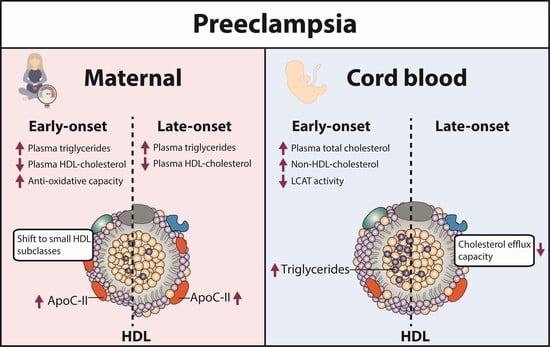
Preeclampsia Affects Lipid Metabolism and HDL Function in Mothers and Their Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment and Group Characteristics
2.2. ApoB-Depleted Plasma and Plasma Lipids
2.3. HDL-Associated Apolipoproteins and Lipids
2.4. HDL Subclass Distribution
2.5. Cholesterol Efflux Capacity Assay of Apob-Depleted Plasma
2.6. Arylesterase Activity of PON1
2.7. Anti-Oxidative Capacity
2.8. LCAT Activity
2.9. VLDL and LDL Subclass Distribution
2.10. Statistical Analysis
3. Results
3.1. Maternal and Fetal Characteristics of the Study Population
3.2. Preeclampsia Is Associated with Altered Plasma Lipid Levels in Mothers and Offspring
3.3. Preeclampsia-Related Changes of HDL-Associated Apolipoprotein and Lipid Composition
3.4. Preeclampsia Affects Maternal HDL Subclass Distribution
3.5. Effect of Preeclampsia on LCAT Activity in Mother and Child
3.6. Effects of Preeclampsia on Parameters of HDL Function
3.7. Association of PE with Alterations in Subclasses of Triglyceride-Rich Lipoproteins
4. Discussion
4.1. PE-Associated Alterations in Lipid Metabolism and HDL Function in Mothers
4.2. PE-Related Alterations in Lipid Metabolism and HDL Function in Neonates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallis, A.B.; Saftlas, A.F.; Hsia, J.; Atrash, H.K. Secular Trends in the Rates of Preeclampsia, Eclampsia, and Gestational Hypertension, United States, 1987–2004. Am. J. Hypertens. 2008, 21, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obset. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Gathiram, P.; Moodley, J. Pre-Eclampsia: Its Pathogenesis and Pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef] [Green Version]
- ACOG Committee on Practice Bulletins--Obstetrics ACOG Practice Bulletin. Diagnosis and Management of Preeclampsia and Eclampsia. Number 33, January 2002. Obstet. Gynecol. 2002, 99, 159–167. [Google Scholar] [CrossRef]
- Hubel, C.A. Oxidative Stress in the Pathogenesis of Preeclampsia. Proc. Soc. Exp. Biol. Med. 1999, 222, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Perico, N.; Remuzzi, G. Mechanisms of Disease: Pre-Eclampsia. Nat. Clin. Pract. Nephrol. 2005, 1, 98–114. [Google Scholar] [CrossRef] [Green Version]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D.J. Pre-Eclampsia and Risk of Cardiovascular Disease and Cancer in Later Life: Systematic Review and Meta-Analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular Disease Risk in Women with Pre-Eclampsia: Systematic Review and Meta-Analysis. Eur. J. Epidemiol. 2013, 28, 1–19. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Macdonald-Wallis, C.; Fraser, A.; Nelson, S.M.; Hingorani, A.; Davey Smith, G.; Sattar, N.; Deanfield, J. Cardiovascular Biomarkers and Vascular Function during Childhood in the Offspring of Mothers with Hypertensive Disorders of Pregnancy: Findings from the Avon Longitudinal Study of Parents and Children. Eur. Heart J. 2012, 33, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Tenhola, S.; Rahiala, E.; Halonen, P.; Vanninen, E.; Voutilainen, R. Maternal Preeclampsia Predicts Elevated Blood Pressure in 12-Year-Old Children: Evaluation by Ambulatory Blood Pressure Monitoring. Pediatr. Res. 2006, 59, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.S.; Nohr, E.A.; Bech, B.H.; Vestergaard, M.; Catov, J.M.; Olsen, J. Health of Children Born to Mothers Who Had Preeclampsia: A Population-Based Cohort Study. Am. J. Obstet. Gynecol. 