [CrossRef]
- Penuela, S.; Bhalla, R.; Gong, X.-Q.; Cowan, K.N.; Celetti, S.J.; Cowan, B.J.; Bai, D.; Shao, Q.; Laird, D.W. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 2007, 120, 3772–3783. [Google Scholar] [CrossRef] [Green Version]
- Beyer, E.C.; Berthoud, V.M. Gap junction gene and protein families: Connexins, innexins, and pannexins. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Civitelli, R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch. Biochem. Biophys. 2008, 473, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Romanello, M.; D’Andrea, P. Dual Mechanism of Intercellular Communication in HOBIT Osteoblastic Cells: A Role for Gap-Junctional Hemichannels. J. Bone Miner. Res. 2001, 16, 1465–1476. [Google Scholar] [CrossRef]
- Jiang, J.X.; Cherian, P.P. Hemichannels Formed by Connexin 43 Play an Important Role in the Release of Prostaglandin E2by Osteocytes in Response to Mechanical Strain. Cell Commun. Adhes. 2009, 10, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Cherian, P.P.; Siller-Jackson, A.J.; Gu, S.; Wang, X.; Bonewald, L.F.; Sprague, E.; Jiang, J.X. Mechanical Strain Opens Connexin 43 Hemichannels in Osteocytes: A Novel Mechanism for the Release of Prostaglandin. Mol. Biol. Cell 2005, 16, 3100–3106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genetos, D.; Kephart, C.J.; Zhang, Y.; Yellowley, C.E.; Donahue, H.J. Oscillating fluid flow activation of gap junction hemichannels induces atp release from MLO-Y4 osteocytes. J. Cell. Physiol. 2007, 212, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, S.; Xie, L.H.; John, S.A.; Subramaniam, S.; Lal, R. A Novel Role for Connexin Hemichannel in Oxidative Stress and Smoking-Induced Cell Injury. PLoS ONE 2007, 2, e712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retamal, M.A.; Schalper, K.A.; Shoji, K.F.; Bennett, M.V.L.; Sáez, J.C. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc. Natl. Acad. Sci. USA 2007, 104, 8322–8327. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, B.S.; Toft-Bertelsen, T.L.; Lolansen, S.D.; Anderson, C.L.; Nielsen, M.S.; Thompson, R.J.; MacAulay, N. Pannexin 1 activation and inhibition is permeant-selective. J. Physiol. 2020, 598, 361–379. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Stains, J.P. Connexins and pannexins in the skeleton: Gap junctions, hemichannels and more. Cell. Mol. Life Sci. 2015, 72, 2853–2867. [Google Scholar] [CrossRef] [Green Version]
- Locovei, S.; Scemes, E.; Qiu, F.; Spray, D.C.; Dahl, G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett. 2007, 581, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Cabahug-Zuckerman, P.; Stout, R.F.; Majeska, R.J.; Thi, M.M.; Spray, D.C.; Weinbaum, S.; Schaffler, M.B. Potential role for a specialized β3integrin-based structure on osteocyte processes in bone mechanosensation. J. Orthop. Res. 2017, 36, 642–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poornima, V.; Madhupriya, M.; Kootar, S.; Sujatha, G.; Kumar, A.; Bera, A.K. P2X7 Receptor–Pannexin 1 Hemichannel Association: Effect of Extracellular Calcium on Membrane Permeabilization. J. Mol. Neurosci. 2011, 46, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef] [Green Version]
- Parzych, K.; Zetterqvist, A.V.; Wright, W.R.; Kirkby, N.S.; Mitchell, J.A.; Paul-Clark, M.J. Differential role of pannexin-1/ATP/ P2X 7 axis in IL-1β release by human monocytes. FASEB J. 2017, 31, 2439–2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, B.L.; Penuela, S. Pannexin 3 channels in health and disease. Purinergic Signal 2021, 17, 577–589. [Google Scholar] [CrossRef]
- Moon, P.M.; Penuela, S.; Barr, K.; Khan, S.; Pin, C.; Welch, I.; Attur, M.; Abramson, S.B.; Laird, D.W.; Beier, F. Deletion of Panx3 Prevents the Development of Surgically Induced Osteoarthritis. J. Mol. Med. 2015, 93, 845–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeppel, J.P.; Crist, J.D.; Anderson, H.C.; Wang, J. Molecular regulation of articular chondrocyte function and its significance in osteoarthritis. Histol. Histopathol. 2011, 26, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Knight, M.M. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J. Orthop. Res. 2009, 28, 510–515. [Google Scholar] [CrossRef] [PubMed]
| Protein | Mechano | pH | OS | Cell Types | Physiological Process | Ref. |
