Safe-Shields: Basal and Anti-UV Protection of Human Keratinocytes by Redox-Active Cerium Oxide Nanoparticles Prevents UVB-Induced Mutagenesis
Abstract
:1. Introduction
2. Materials and Methods
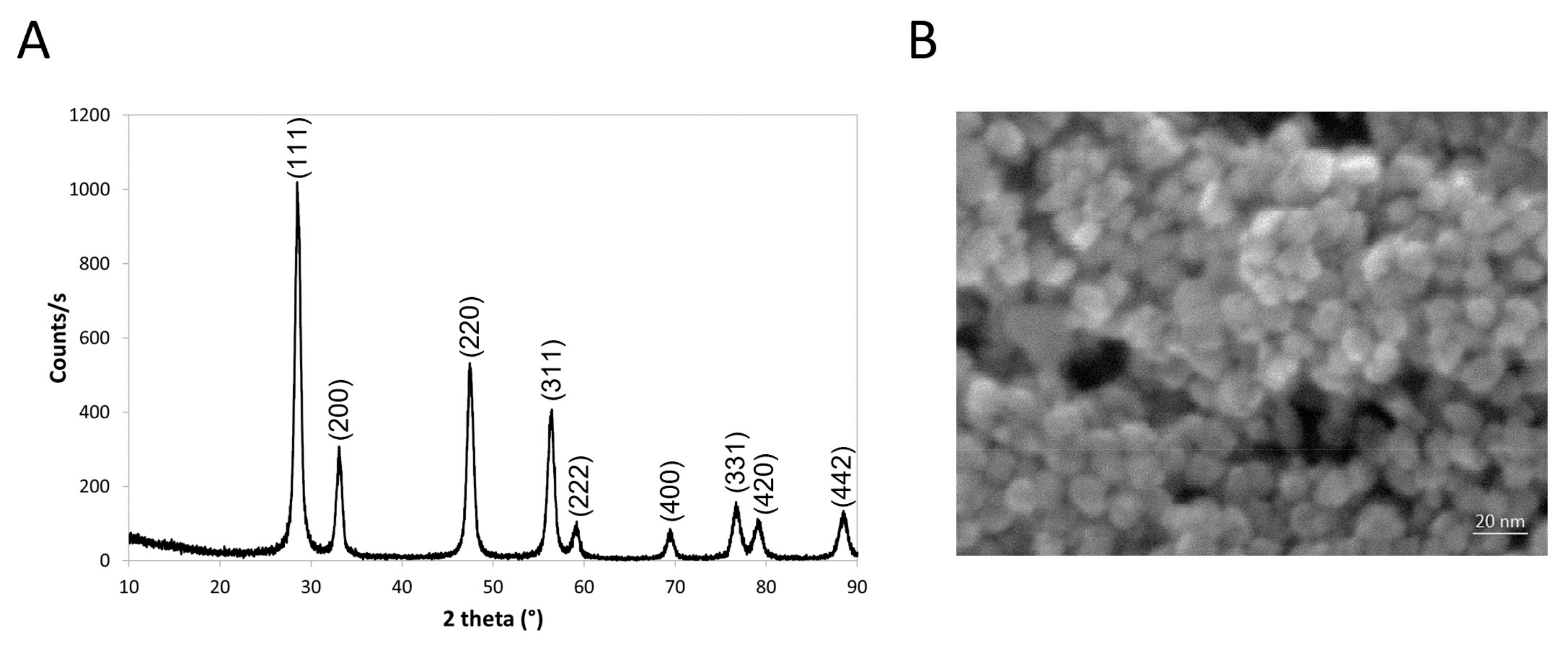
2.1. Nanoparticle Synthesis and Characterization
2.1.1. Cerium Oxide Synthesis
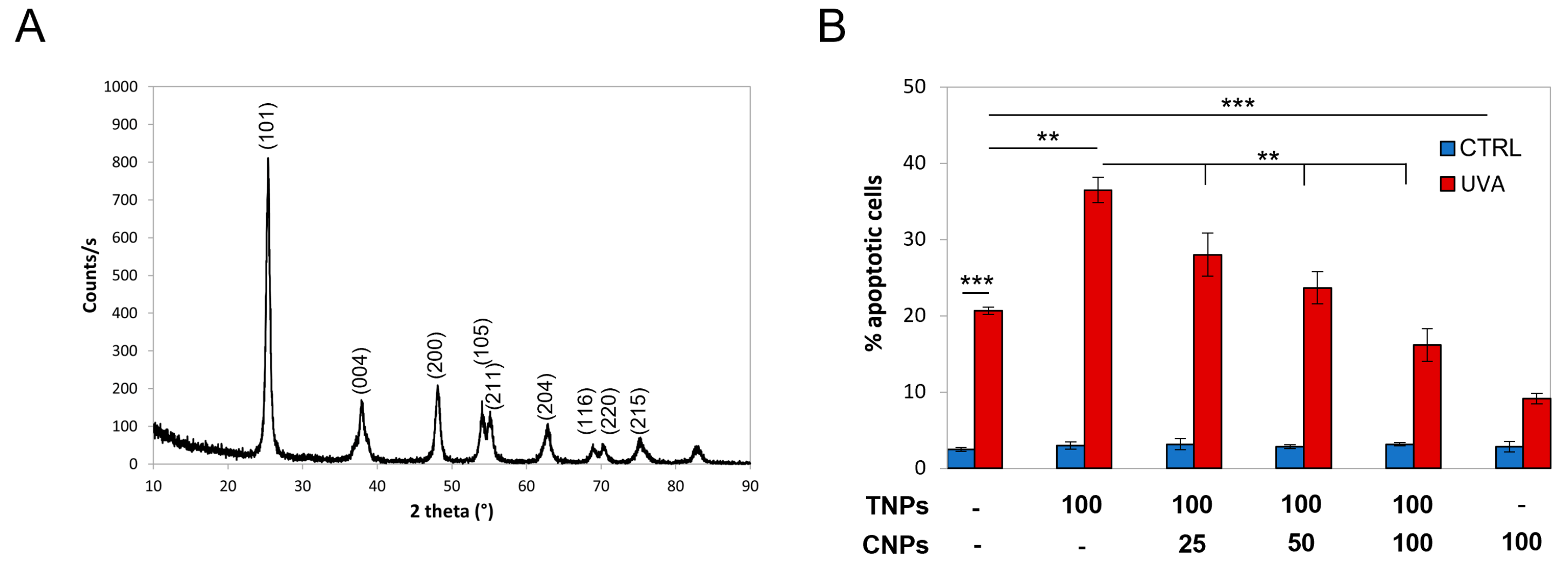
2.1.2. Titanium Dioxide Nanoparticles Synthesis
2.1.3. Nanoparticles Physicochemical Characterization
2.2. Cell Culture
2.2.1. Cells
2.2.2. Nanoparticle Administration
2.2.3. Cell Irradiation Protocols
2.2.4. Evaluation of Apoptosis
2.2.5. DNA Damage Analysis by the Alkaline Comet Assay
2.2.6. Phospho-Histone H2AX: Microscope Immunofluorescence
2.2.7. Micronucleus Cytome Assay
2.3. Statistical Analysis
3. Results
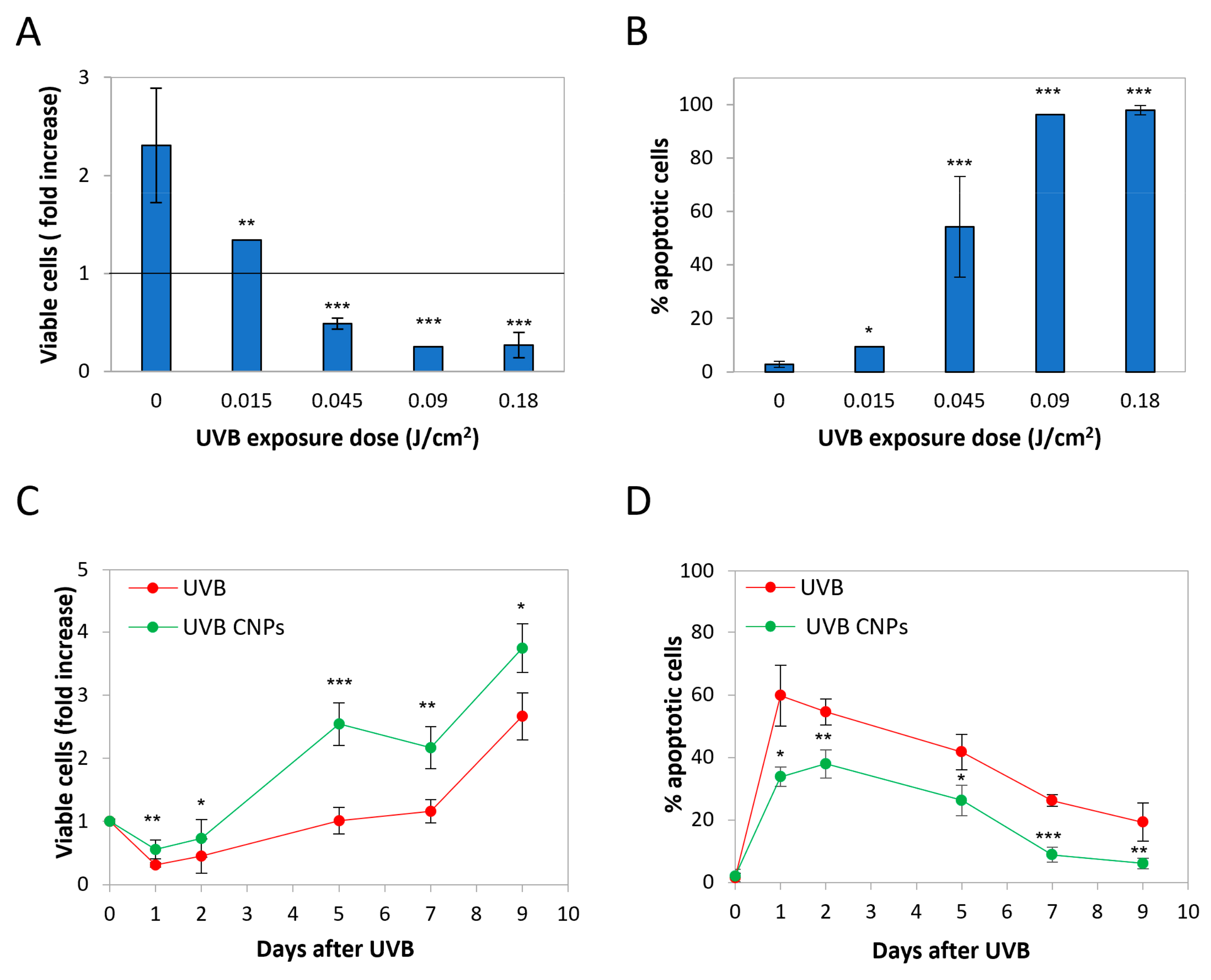
3.1. Nanoceria Reduce UVB-Induced Cytotoxicity in HaCaT Cells
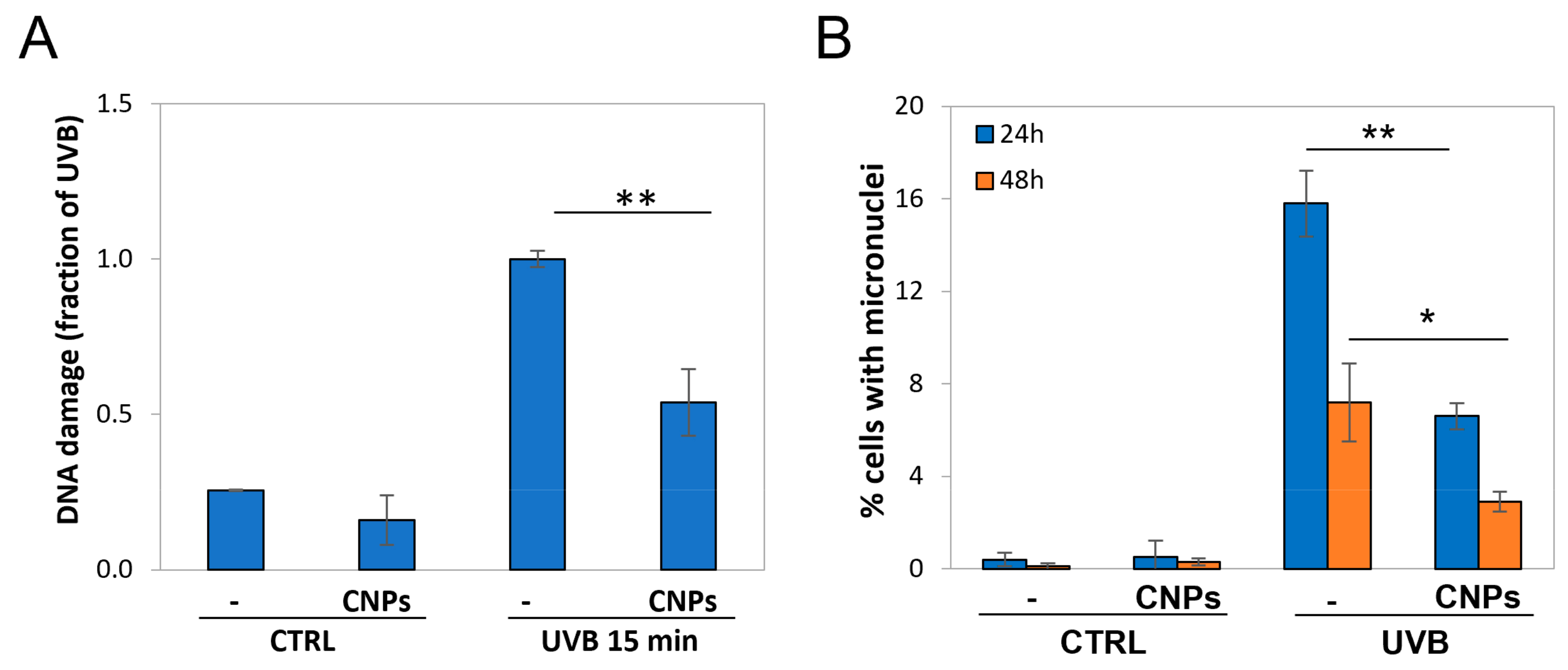
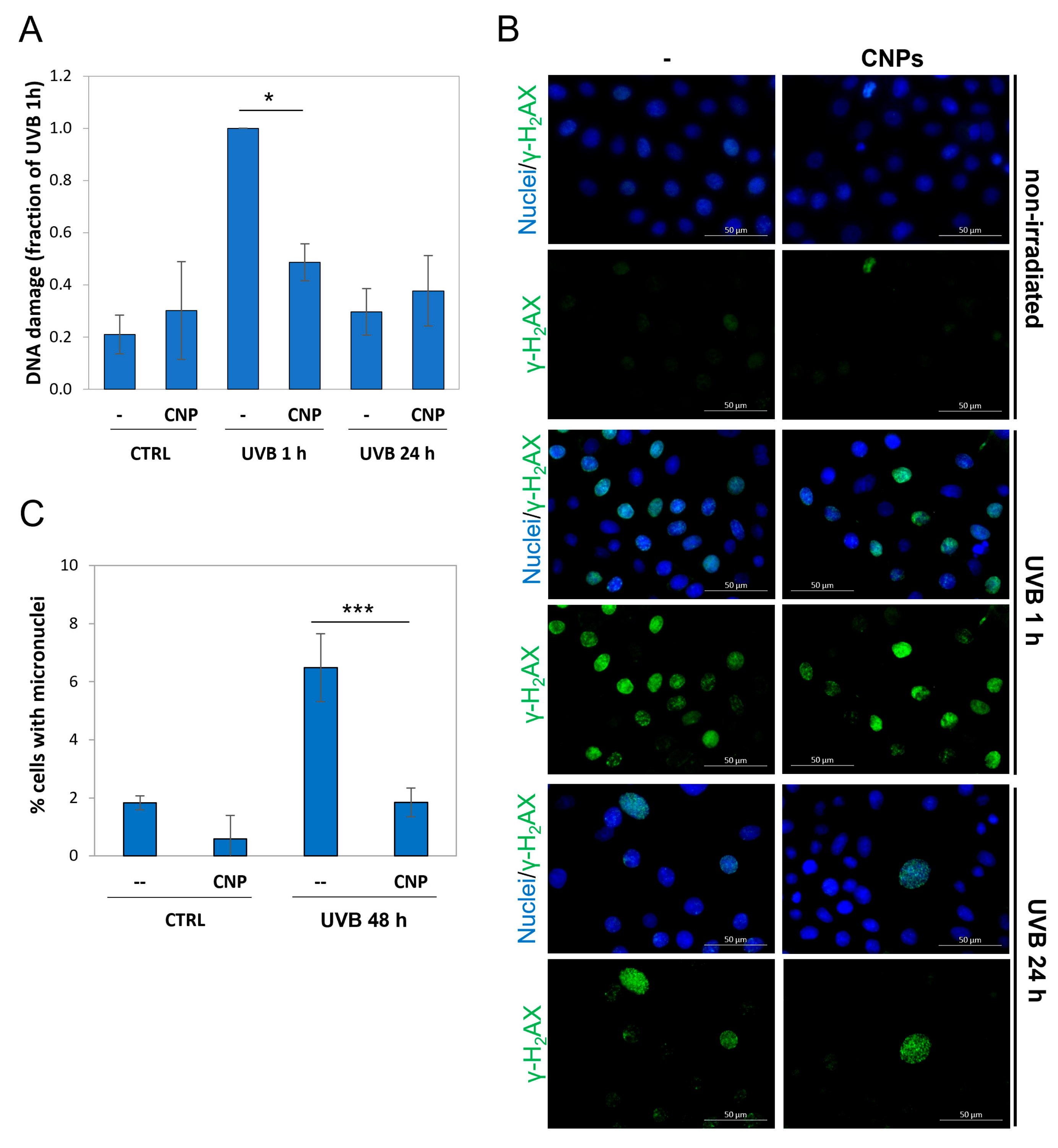
3.2. Nanoceria Reduce DNA Damage and Mutagenesis in HaCaT Cells
3.3. Nanotitania Increase UVA-Cytotoxicity, Which Is Prevented by Nanoceria
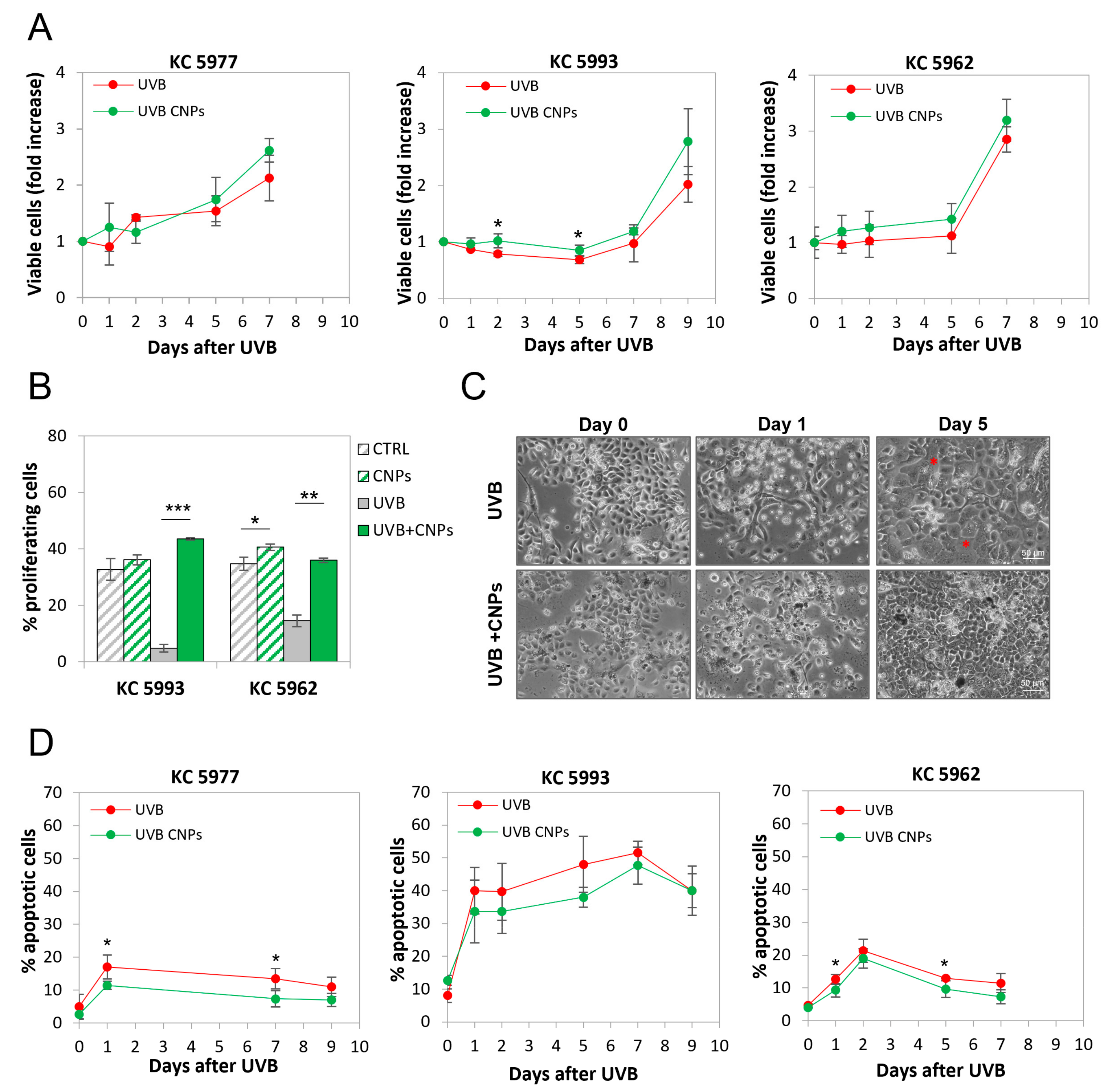
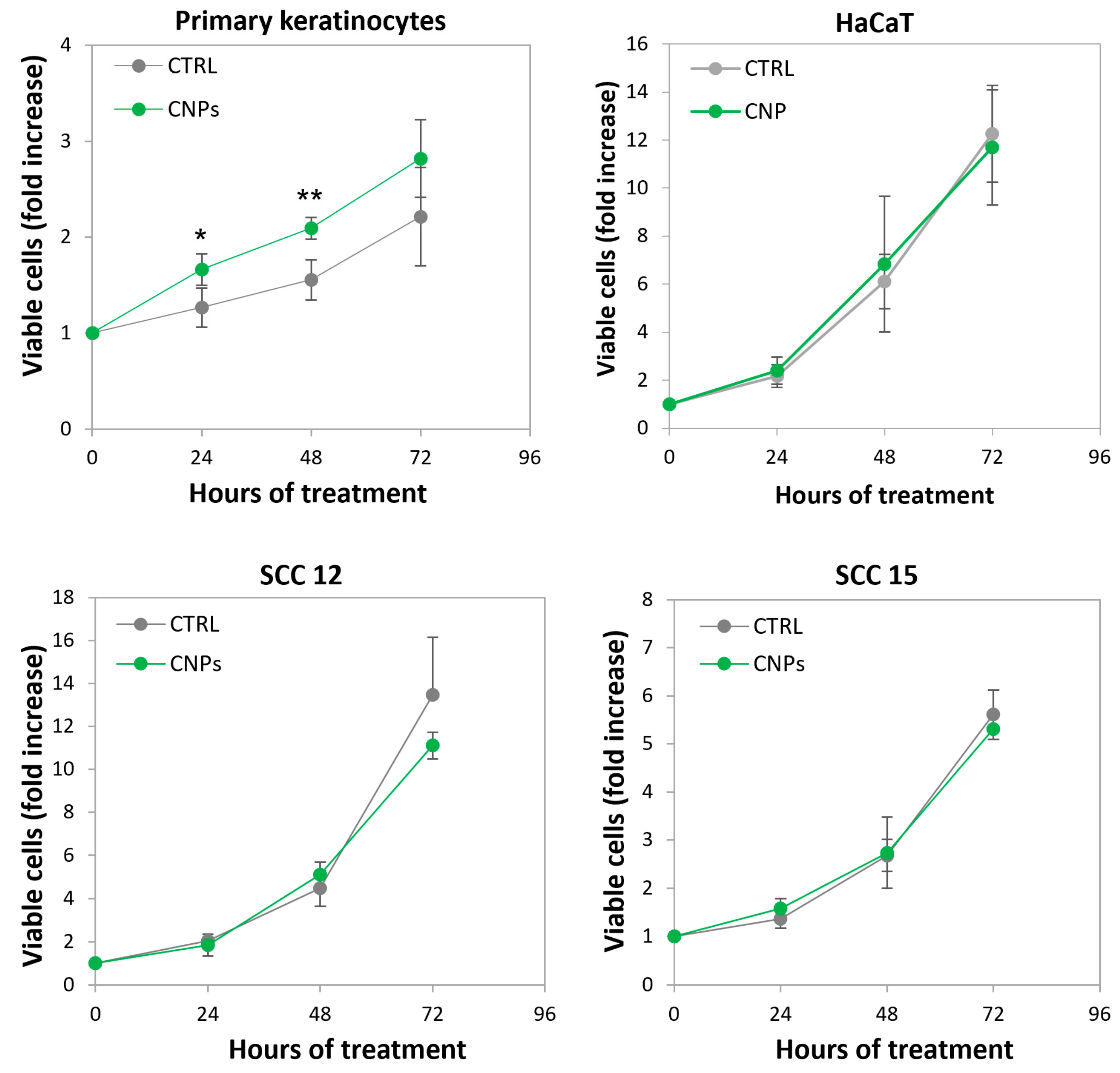
3.4. Nanoceria Reduce UVB-Induced Cell Loss in Primary Human Keratinocytes
3.5. Nanoceria Reduce DNA Damage and Prevent Mutagenesis in Primary Human Keratinocytes
3.6. Nanoceria Favour Basal Proliferation of Primary Human Keratinocytes but Not of HaCaT or Squamous Cell Carcinoma Keratinocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yang, W.; Yang, X.; Zhu, L.; Chu, H.; Li, X.; Xu, W. Nanozymes: Activity Origin, Catalytic Mechanism, and Biological Application. Coord. Chem. Rev. 2021, 448, 214170. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gao, L.; Fan, K.; Liu, J.; He, J.; Qu, X.; Dong, S.; Wang, E.; Yan, X. Nanozymes: A Clear Definition with Fuzzy Edges. Nano Today 2021, 40, 101269. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The Role of Cerium Redox State in the SOD Mimetic Activity of Nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef] [Green Version]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.S.; Seal, S.; Self, W.T. Nanoceria Exhibit Redox State-Dependent Catalase Mimetic Activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [Green Version]
