The Extracellular Matrix Vitalizer RATM Increased Skin Elasticity by Modulating Mitochondrial Function in Aged Animal Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of RA
2.2. In Vitro Model
2.2.1. Cell Culture
2.2.2. NA, AA, and RA Treatment
2.3. In Vivo Model
2.3.1. Mouse Conditions
2.3.2. RA Treatment
2.3.3. Skin Elasticity
2.4. Sample Preparation
2.4.1. Protein Isolation
2.4.2. RNA Extraction and cDNA Synthesis
2.4.3. Paraffin-Embedded Tissue
2.5. Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase and SOD Activity
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Western Blotting
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT–PCR)
2.9. Immunohistochemistry
2.10. Histological Analysis
2.10.1. Periodic Acid-Schiff (PAS) Staining
2.10.2. Masson’s Trichrome Staining
2.11. Transmission Electron Microscopy (TEM)
2.12. Statistical Analysis
3. Results
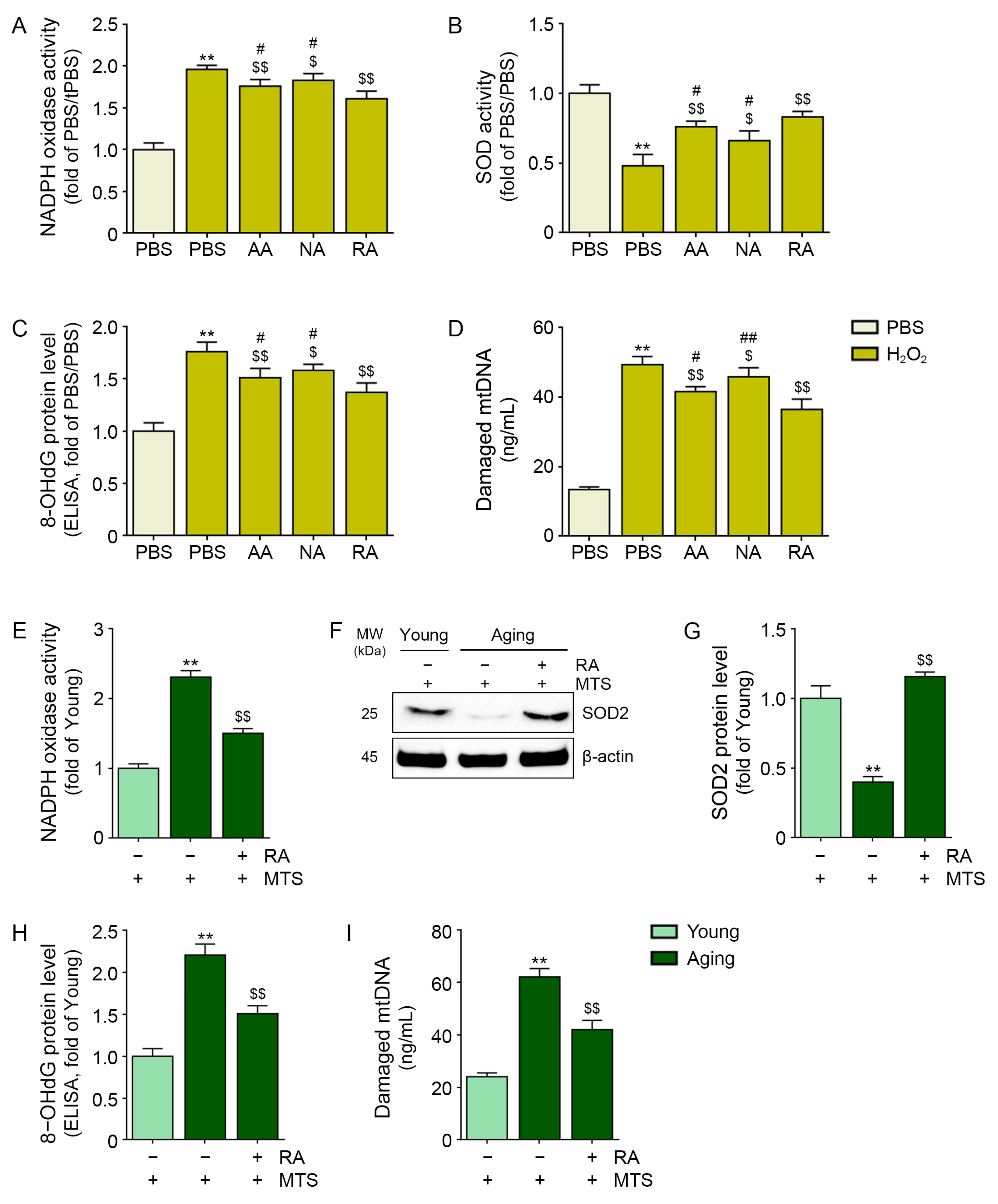
3.1. RA Decreased Oxidative Stress in H2O2-Treated Keratinocytes and Aged Animal Skin
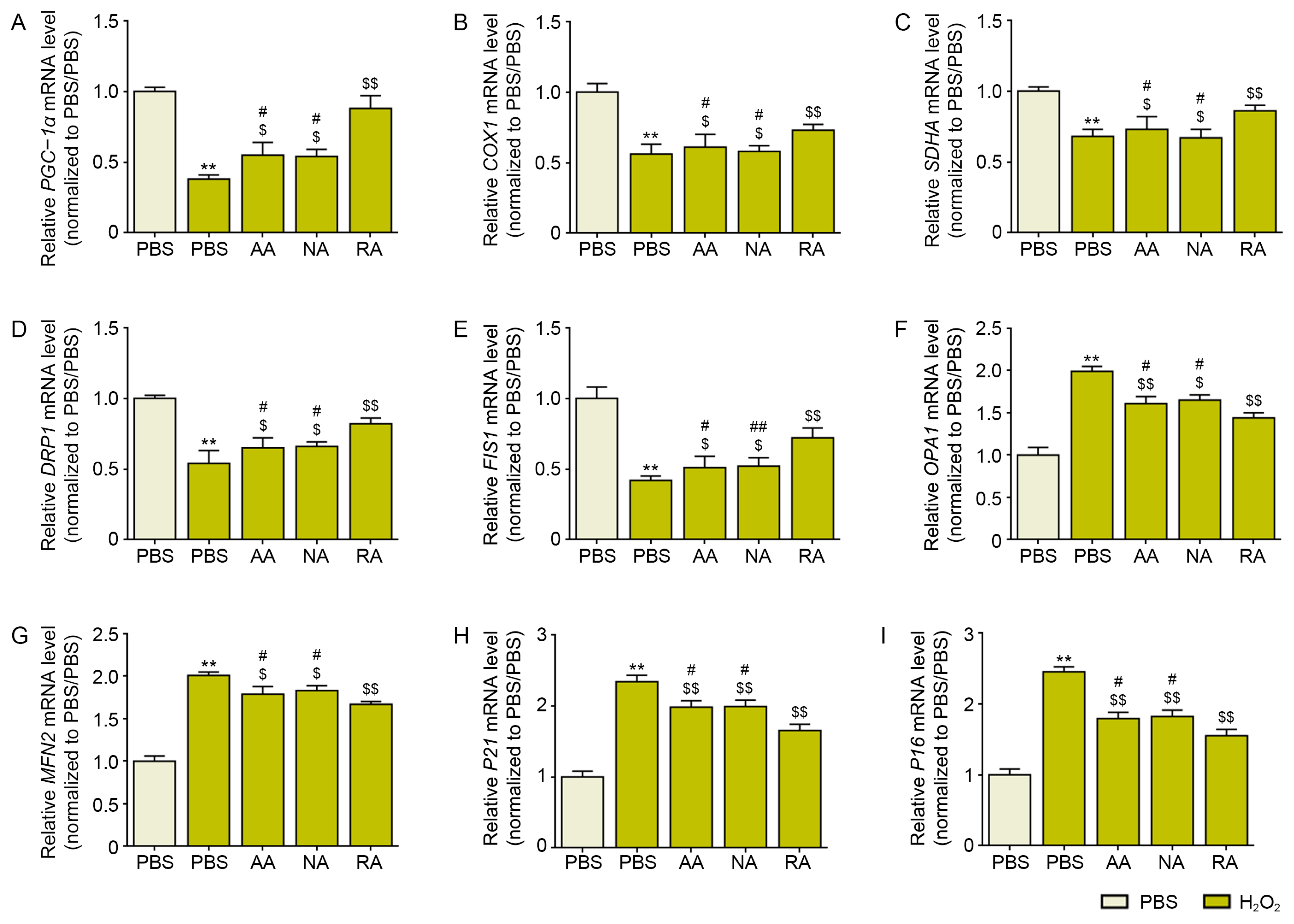
3.2. RA Decreased Mitochondrial Dysfunction and Cellular Senescence in H2O2-Treated Keratinocytes and Aged Skin
3.3. RA Decreased NF-Κb/AP-1 and MMP1/2/3/9 Expression in Aged Skin
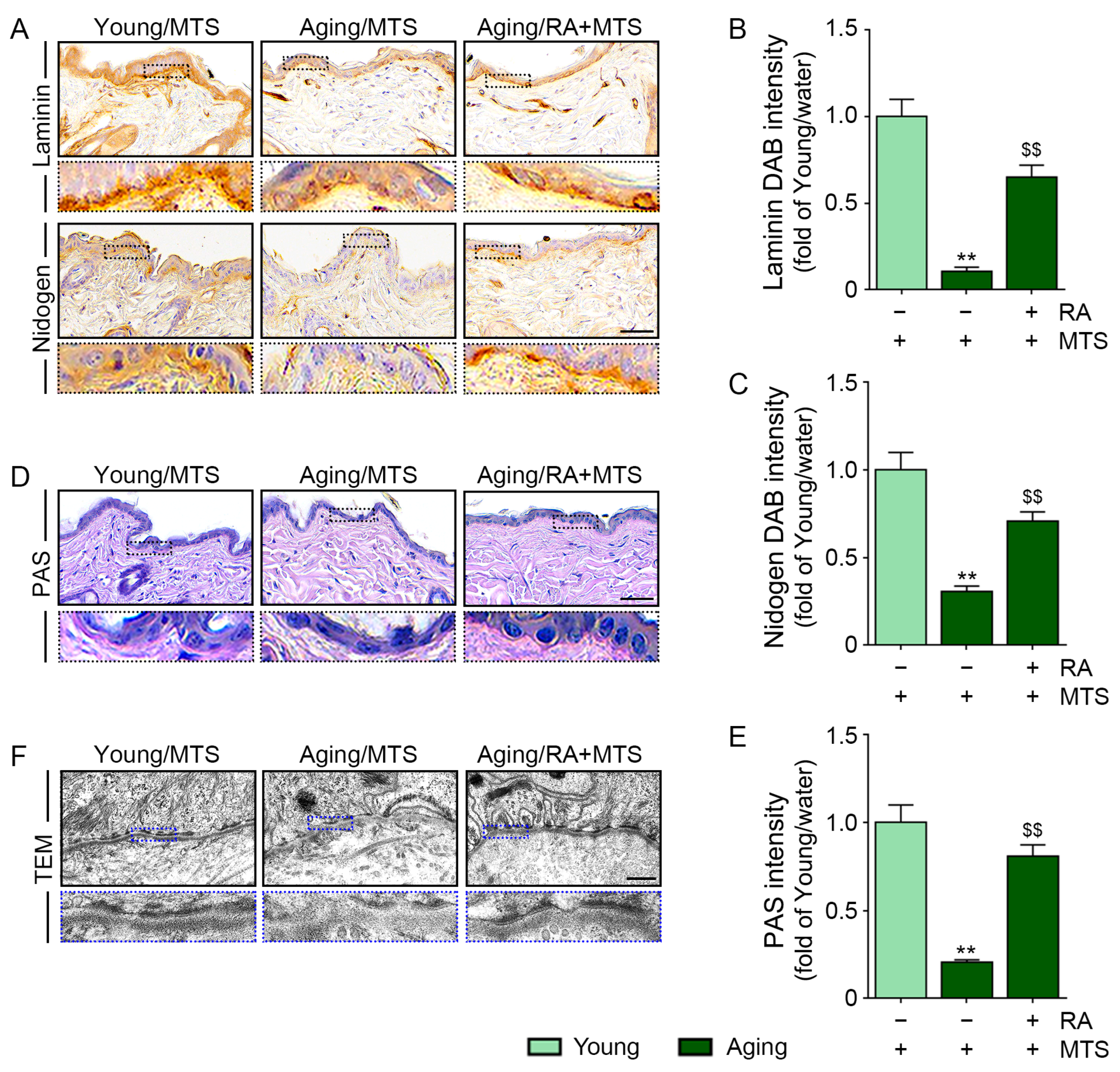
3.4. RA Increased the Expression of Laminin and Nidogen and the BM Density
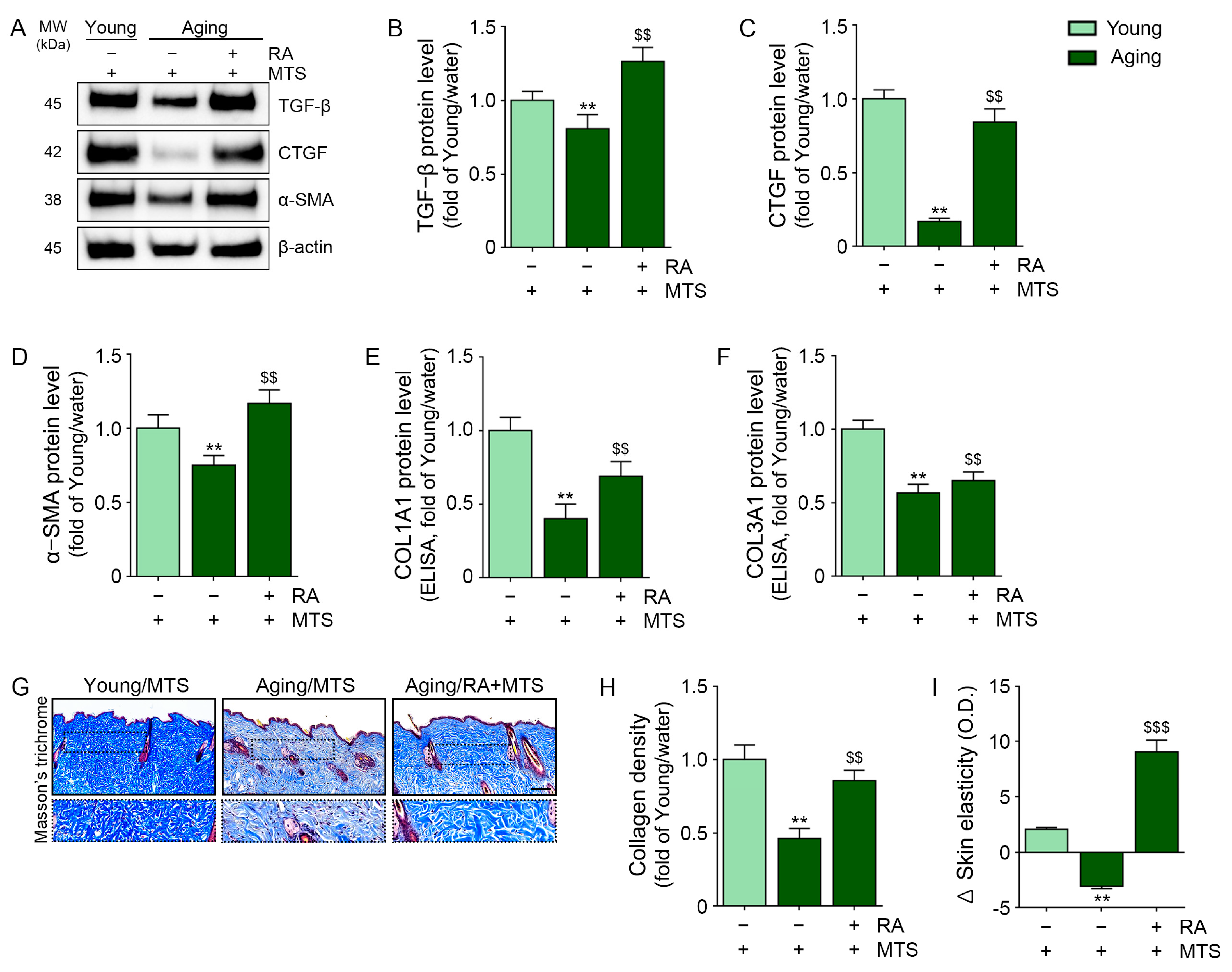
3.5. RA Upregulated the Expression of TGF-Β, CTGF, and α-Smooth Muscle Actin (A-SMA) and Collagen Fiber Accumulation in Aged Skin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kandola, K.; Bowman, A.; Birch-Machin, M.A. Oxidative stress—A key emerging impact factor in health, ageing, lifestyle and aesthetics. Int. J. Cosmet. Sci. 2015, 37 (Suppl. 2), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthet. Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Ahsanuddin, S.; Lam, M.; Baron, E.D. Skin aging and oxidative stress. AIMS Mol. Sci. 2016, 3, 187–195. [Google Scholar] [CrossRef]