2009, 201, 269.e1–269.e10. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.; Donald, A.; Ferri, C.; Giannattasio, C.; Halcox, J.; Halligan, S.; Lerman, A.; Mancia, G.; Oliver, J.J.; Pessina, A.C.; et al. Endothelial Function and Dysfunction. Part I: Methodological Issues for Assessment in the Different Vascular Beds: A Statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005, 23, 7–17. [Google Scholar] [CrossRef]
- Wojcik-Baszko, D.; Charkiewicz, K.; Laudanski, P. Role of Dyslipidemia in Preeclampsia-A Review of Lipidomic Analysis of Blood, Placenta, Syncytiotrophoblast Microvesicles and Umbilical Cord Artery from Women with Preeclampsia. Prostaglandins Other Lipid Mediat. 2018, 139, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Abraham, R.; Vedavalli, R.; Daniel, M. Study of Lipid Profile, Lipid Peroxidation and Vitamin E in Pregnancy Induced Hypertension. Indian J. Physiol. Pharmacol. 2009, 53, 365–369. [Google Scholar] [PubMed]
- Bayhan, G.; Koçyigit, Y.; Atamer, A.; Atamer, Y.; Akkus, Z. Potential Atherogenic Roles of Lipids, Lipoprotein(a) and Lipid Peroxidation in Preeclampsia. Gynecol. Endocrinol. 2005, 21, 1–6. [Google Scholar] [CrossRef]
- Iftikhar, U.; Iqbal, A.; Shakoor, S. Relationship between Leptin and Lipids during Pre-Eclampsia. J. Pak. Med. Assoc. 2010, 60, 432–435. [Google Scholar]
- Fantone, S.; Mazzucchelli, R.; Giannubilo, S.R.; Ciavattini, A.; Marzioni, D.; Tossetta, G. AT-Rich Interactive Domain 1A Protein Expression in Normal and Pathological Pregnancies Complicated by Preeclampsia. Histochem. Cell Biol. 2020, 154, 339–346. [Google Scholar] [CrossRef]
- Sørensen, A.; Sinding, M. Preeclamptic Placenta. Hypertension 2020, 75, 1412–1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Rana, S.; Karumanchi, S.A. Preeclampsia: The Role of Angiogenic Factors in Its Pathogenesis. Physiology 2009, 24, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef]
- Raymond, D.; Peterson, E. A Critical Review of Early-Onset and Late-Onset Preeclampsia. Obstet. Gynecol. Surv. 2011, 66, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Melchior, J.T.; Swertfeger, D.K.; Morris, J.; Street, S.E.; Warshak, C.R.; Welge, J.A.; Remaley, A.T.; Catov, J.M.; Davidson, W.S.; Woollett, L.A. Pregnancy Is Accompanied by Larger High Density Lipoprotein Particles and Compositionally Distinct Subspecies. J. Lipid Res. 2021, 62, 100107. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Tan, L.; Long, J.L.; Davidson, W.S. Proteomic Diversity of High Density Lipoproteins: Our Emerging Understanding of Its Importance in Lipid Transport and Beyond. J. Lipid Res. 2013, 54, 2575–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagao, M.; Nakajima, H.; Toh, R.; Hirata, K.; Ishida, T. Cardioprotective Effects of High-Density Lipoprotein Beyond Its Anti-Atherogenic Action. J. Atheroscler. Thromb. 