- Amin, K.A.; Hassan, M.S.; Awad, E.S.T.; Hashem, K.S. The Protective Effects of Cerium Oxide Nanoparticles against Hepatic Oxidative Damage Induced by Monocrotaline. Int. J. Nanomed. 2011, 6, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Salcedo, A.; Poggio-Fraccari, E.; Mariño, F.; Irigoyen, B. Tuning the Selectivity of Cerium Oxide for Ethanol Dehydration to Ethylene. Appl. Surf. Sci. 2022, 599, 153963. [Google Scholar] [CrossRef]
- Singh, R.; Karakoti, A.S.; Self, W.; Seal, S.; Singh, S. Redox-Sensitive Cerium Oxide Nanoparticles Protect Human Keratinocytes from Oxidative Stress Induced by Glutathione Depletion. Langmuir 2016, 32, 12202–12211. [Google Scholar] [CrossRef]
- Corsi, F.; Caputo, F.; Traversa, E.; Ghibelli, L. Not Only Redox: The Multifaceted Activity of Cerium Oxide Nanoparticles in Cancer Prevention and Therapy. Front. Oncol. 2018, 8, 309. [Google Scholar] [CrossRef] [Green Version]
- Baldim, V.; Bedioui, F.; Mignet, N.; Margaill, I.; Berret, J.F. The Enzyme-like Catalytic Activity of Cerium Oxide Nanoparticles and Its Dependency on Ce3+ Surface Area Concentration. Nanoscale 2018, 10, 6971–6980. [Google Scholar] [CrossRef] [Green Version]
- Lord, M.S.; Berret, J.F.; Singh, S.; Vinu, A.; Karakoti, A.S. Redox Active Cerium Oxide Nanoparticles: Current Status and Burning Issues. Small 2021, 17, 2102342. [Google Scholar] [CrossRef]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological Potential of Cerium Oxide Nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef]
- Campbell, C.T.; Peden, C.H.F. Oxygen Vacancies and Catalysis on Ceria Surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef]