|---|---|---|---|---|---|---|
| Piezo1 | X | MSC | ↑ osteoblast differentiation | [17] | ||
| ↓ adipogenic differentiation | ||||||
| ↓ osteoporosis | [18] | |||||
| X | osteoblast | ↑ osteoblast differentiation | [19] | |||
| mechanotransductive processes | ||||||
| X | osteocyte | ↑ osteogenesis | [20] | |||
| mechanotransductive processes | ||||||
| chondrocyte (in vivo mouse model) | ↑ cartilage degradation | [19] | ||||
| X | HEK (transfected) | [21] | ||||
| TRPV4 | X | MSC | ↑ osteogenesis | [22] | ||
| X | mechanotransductive processes | [23] | ||||
| X | ↓ osteoblast differentiation | [24] | ||||
| X | osteoblast | mechanotransductive processes | [25,26] | |||
| X | osteoclast | ↑ osteoclast survival | [27] | |||
| X | X | ↑ osteoclast differentiation | [28,29] | |||
| X | X | osteocyte | ↑ osteogenesis | [30] | ||
| X | mechanotransductive processes | [31] | ||||
| X | X | chondrocyte | ↑ macrophages infiltration | [32,33] | ||
| X | mechanotransductive process | [34] | ||||
| X | X | synoviocyte | [32,35] | |||
| TRPV1 | X | X | osteoblast | ↑ osteoblast proliferation ↑ osteoblast differentiation | [36,37] | |
| TRPV1 TRPV2 TRPV4 | X | osteoclast | ↑ osteoclast formation | [29,37,38,39,40] | ||
| TRPA1 | X | osteoclast | ↑ osteoclast formation | [41,42] | ||
| MSC | [43] | |||||
| X | periodontal ligament cells | mechanotransduction | [41,42] | |||
| X | odontoblast-like cell | inflammation | [44] | |||
| bone cancer cell | [45,46] | |||||
| X | artificial bilayer | mechanotransduction | [47] | |||
| X | prostate CAF | [48] | ||||
| TRPM7 | X | MSC | ↑ osteoblast differentiation | [49] | ||
| ↑ chondrogenesis | [50] | |||||
| pre osteoblast | ↑ osteoblastic differentiation | [51] | ||||
| X | odontoblast (in vivo mouse model) | mechanotransductive processes | [52,53] | |||
| X | osteoblast | [49,54] | ||||
| ASIC1-4 | X | MSC | ↑ osteoclastogenesis (ASIC2) ↓ osteoblastogenesis | [55] | ||
| X | MSC | mechanotransductive processes | [56] | |||
| X | bone cancer cell | [57] | ||||
| ENaC | osteoblast | ↑ osteoblast proliferation and differentiation | [58,59] | |||
| X | chondrocyte | mechanotransductive processes | [60,61] | |||
| osteoclast | ↑ osteoclast differentiation | [62] | ||||
| X | mpkCCDc14 | [63] | ||||
| P2X7 | X | MSC | mechanotransductive processes | [64] | ||
| X | osteoblast | [65] | ||||
| X | osteocyte | [45] | ||||
| X | osteoblast | ↑ osteoblast differentiation | [66,67] | |||
| X | osteoclast | ↑ osteoclast survival and differentiation | [64,68] | |||
| P2X2/3/5 | osteoclast | [69,70] | ||||
| Cx43 | X | osteoblast | ↑ osteoblast differentiation | [71] | ||
| X | osteocyte | [72,73] | ||||
| X | HeLa | [74] | ||||
| Panx1 | X | osteoblast | mechanotransductive processes (in complex with P2X7) | [45,71,75,76] | ||
| osteocyte | ||||||
| X | zebrafish retina (in vivo) | [77,78] | ||||
| Panx3 | osteoblast | ↑ osteogenesis | [79,80] | |||
| MSC | ↑ osteoblast differentiation | |||||
| X | osteoblast (MC3T3 and in vivo mouse model) | ↑ osteoblastogenesis | [79,81,82] | |||
| X | X | chondrocyte (in vivo mouse model) | ↑ chondrogenesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perin, M.; Chinigò, G.; Genova, T.; Mussano, F.; Munaron, L. The Impact of Plasma Membrane Ion Channels on Bone Remodeling in Response to Mechanical Stress, Oxidative Imbalance, and Acidosis. Antioxidants 2023, 12, 689. https://doi.org/10.3390/antiox12030689
Perin M, Chinigò G, Genova T, Mussano F, Munaron L. The Impact of Plasma Membrane Ion Channels on Bone Remodeling in Response to Mechanical Stress, Oxidative Imbalance, and Acidosis. Antioxidants. 2023; 12(3):689. https://doi.org/10.3390/antiox12030689
Chicago/Turabian StylePerin, Martina, Giorgia Chinigò, Tullio Genova, Federico Mussano, and Luca Munaron. 2023. "The Impact of Plasma Membrane Ion Channels on Bone Remodeling in Response to Mechanical Stress, Oxidative Imbalance, and Acidosis" Antioxidants 12, no. 3: 689. https://doi.org/10.3390/antiox12030689
APA StylePerin, M., Chinigò, G., Genova, T., Mussano, F., & Munaron, L. (2023). The Impact of Plasma Membrane Ion Channels on Bone Remodeling in Response to Mechanical Stress, Oxidative Imbalance, and Acidosis. Antioxidants, 12(3), 689. https://doi.org/10.3390/antiox12030689