- Wlaschek, M.; Briviba, K.; Stricklin, G.P.; Sies, H.; Scharffetter-Kochanek, K. Singlet oxygen may mediate the ultraviolet A-induced synthesis of interstitial collagenase. J. Investig. Dermatol. 1995, 104, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenneisen, P.; Briviba, K.; Wlaschek, M.; Wenk, J.; Scharffetter-Kochanek, K. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radic. Biol. Med. 1997, 22, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.K.; Melendez, J.A. Mitochondrial redox control of matrix metalloproteinases. Free Radic. Biol. Med. 2004, 37, 768–784. [Google Scholar] [CrossRef]
- He, T.; Quan, T.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Oxidative exposure impairs TGF-β pathway via reduction of type II receptor and SMAD3 in human skin fibroblasts. Age 2014, 36, 9623. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Birch-Machin, M.A. The role of mitochondria in ageing and carcinogenesis. Clin. Exp. Dermatol. 2006, 31, 548–552. [Google Scholar] [CrossRef]
- Anderson, A.; Bowman, A.; Boulton, S.J.; Manning, P.; Birch-Machin, M.A. A role for human mitochondrial complex II in the production of reactive oxygen species in human skin. Redox Biol. 2014, 2, 1016–1022. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Woo, S.W.; Kim, M.B.; Kim, C.; Hwang, J.K. Standardized Kaempferia parviflora Extract Inhibits Intrinsic Aging Process in Human Dermal Fibroblasts and Hairless Mice by Inhibiting Cellular Senescence and Mitochondrial Dysfunction. Evid. Based Complement. Alternat. Med. 2017, 2017, 6861085. [Google Scholar] [CrossRef] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Berneburg, M.; Lehmann, A.R. Xeroderma pigmentosum and related disorders: Defects in DNA repair and transcription. Adv. Genet. 2001, 43, 71–102. [Google Scholar]