- Chung, C.H.; Tu, F.Y.; Chiu, T.A.; Wu, T.T.; Yu, W.Y. Critical Roles of Surface Oxygen Vacancy in Heterogeneous Catalysis over Ceria-Based Materials: A Selected Review. Chem. Lett. 2021, 50, 856–865. [Google Scholar] [CrossRef]
- Singh, S.; Dosani, T.; Karakoti, A.S.; Kumar, A.; Seal, S.; Self, W.T. A Phosphate-Dependent Shift in Redox State of Cerium Oxide Nanoparticles and Its Effects on Catalytic Properties. Biomaterials 2011, 32, 6745–6753. [Google Scholar] [CrossRef] [Green Version]
- Perez, J.M.; Asati, A.; Nath, S.; Kaittanis, C. Synthesis of Biocompatible Dextran-Coated Nanoceria with PH-Dependent Antioxidant Properties. Small 2008, 4, 552–556. [Google Scholar] [CrossRef]
- Demokritou, P.; Gass, S.; Pyrgiotakis, G.; Cohen, J.M.; Goldsmith, W.; McKinney, W.; Frazer, D.; Ma, J.; Schwegler-Berry, D.; Brain, J.; et al. An in Vivo and in Vitro Toxicological Characterisation of Realistic Nanoscale CeO2 Inhalation Exposures. Nanotoxicology 2013, 7, 1338–1350. [Google Scholar] [CrossRef] [Green Version]
- Yabe, S.; Sato, T. Cerium Oxide for Sunscreen Cosmetics. J. Solid State Chem. 2003, 171, 7–11. [Google Scholar] [CrossRef]
- Lewicka, Z.A.; Benedetto, A.F.; Benoit, D.N.; Yu, W.W.; Fortner, J.D.; Colvin, V.L. The Structure, Composition, and Dimensions of TiO2 and ZnO Nanomaterials in Commercial Sunscreens. J. Nanopart. Res. 2011, 13, 3607–3617. [Google Scholar] [CrossRef]
- Cole, C.; Shyr, T.; Ou-Yang, H. Metal Oxide Sunscreens Protect Skin by Absorption, Not by Reflection or Scattering. Photodermatol. Photoimmunol. Photomed. 2016, 32, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Caputo, F.; De Nicola, M.; Sienkiewicz, A.; Giovanetti, A.; Bejarano, I.; Licoccia, S.; Traversa, E.; Ghibelli, L. Cerium Oxide Nanoparticles, Combining Antioxidant and UV Shielding Properties, Prevent UV-Induced Cell Damage and Mutagenesis. Nanoscale 2015, 7, 15643–15656. [Google Scholar] [CrossRef] [PubMed]
- Aldahan, A.S.; Shah, V.V.; Mlacker, S.; Nouri, K. The History of Sunscreen. JAMA Dermatol. 2015, 151, 1316. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef] [Green Version]