2018, 25, 985–993. [Google Scholar] [CrossRef] [Green Version]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Barter, P.J.; Nicholls, S.; Rye, K.-A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Antiinflammatory Properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative Activity of High-Density Lipoprotein (HDL): Mechanistic Insights into Potential Clinical Benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Mineo, C.; Shaul, P.W. HDL Stimulation of Endothelial Nitric Oxide Synthase: A Novel Mechanism of HDL Action. Trends Cardiovasc. Med. 2003, 13, 226–231. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-Density Lipoprotein Revisited: Biological Functions and Clinical Relevance. Eur. Heart J. 2022, ehac605. [Google Scholar] [CrossRef]
- Litvinov, D.; Mahini, H.; Garelnabi, M. Antioxidant and Anti-Inflammatory Role of Paraoxonase 1: Implication in Arteriosclerosis Diseases. N. Am. J. Med. Sci. 2012, 4, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Huesca-Gómez, C.; Carreón-Torres, E.; Nepomuceno-Mejía, T.; Sánchez-Solorio, M.; Galicia-Hidalgo, M.; Mejía, A.M.; Montaño, L.-F.; Franco, M.; Posadas-Romero, C.; Pérez-Méndez, O. Contribution of Cholesteryl Ester Transfer Protein and Lecithin:Cholesterol Acyltransferase to HDL Size Distribution. Endocr. Res. 2004, 30, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Sorci-Thomas, M.G.; Bhat, S.; Thomas, M.J. Activation of Lecithin: Cholesterol Acyltransferase by HDL ApoA-I Central Helices. Clin. Lipidol. 2009, 4, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Borggreve, S.E.; De Vries, R.; Dullaart, R.P.F. Alterations in High-Density Lipoprotein Metabolism and Reverse Cholesterol Transport in Insulin Resistance and Type 2 Diabetes Mellitus: Role of Lipolytic Enzymes, Lecithin: Cholesterol Acyltransferase and Lipid Transfer Proteins. Eur. J. Clin. Investig. 2003, 33, 1051–1069. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.E.; Fisher, E.A. Lipoprotein Metabolism, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2013, 33, 380–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadler, J.T.; Mangge, H.; Rani, A.; Curcic, P.; Herrmann, M.; Prüller, F.; Marsche, G. Low HDL Cholesterol Efflux Capacity Indicates a Fatal Course of COVID-19. Antioxidants 2022, 11, 1858. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Schilcher, G.; Curcic, S.; Trieb, M.; Ljubojevic, S.; Stojakovic, T.; Scharnagl, H.; Kopecky, C.M.; Rosenkranz, A.R.; Heinemann, A.; et al. Dialysis Modalities and HDL Composition and Function. J. Am. Soc. Nephrol. 2015, 26, 2267–2276. [Google Scholar] [CrossRef] [Green Version]
- Hollstein, T.; Vogt, A.; Grenkowitz, T.; Stojakovic, T.; März, W.; Laufs, U.; Bölükbasi, B.; Steinhagen-Thiessen, E.; Scharnagl, H.; Kassner, U. Treatment with PCSK9 Inhibitors Reduces Atherogenic VLDL Remnants in a Real-World Study. Vasc. Pharmacol. 2019, 116, 8–15. [Google Scholar] [CrossRef]
- Harangi, M.; Szentpéteri, A.; Nádró, B.; Lőrincz, H.; Seres, I.; Páll, D.; Paragh, G. HDL Subfraction Distribution and HDL Function in Untreated Dyslipidemic Patients. Vessel. Plus 2017, 1, 166–173. [Google Scholar] [CrossRef]