- Berneburg, M.; Krutmann, J. Photoaging-associated large-scale deletions of mitochondrial DNA. Methods Enzymol. 2000, 319, 366–376. [Google Scholar]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Kornfeld, O.S.; Qvit, N.; Haileselassie, B.; Shamloo, M.; Bernardi, P.; Mochly-Rosen, D. Interaction of mitochondrial fission factor with dynamin related protein 1 governs physiological mitochondrial function in vivo. Sci. Rep. 2018, 8, 14034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, Y.S.; Yoon, D.S.; Lim, I.K.; Yoon, S.-H.; Chung, H.-Y.; Rojo, M.; Malka, F.; Jou, M.-J.; Martinou, J.-C.; Yoon, G. Formation of elongated giant mitochondria in DFO-induced cellular senescence: Involvement of enhanced fusion process through modulation of Fis1. J. Cell. Physiol. 2006, 209, 468–480. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, S.Y.; Lim, W.C.; Kim, S.; Park, Y.-Y.; Sun, X.; Youle, R.J.; Cho, H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J. Biol. Chem. 2007, 282, 22977–22983. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Hong, Y.; Tran, Q.; Cho, H.; Kim, M.; Kim, C.; Kwon, S.H.; Park, S.; Park, J.; Park, J. A new role for the ginsenoside RG3 in antiaging via mitochondria function in ultraviolet-irradiated human dermal fibroblasts. J. Ginseng. Res. 2019, 43, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Berneburg, M.; Gattermann, N.; Stege, H.; Grewe, M.; Vogelsang, K.; Ruzicka, T.; Krutmann, J. Chronically ultraviolet-exposed human skin shows a higher mutation frequency of mitochondrial DNA as compared to unexposed skin and the hematopoietic system. Photochem. Photobiol. 1997, 66, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Barnes, P.J.; Passos, J.F. Mitochondria, telomeres and cell senescence: Implications for lung ageing and disease. Pharmacol. Ther. 2018, 183, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Birch-Machin, M.A.; Tindall, M.; Turner, R.; Haldane, F.; Rees, J.L. Mitochondrial DNA deletions in human skin reflect photo- rather than chronologic aging. J. Investig. Dermatol. 