- Burnett, M.E.; Wang, S.Q. Current Sunscreen Controversies: A Critical Review. Photodermatol. Photoimmunol. Photomed. 2011, 27, 58–67. [Google Scholar] [CrossRef]
- Traversa, E.; Di Vona, M.L.; Licoccia, S.; Sacerdoti, M.; Carotta, M.C.; Crema, L.; Martinelli, G. Sol-Gel Processed TiO2-Based Nano-Sized Powders for Use in Thick-Film Gas Sensors for Atmospheric Pollutant Monitoring. J. Sol-Gel Sci. Technol. 2001, 22, 167–179. [Google Scholar] [CrossRef]
- Licoccia, S.; Traversa, E. Increasing the Operation Temperature of Polymer Electrolyte Membranes for Fuel Cells: From Nanocomposites to Hybrids. J. Power Sources 2006, 159, 12–20. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Rheinwatd, J.G.; Green, H. Seria Cultivation of Strains of Human Epidemal Keratinocytes: The Formation Keratinizin Colonies from Single Cell Is. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Beckett, M.A. Tumorigenic Keratinocyte Lines Requiring Anchorage and Fibroblast Support Cultured from Human Squamous Cell Carcinomas. Cancer Res. 1981, 41, 1657–1663. [Google Scholar]
- Dini, L.; Coppola, S.; Ruzittu, M.T.; Ghibelli, L. Multiple Pathways for Apoptotic Nuclear Fragmentation. Exp. Cell Res. 1996, 223, 340–347. [Google Scholar] [CrossRef]
- Ghibelli, L.; Coppola, S.; Rotilio, G.; Lafavia, E.; Maresca, V.; Ciriolo, M.R. Non-Oxidative Loss of Glutathione in Apoptosis via GSH Extrusion. Biochem. Biophys. Res. Commun. 1995, 216, 313–320. [Google Scholar] [CrossRef]
- Wischermann, K.; Boukamp, P.; Schmezer, P. Improved Alkaline Comet Assay Protocol for Adherent HaCaT Keratinocytes to Study UVA-Induced DNA Damage. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 630, 122–128. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for in Vitro and in Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- García, O.; Mandina, T.; Lamadrid, A.I.; Diaz, A.; Remigio, A.; Gonzalez, Y.; Piloto, J.; Gonzalez, J.E.; Alvarez, A. Sensitivity and Variability of Visual Scoring in the Comet Assay: Results of an Inter-Laboratory Scoring Exercise with the Use of Silver Staining. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 556, 25–34. [Google Scholar] [CrossRef]