- Marsche, G.; Zelzer, S.; Meinitzer, A.; Kern, S.; Meissl, S.; Pregartner, G.; Weghuber, D.; Almer, G.; Mangge, H. Adiponectin Predicts High-Density Lipoprotein Cholesterol Efflux Capacity in Adults Irrespective of Body Mass Index and Fat Distribution. J. Clin. Endocrinol. Metab. 2017, 102, 4117–4123. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [Green Version]
- Trieb, M.; Wolf, P.; Knuplez, E.; Weger, W.; Schuster, C.; Peinhaupt, M.; Holzer, M.; Trakaki, A.; Eichmann, T.; Lass, A.; et al. Abnormal Composition and Function of High-Density Lipoproteins in Atopic Dermatitis Patients. Allergy 2019, 74, 398–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trakaki, A.; Sturm, G.J.; Pregartner, G.; Scharnagl, H.; Eichmann, T.O.; Trieb, M.; Knuplez, E.; Holzer, M.; Stadler, J.T.; Heinemann, A.; et al. Allergic Rhinitis Is Associated with Complex Alterations in High-Density Lipoprotein Composition and Function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.T.; van Poppel, M.N.M.; Wadsack, C.; Holzer, M.; Pammer, A.; Simmons, D.; Hill, D.; Desoye, G.; Marsche, G.; DALI Core Investigator Group. Obesity Affects Maternal and Neonatal HDL Metabolism and Function. Antioxidants 2023, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Serrano, N.C.; Guio-Mahecha, E.; Quintero-Lesmes, D.C.; Becerra- Bayona, S.; Paez, M.C.; Beltran, M.; Herrera, V.M.; Leon, L.J.; Williams, D.; Casas, J.P. Lipid Profile, Plasma Apolipoproteins, and Pre-Eclampsia Risk in the GenPE Case-Control Study. Atherosclerosis 2018, 276, 189–194. [Google Scholar] [CrossRef]
- Spracklen, C.N.; Smith, C.J.; Saftlas, A.F.; Robinson, J.G.; Ryckman, K.K. Maternal Hyperlipidemia and the Risk of Preeclampsia: A Meta-Analysis. Am. J. Epidemiol. 2014, 180, 346–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsche, G.; Saemann, M.D.; Heinemann, A.; Holzer, M. Inflammation Alters HDL Composition and Function: Implications for HDL-Raising Therapies. Pharmacol. Ther. 2013, 137, 341–351. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Scheraldi, C.A.; Yacoub, L.K.; Saxena, U.; Bisgaier, C.L. Lipoprotein ApoC-II Activation of Lipoprotein Lipase. Modulation by Apolipoprotein A-IV. J. Biol. Chem. 1990, 265, 4266–4272. [Google Scholar] [CrossRef]
- Camont, L.; Chapman, M.J.; Kontush, A. Biological Activities of HDL Subpopulations and Their Relevance to Cardiovascular Disease. Trends Mol. Med. 2011, 17, 594–603. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M.J. Antiatherogenic Small, Dense HDL--Guardian Angel of the Arterial Wall? Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 144–153. [Google Scholar] [CrossRef]
- Zhang, B.; Kawachi, E.; Miura, S.; Uehara, Y.; Matsunaga, A.; Kuroki, M.; Saku, K. Therapeutic Approaches to the Regulation of Metabolism of High-Density Lipoprotein. Circ. J. 2013, 77, 2651–2663. [Google Scholar] [CrossRef] [Green Version]