1998, 110, 149–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passos, J.F.; Saretzki, G.; von Zglinicki, T. DNA damage in telomeres and mitochondria during cellular senescence: Is there a connection? Nucleic Acids Res. 2007, 35, 7505–7513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ressler, S.; Bartkova, J.; Niederegger, H.; Bartek, J.; Scharffetter-Kochanek, K.; Jansen-Durr, P.; Wlaschek, M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 2006, 5, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Waaijer, M.E.; Gunn, D.A.; Adams, P.D.; Pawlikowski, J.S.; Griffiths, C.E.M.; van Heemst, D.; Slagboom, P.E.; Westendorp, R.G.J.; Maier, A.B. P16INK4a Positive Cells in Human Skin Are Indicative of Local Elastic Fiber Morphology, Facial Wrinkling, and Perceived Age. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1022–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurchenco, P.D.; Patton, B.L. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 2009, 15, 1277–1294. [Google Scholar] [CrossRef] [Green Version]
- Amano, S. Possible involvement of basement membrane damage in skin photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Iriyama, S.; Matsunaga, Y.; Takahashi, K.; Matsuzaki, K.; Kumagai, N.; Amano, S. Activation of heparanase by ultraviolet B irradiation leads to functional loss of basement membrane at the dermal-epidermal junction in human skin. Arch. Dermatol. Res. 2011, 303, 253–261. [Google Scholar] [CrossRef]
- Lavker, R.M. Structural alterations in exposed and unexposed aged skin. J. Investig. Dermatol. 1979, 73, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Q.; Mauviel, A.; Ryynänen, J.; Sollberg, S.; Uitto, J. Type VII collagen gene expression by human skin fibroblasts and keratinocytes in culture: Influence of donor age and cytokine responses. J. Investig. Dermatol. 1994, 102, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Feru, J.; Delobbe, E.; Ramont, L.; Brassart, B.; Terryn, C.; Dupont-Deshorgue, A.; Garbar, C.; Monboisse, J.-C.; Maquart, F.-X.; Brassart-Pasco, S. Aging decreases collagen IV expression in vivo in the dermo-epidermal junction and in vitro in dermal fibroblasts: Possible involvement of TGF-β1. Eur. J. Dermatol. 2016, 26, 350–360. [Google Scholar] [CrossRef]