- Pelliccia, A.; Capradossi, F.; Corsi, F.; Tarquini, G.D.; Bruni, E.; Reichle, A.; Torino, F.; Ghibelli, L. Androgen Deprivation Freezes Hormone-Sensitive Prostate Cancer Cells in a Reversible, Genetically Unstable Quasi-Apoptotic State, Bursting into Full Apoptosis upon Poly(ADP-Ribose) Polymerase Inhibition. Int. J. Mol. Sci. 2023, 24, 2040. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [Green Version]
- Lehman, T.A.; Modali, R.; Boukamp, P.; Stanek, J.; Bennett, W.P.; Welsh, J.A.; Metcalf, R.A.; Stampfer, M.R.; Fusenig, N.; Rogan, E.M.; et al. P53 Mutations in Human Immortalized Epithelial Cell Lines. Carcinogenesis 1993, 14, 833–839. [Google Scholar] [CrossRef]
- Caputo, F.; Mameli, M.; Sienkiewicz, A.; Licoccia, S.; Stellacci, F.; Ghibelli, L.; Traversa, E. A Novel Synthetic Approach of Cerium Oxide Nanoparticles with Improved Biomedical Activity. Sci. Rep. 2017, 7, 4636. [Google Scholar] [CrossRef] [Green Version]
- Celardo, I.; De Nicola, M.; Mandoli, C.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Ce3+ Ions Determine Redox-Dependent Anti-Apoptotic Effect of Cerium Oxide Nanoparticles. ACS Nano 2011, 5, 4537–4549. [Google Scholar] [CrossRef]
- Feehan, R.P.; Shantz, L.M. Molecular Signaling Cascades Involved in Nonmelanoma Skin Carcinogenesis. Biochem. J. 2016, 473, 2973–2994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solar, I. Ultraviolet Radiation. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer, World Health Organization: Lyon, France, 1992. [Google Scholar]