- Bauer, L.; Kern, S.; Rogacev, K.S.; Emrich, I.E.; Zawada, A.; Fliser, D.; Heinemann, A.; Heine, G.H.; Marsche, G. HDL Cholesterol Efflux Capacity and Cardiovascular Events in Patients With Chronic Kidney Disease. J. Am. Coll. Cardiol. 2017, 69, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Trieb, M.; Horvath, A.; Birner-Gruenberger, R.; Spindelboeck, W.; Stadlbauer, V.; Taschler, U.; Curcic, S.; Stauber, R.E.; Holzer, M.; Pasterk, L.; et al. Liver Disease Alters High-Density Lipoprotein Composition, Metabolism and Function. Biochim. Biophys. Acta 2016, 1861, 630–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, C.; Karasik, O.; King-Morris, K.; Asmar, A. Uric Acid as a Marker of Kidney Disease: Review of the Current Literature. Dis. Markers 2015, 2015, e382918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric Acid Provides an Antioxidant Defense in Humans against Oxidant- and Radical-Caused Aging and Cancer: A Hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.A.; Breslow, J.L.; Hennekens, C.H.; Buring, J.E.; Willett, W.C.; Krauss, R.M. Low-Density Lipoprotein Subclass Patterns and Risk of Myocardial Infarction. JAMA 1988, 260, 1917–1921. [Google Scholar] [CrossRef]
- Campos, H.; Genest, J.J.; Blijlevens, E.; McNamara, J.R.; Jenner, J.L.; Ordovas, J.M.; Wilson, P.W.; Schaefer, E.J. Low Density Lipoprotein Particle Size and Coronary Artery Disease. Arterioscler. Thromb. A J. Vasc. Biol. 1992, 12, 187–195. [Google Scholar] [CrossRef]
- Heidemann, B.E.; Koopal, C.; Bots, M.L.; Asselbergs, F.W.; Westerink, J.; Visseren, F.L.J. The Relation between VLDL-Cholesterol and Risk of Cardiovascular Events in Patients with Manifest Cardiovascular Disease. Int. J. Cardiol. 2021, 322, 251–257. [Google Scholar] [CrossRef]
- Davis, P.A.; Forte, T.M. Neonatal Umbilical Cord Blood Lipoproteins. Isolation and Characterization of Intermediate Density and Low Density Lipoproteins. Arteriosclerosis 1982, 2, 37–43. [Google Scholar] [CrossRef] [Green Version]
- de Lima, V.J.; de Andrade, C.R.; Ruschi, G.E.; Sass, N. Serum Lipid Levels in Pregnancies Complicated by Preeclampsia. Sao Paulo Med. J. 2011, 129, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adiga, U.; D’souza, V.; Kamath, A.; Mangalore, N. Antioxidant Activity and Lipid Peroxidation in Preeclampsia. J. Chin. Med. Assoc. 2007, 70, 435–438. [Google Scholar] [CrossRef] [Green Version]
- Gohil, J.T.; Patel, P.K.; Gupta, P. Estimation of Lipid Profile in Subjects of Preeclampsia. J. Obstet. Gynaecol. India 2011, 61, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Endresen, M.J.; Lorentzen, B.; Henriksen, T. Increased Lipolytic Activity and High Ratio of Free Fatty Acids to Albumin in Sera from Women with Preeclampsia Leads to Triglyceride Accumulation in Cultured Endothelial Cells. Am. J. Obstet. Gynecol. 1992, 167, 440–447. [Google Scholar] [CrossRef]
- Kaaja, R. Lipid Abnormalities in Pre-Eclampsia:Implications for Vascular Health. Clin. Lipidol. 2011, 6, 71–78. [Google Scholar] [CrossRef]
- Kaaja, R.; Tikkanen, M.J.; Viinikka, L.; Ylikorkala, O. Serum Lipoproteins, Insulin, and Urinary Prostanoid Metabolites in Normal and Hypertensive Pregnant Women. Obstet. Gynecol. 1995, 85, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Greer, I.A.; Louden, J.; Lindsay, G.; McConnell, M.; Shepherd, J.; Packard, C.J. Lipoprotein Subfraction Changes in Normal Pregnancy: Threshold Effect of Plasma Triglyceride on Appearance of Small, Dense Low Density Lipoprotein. J. Clin. Endocrinol. Metab. 1997, 82, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.S.; Shah, A.S.; Sexmith, H.; Gordon, S.M. The HDL Proteome Watch: Compilation of Studies Leads to New Insights on HDL Function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159072. [Google Scholar] [CrossRef]