- Jeong, S.; Yoon, S.; Kim, S.; Jung, J.; Kor, M.; Shin, K.; Lim, C.; Han, H.S.; Lee, H.; Park, K.-Y.; et al. Anti-Wrinkle Benefits of Peptides Complex Stimulating Skin Basement Membrane Proteins Expression. Int. J. Mol. Sci. 2019, 21, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blake, S. Vitamins and Minerals Demystifie; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Combs, G.F.; McClung, J.P. The Vitamins. Fundamental Aspects in Nutrition and Health, 4th ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef] [Green Version]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD+ in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef]
- Iraji, F.; Nasimi, M.; Asilian, A.; Faghihi, G.; Mozafarpoor, S.; Hafezi, H. Efficacy of mesotherapy with tranexamic acid and ascorbic acid with and without glutathione in treatment of melasma: A split face comparative trial. J. Cosmet. Dermatol. 2019, 10, 1416–1421. [Google Scholar] [CrossRef]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- Ardizzoni, A.; Neglia, R.G.; Baschieri, M.C.; Cermelli, C.; Caratozzolo, M.; Righi, E.; Palmieri, B.; Blasi, E. Influence of hyaluronic acid on bacterial and fungal species, including clinically relevant opportunistic pathogens. J. Mater. Sci. Mater. Med. 2011, 22, 2329–2338. [Google Scholar] [CrossRef] [Green Version]
- Jäger, C.; Brenner, C.; Habicht, J.; Wallich, R. Bioactive reagents used in mesotherapy for skin rejuvenation in vivo induce diverse physiological processes in human skin fibroblasts in vitro- a pilot study. Exp. Dermatol. 2012, 21, 72–75. [Google Scholar] [CrossRef]
- Du, L.; Chen, E.; Wu, T.; Ruan, Y.; Wu, S. Resveratrol attenuates hydrogen peroxide-induced aging through upregulation of autophagy in human umbilical vein endothelial cells. Drug Des. Devel. Ther. 2019, 13, 747–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, Y.A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Cividini, F.; Scott, B.T.; Dai, A.; Han, W.; Suarez, J.; Diaz-Juarez, J.; Diemer, T.; Casteel, D.; Dillmann, W. O-GlcNAcylation