- Caputo, F.; Giovanetti, A.; Corsi, F.; Maresca, V.; Briganti, S.; Licoccia, S.; Traversa, E.; Ghibelli, L. Cerium Oxide Nanoparticles Reestablish Cell Integrity Checkpoints and Apoptosis Competence in Irradiated HaCaT Cells via Novel Redox-Independent Activity. Front. Pharmacol. 2018, 9, 1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsi, F.; Capradossi, F.; Pelliccia, A.; Briganti, S.; Bruni, E.; Traversa, E.; Torino, F.; Reichle, A.; Ghibelli, L. Apoptosis as a Driver of Therapy-Induced Cancer Repopulation and Acquired Cell-Resistance (CRAC): A Simple In Vitro Model of Phoenix Rising in Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 1152. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L.; Meunier, J.R. Skin DNA Photodamage and Its Biological Consequences. J. Am. Acad. Dermatol. 2008, 58, S139–S148. [Google Scholar] [CrossRef]
- Daré, R.G.; Kolanthai, E.; Neal, C.J.; Fu, Y.; Seal, S.; Nakamura, C.V.; Lautenschlager, S.O.S. Cerium Oxide Nanoparticles Conjugated with Tannic Acid Prevent UVB-Induced Oxidative Stress in Fibroblasts: Evidence of a Promising Anti-Photodamage Agent. Antioxidants 2023, 12, 190. [Google Scholar] [CrossRef]
- Ribeiro, F.M.; de Oliveira, M.M.; Singh, S.; Sakthivel, T.S.; Neal, C.J.; Seal, S.; Ueda-Nakamura, T.; de Oliveira Silva Lautenschlager, S.; Nakamura, C.V. Ceria Nanoparticles Decrease UVA-Induced Fibroblast Death Through Cell Redox Regulation Leading to Cell Survival, Migration and Proliferation. Front. Bioeng. Biotechnol. 2020, 8, 577557. [Google Scholar] [CrossRef]
- Pozzi, D.; Grimaldi, P.; Gaudenzi, S.; Di Giambattista, L.; Silvestri, I.; Morrone, S.; Congiu Castellano, A. UVB-Radiation-Induced Apoptosis in Jurkat Cells: A Coordinated Fourier Transform Infrared Spectroscopy-Flow Cytometry Study. Radiat. Res. 2007, 168, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Chigurupati, S.; Mughal, M.R.; Okun, E.; Das, S.; Kumar, A.; McCaffery, M.; Seal, S.; Mattson, M.P. Effects of Cerium Oxide Nanoparticles on the Growth of Keratinocytes, Fibroblasts and Vascular Endothelial Cells in Cutaneous Wound Healing. Biomaterials 2013, 34, 2194–2201. [Google Scholar] [CrossRef] [Green Version]