- Ronsein, G.E.; Vaisar, T. Deepening Our Understanding of HDL Proteome. Expert Rev. Proteom. 2019, 16, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Marsche, G.; Heine, G.H.; Stadler, J.T.; Holzer, M. Current Understanding of the Relationship of HDL Composition, Structure and Function to Their Cardioprotective Properties in Chronic Kidney Disease. Biomolecules 2020, 10, 1348. [Google Scholar] [CrossRef]
- Wolska, A.; Dunbar, R.L.; Freeman, L.A.; Ueda, M.; Amar, M.J.; Sviridov, D.O.; Remaley, A.T. Apolipoprotein C-II: New Findings Related to Genetics, Biochemistry, and Role in Triglyceride Metabolism. Atherosclerosis 2017, 267, 49–60. [Google Scholar] [CrossRef]
- Winkler, K.; Wetzka, B.; Hoffmann, M.M.; Friedrich, I.; Kinner, M.; Baumstark, M.W.; Zahradnik, H.-P.; Wieland, H.; März, W. Triglyceride-Rich Lipoproteins Are Associated with Hypertension in Preeclampsia. J. Clin. Endocrinol. Metab. 2003, 88, 1162–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontush, A.; Chantepie, S.; Chapman, M.J. Small, Dense HDL Particles Exert Potent Protection of Atherogenic LDL Against Oxidative Stress. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Fabbrini, E.; Serafini, M.; Colic Baric, I.; Hazen, S.L.; Klein, S. Effect of Plasma Uric Acid on Antioxidant Capacity, Oxidative Stress, and Insulin Sensitivity in Obese Subjects. Diabetes 2014, 63, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Untersteller, K.; Meissl, S.; Trieb, M.; Emrich, I.E.; Zawada, A.M.; Holzer, M.; Knuplez, E.; Fliser, D.; Heine, G.H.; Marsche, G. HDL Functionality and Cardiovascular Outcome among Nondialysis Chronic Kidney Disease Patients. J. Lipid Res. 2018, 59, 1256–1265. [Google Scholar] [CrossRef] [Green Version]
- Einbinder, Y.; Biron-Shental, T.; Agassi-Zaitler, M.; Tzadikevitch-Geffen, K.; Vaya, J.; Khatib, S.; Ohana, M.; Benchetrit, S.; Zitman-Gal, T. High-Density Lipoproteins (HDL) Composition and Function in Preeclampsia. Arch. Gynecol. Obstet. 2018, 298, 405–413. [Google Scholar] [CrossRef]
- Aksoy, A.N.; Ozturk, N.; Aksoy, H.; Akcay, F. Paraoxonase and Arylesterase Activities in Patients with Preeclampsia. Eurasian J. Med. 2008, 40, 10–13. [Google Scholar] [PubMed]
- Song, L.; Wang, N.; Peng, Y.; Sun, B.; Cui, W. Placental Lipid Transport and Content in Response to Maternal Overweight and Gestational Diabetes Mellitus in Human Term Placenta. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Amusquivar, E.; López-Soldado, I.; Ortega, H. Maternal Lipid Metabolism and Placental Lipid Transfer. Horm. Res. Paediatr. 2006, 65, 59–64. [Google Scholar] [CrossRef]
- León-Reyes, G.; Sosa, S.E.Y.; Medina-Navarro, R.; Guzmán-Grenfell, A.M.; Medina-Urrutia, A.X.; Fuentes-García, S.; Hicks, G.J.; Torres-Ramos, Y.D. Oxidative Modifications of Foetal LDL-c and HDL-c Lipoproteins in Preeclampsia. Lipids Health Dis. 2018, 17, 110. [Google Scholar] [CrossRef] [Green Version]