of 8-Oxoguanine DNA Glycosylase (Ogg1) Impairs Oxidative Mitochondrial DNA Lesion Repair in Diabetic Hearts. J. Biol. Chem. 2016, 291, 26515–26528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustyniak, J.; Lenart, J.; Zychowicz, M.; Stepien, P.P.; Buzanska, L. Mitochondrial biogenesis and neural differentiation of human iPSC is modulated by idebenone in a developmental stage-dependent manner. Biogerontology 2017, 18, 665–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef]
- Inoue, S. Ultrastructure of basement membranes. Int. Rev. Cytol. 1989, 117, 57–98. [Google Scholar] [PubMed]
- Bagalad, B.S.; Mohan Kumar, K.P.; Puneeth, H.K. Myofibroblasts: Master of disguise. J. Oral Maxillofac. Pathol. 2017, 21, 462–463. [Google Scholar] [CrossRef]
- Lombardi, F.; Palumbo, P.; Augello, F.R.; Giusti, I.; Dolo, V.; Guerrini, L.; Cifone, M.G.; Giuliani, M.; Cinque, B. Type I Collagen Suspension Induces Neocollagenesis and Myodifferentiation in Fibroblasts In Vitro. BioMed Res. Int. 2020, 2020, 6093974. [Google Scholar] [CrossRef]
- Watanabe, S.; Kawamoto, S.; Ohtani, N.; Hara, E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017, 108, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23 (Suppl. 1), 7–12. [Google Scholar] [CrossRef]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef] [PubMed]
- Fleischmajer, R.; Perlish, J.S.; Timpl, R. Collagen fibrillogenesis in human skin. Ann. N. Y. Acad. Sci. 1985, 460, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Licia, G. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Nolte, S.V.; Xu, W.; Rennekampff, H.O.; Rodemann, H.P. Diversity of fibroblasts--a review on implications for skin tissue engineering. Cells Tissues Organs 2008, 187, 165–176. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Oikarinen, A. The aging of skin: Chronoaging versus photoaging. Photodermatol. Photoimmunol. Photomed. 1990, 7, 3–4. [Google Scholar] [PubMed]