- Martincorena, I. Somatic Mutation and Clonal Expansions in Human Tissues. Genome Med. 2019, 11, 35. [Google Scholar] [CrossRef]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and Organic UV Filters: Their Role and Efficacy in Sunscreens and Suncare Products. Inorg. Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Horie, M.; Sugino, S.; Kato, H.; Tabei, Y.; Nakamura, A.; Yoshida, Y. Does Photocatalytic Activity of TiO2 Nanoparticles Correspond to Photo-Cytotoxicity? Cellular Uptake of TiO2 Nanoparticles Is Important in Their Photo-Cytotoxicity. Toxicol. Mech. Methods 2016, 26, 284–294. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of Noncytotoxic Chitosan-Gold Nanocomposites as Efficient Antibacterial Materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef]
- Skotarczak, K.; Osmola-Mańkowska, A.; Lodyga, M.; Polańska, A.; Mazur, M.; Adamski, Z. Photoprotection: Facts and Controversies. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 98–112. [Google Scholar]
- Compton, D.L.; Evans, K.O.; Appell, M.; Goodell, J.R. Protection of Antioxidants, Vitamins E and C, from Ultraviolet Degradation Using Feruloylated Vegetable Oil. JAOCS J. Am. Oil Chem. Soc. 2019, 96, 999–1009. [Google Scholar] [CrossRef]
- Caruso, F.; Incerpi, S.; Pedersen, J.; Belli, S.; Kaur, S.; Rossi, M. Aromatic Polyphenol π-π Interactions with Superoxide Radicals Contribute to Radical Scavenging and Can Make Polyphenols Mimic Superoxide Dismutase Activity. Curr. Issues Mol. Biol. 2022, 44, 5209–5220. [Google Scholar] [CrossRef]
- Kim, M.S.; Stees, M.; Karuturi, B.V.K.; Vijayaraghavalu, S.; Peterson, R.E.; Madsen, G.L.; Labhasetwar, V. Pro-NP™ Protect against TiO2 Nanoparticle-Induced Phototoxicity in Zebrafish Model: Exploring Potential Application for Skin Care. Drug Deliv. Transl. Res. 2017, 7, 372–382. [Google Scholar] [CrossRef]
- Pagliari, F.; Mandoli, C.; Forte, G.; Magnani, E.; Pagliari, S.; Nardone, G.; Licoccia, S.; Minieri, M.; Di Nardo, P.; Traversa, E. Cerium Oxide Nanoparticles Protect Cardiac Progenitor Cells from Oxidative Stress. ACS Nano 2012, 6, 3767–3775. [Google Scholar] [CrossRef] [Green Version]