- Stadler, J.T.; Wadsack, C.; Marsche, G. Fetal High-Density Lipoproteins: Current Knowledge on Particle Metabolism, Composition and Function in Health and Disease. Biomedicines 2021, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.T.; van Poppel, M.N.M.; Christoffersen, C.; Hill, D.; Wadsack, C.; Simmons, D.; Desoye, G.; Marsche, G.; DALI Core Investigator Group. Gestational Hypertension and High-Density Lipoprotein Function: An Explorative Study in Overweight/Obese Women of the DALI Cohort. Antioxidants 2023, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Kurlak, L.O.; Mansour, Y.T.; Zurkinden, L.; Mohaupt, M.G.; Escher, G. Increased Maternal and Fetal Cholesterol Efflux Capacity and Placental CYP27A1 Expression in Preeclampsia. J. Lipid Res. 2017, 58, 1186–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asztalos, B.F.; Schaefer, E.J.; Horvath, K.V.; Yamashita, S.; Miller, M.; Franceschini, G.; Calabresi, L. Role of LCAT in HDL Remodeling: Investigation of LCAT Deficiency States. J. Lipid Res. 2007, 48, 592–599. [Google Scholar] [CrossRef] [Green Version]







| Maternal Characteristics | Normal Pregnancy | Early-Onset PE | p-Value | Late-Onset PE | p-Value |
|---|---|---|---|---|---|
| Number of matched samples | 32 | 18 | 14 | ||
| Maternal age (years) | 30 (27–33) | 34 (28–37) | ns | 31 (30–36) | ns |
| Pre-pregnancy BMI (kg/m2) | 22.5 (20.7–28.3) | 25.4 (22.0–27.4) | ns | 26.5 (22.8–29.3) | ns |
| Gestational age (weeks) | 38.8 (38.1–39.1) | 33.6 (31.7–34.3) † | <0.001 | 37.2 (35.5–37.9) | ns |
| Mode of delivery (% C-section) | 88 | 100 | ns | 72 | ns |
| Systolic blood pressure (mmHg) | 117 (110–122) | 163 (156–185) | <0.001 | 153 (143–169) | <0.001 |
| Diastolic blood pressure (mmHg) | 74 (66–79) | 107 (100–115) | <0.001 | 101 (94–108) | <0.001 |
| CRP (mg/mL) | 4.8 (2.5–7.9) | 5.1 (3.4–19.9) | ns | 7.0 (2.5–17.1) | ns |
| Sflt-1 (pg/mL) | - | 15,721 (12,184–21,500) † | - | 9542 (8125–154,001) | - |
| PLGF (pg/mL) | - | 44.5 (29.1–56.8) † | - | 67.3 (36.9–86.6) | - |
| Platelets | 212 (174–244) | 202 (150–239) | ns | 189 (140–220) | ns |
| Uric acid (mg/dL) | - | 6.2 (5.5–7.0) | - | 5.9 (5.1–7.1) | - |
| ALT (U/L) | - | 30.0 (21.8–44.3) | - | 24.5 (16.8–38.8) | - |
| AST (U/L) | - | 25.0 (18.8–51.0) † | - | 13.5 (8.8–45.8) | - |
| Fetal characteristics | |||||
| Sex (% female) | 59 | 50 | ns | 57 | ns |
| Weight at birth (g) | 3230 (2951–3683) | 1660 (1438–2021) | <0.001 | 2545 (2303–3185) | 0.054 |
| Placenta weight (g) | 615 (563–665) | 360 (320–455) | <0.001 | 495 (448–533) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stadler, J.T.; Scharnagl, H.; Wadsack, C.; Marsche, G. Preeclampsia Affects Lipid Metabolism and HDL Function in Mothers and Their Offspring. Antioxidants 2023, 12, 795. https://doi.org/10.3390/antiox12040795
Stadler JT, Scharnagl H, Wadsack C, Marsche G. Preeclampsia Affects Lipid Metabolism and HDL Function in Mothers and Their Offspring. Antioxidants. 2023; 12(4):795. https://doi.org/10.3390/antiox12040795
Chicago/Turabian StyleStadler, Julia T., Hubert Scharnagl, Christian Wadsack, and Gunther Marsche. 2023. "Preeclampsia Affects Lipid Metabolism and HDL Function in Mothers and Their Offspring" Antioxidants 12, no. 4: 795. https://doi.org/10.3390/antiox12040795