- Wall, I.B.; Bhadal, N.; Broad, S.; Whawell, S.A.; Mudera, V.; Lewis, M.P. Force generation and protease gene expression in organotypic co-cultures of fibroblasts and keratinocytes. J. Tissue Eng. Regen. Med. 2009, 3, 647–650. [Google Scholar] [CrossRef]
- Tandara, A.A.; Kloeters, O.; Mogford, J.E.; Mustoe, T.A. Hydrated keratinocytes reduce collagen synthesis by fibroblasts via paracrine mechanisms. Wound Repair Regen. 2007, 15, 497–504. [Google Scholar] [CrossRef]
- Smola, H.; Thiekötter, G.; Fusenig, N.E. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J. Cell Biol. 1993, 122, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Yokota, M.; Kamiya, Y.; Suzuki, T.; Ishikawa, S.; Takeda, A.; Kondo, S.; Tohgasaki, T.; Nakashima, T.; Takahashi, Y.; Ōmura, S.; et al. Trehangelins ameliorate inflammation-induced skin senescence by suppressing the epidermal YAP-CCN1 axis. Sci. Rep. 2022, 12, 952. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poljsak, B.; Milisav, I. The neglected significance of “antioxidative stress”. Oxid. Med. Cell. Longev. 2012, 2012, 480895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandy, B.; Davison, A.J. Mitochondrial mutations may increase oxidative stress: Implications for carcinogenesis and aging? Free Radic. Biol. Med. 1990, 8, 523–539. [Google Scholar] [CrossRef]
- Hiona, A.; Leeuwenburgh, C. The role of mitochondrial DNA mutations in aging and sarcopenia: Implications for the mitochondrial vicious cycle theory of aging. Exp. Gerontol. 2008, 43, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokolenko, I.; Venediktova, N.; Bochkareva, A.; Wilson, G.L.; Alexeyev, M.F. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009, 37, 2539–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iriyama, S.; Nishikawa, S.; Hosoi, J.; Amano, S. Basement Membrane Helps Maintain Epidermal Hyaluronan Content. Am. J. Pathol. 2021, 191, 1010–1019. [Google Scholar] [CrossRef]