- Zholobak, N.M.; Ivanov, V.K.; Shcherbakov, A.B.; Shaporev, A.S.; Polezhaeva, O.S.; Baranchikov, A.Y.; Spivak, N.Y.; Tretyakov, Y.D. UV-Shielding Property, Photocatalytic Activity and Photocytotoxicity of Ceria Colloid Solutions. J. Photochem. Photobiol. B Biol. 2011, 102, 32–38. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsi, F.; Di Meo, E.; Lulli, D.; Deidda Tarquini, G.; Capradossi, F.; Bruni, E.; Pelliccia, A.; Traversa, E.; Dellambra, E.; Failla, C.M.; et al. Safe-Shields: Basal and Anti-UV Protection of Human Keratinocytes by Redox-Active Cerium Oxide Nanoparticles Prevents UVB-Induced Mutagenesis. Antioxidants 2023, 12, 757. https://doi.org/10.3390/antiox12030757
Corsi F, Di Meo E, Lulli D, Deidda Tarquini G, Capradossi F, Bruni E, Pelliccia A, Traversa E, Dellambra E, Failla CM, et al. Safe-Shields: Basal and Anti-UV Protection of Human Keratinocytes by Redox-Active Cerium Oxide Nanoparticles Prevents UVB-Induced Mutagenesis. Antioxidants. 2023; 12(3):757. https://doi.org/10.3390/antiox12030757
Chicago/Turabian StyleCorsi, Francesca, Erika Di Meo, Daniela Lulli, Greta Deidda Tarquini, Francesco Capradossi, Emanuele Bruni, Andrea Pelliccia, Enrico Traversa, Elena Dellambra, Cristina Maria Failla, and et al. 2023. "Safe-Shields: Basal and Anti-UV Protection of Human Keratinocytes by Redox-Active Cerium Oxide Nanoparticles Prevents UVB-Induced Mutagenesis" Antioxidants 12, no. 3: 757. https://doi.org/10.3390/antiox12030757
APA StyleCorsi, F., Di Meo, E., Lulli, D., Deidda Tarquini, G., Capradossi, F., Bruni, E., Pelliccia, A., Traversa, E., Dellambra, E., Failla, C. M., & Ghibelli, L. (2023). Safe-Shields: Basal and Anti-UV Protection of Human Keratinocytes by Redox-Active Cerium Oxide Nanoparticles Prevents UVB-Induced Mutagenesis. Antioxidants, 12(3), 757. https://doi.org/10.3390/antiox12030757