- Mondon, P.; Hillion, M.; Peschard, O.; Andre, N.; Marchand, T.; Doridot, E.; Feuilloley, M.G.; Pionneau, C.; Chardonnet, S. Evaluation of dermal extracellular matrix and epidermal-dermal junction modifications using matrix-assisted laser desorption/ionization mass spectrometric imaging, in vivo reflectance confocal microscopy, echography, and histology: Effect of age and peptide applications. J. Cosmet. Dermatol. 2015, 14, 152–160. [Google Scholar] [PubMed]
- Watanabe, M.; Natsuga, K.; Nishie, W.; Kobayashi, Y.; Donati, G.; Suzuki, S.; Fujimura, Y.; Tsukiyama, T.; Ujiie, H.; Shinkuma, S.; et al. Type XVII collagen coordinates proliferation in the interfollicular epidermis. Elife 2017, 6, e26635. [Google Scholar] [CrossRef] [PubMed]
- Iorio, V.; Troughton, L.D.; Hamill, K.J. Laminins: Roles and Utility in Wound Repair. Adv. Wound Care (New Rochelle) 2015, 4, 250–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, A.J.; Al-Bader, T.; Kerrigan, D.; Hickey, S.; Laloeuf, A.; Rawlings, A.V. Synergistic action of a triple peptide complex on an essential extracellular matrix protein exhibits significant anti-aging benefits. J. Cosmet. Dermatol. 2010, 9, 108–116. [Google Scholar] [CrossRef]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57–58, 1–11. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, K.-A.; Oh, S.; Batsukh, S.; Kim, M.J.; Lee, J.H.; Park, H.J.; Chung, M.S.; Son, K.H.; Byun, K. The Extracellular Matrix Vitalizer RATM Increased Skin Elasticity by Modulating Mitochondrial Function in Aged Animal Skin. Antioxidants 2023, 12, 694. https://doi.org/10.3390/antiox12030694
Byun K-A, Oh S, Batsukh S, Kim MJ, Lee JH, Park HJ, Chung MS, Son KH, Byun K. The Extracellular Matrix Vitalizer RATM Increased Skin Elasticity by Modulating Mitochondrial Function in Aged Animal Skin. Antioxidants. 2023; 12(3):694. https://doi.org/10.3390/antiox12030694
Chicago/Turabian StyleByun, Kyung-A, Seyeon Oh, Sosorburam Batsukh, Min Jeong Kim, Je Hyuk Lee, Hyun Jun Park, Moon Suk Chung, Kuk Hui Son, and Kyunghee Byun. 2023. "The Extracellular Matrix Vitalizer RATM Increased Skin Elasticity by Modulating Mitochondrial Function in Aged Animal Skin" Antioxidants 12, no. 3: 694. https://doi.org/10.3390/antiox12030694




