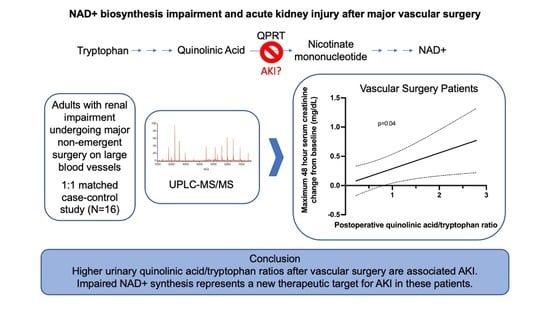
NAD+ Biosynthesis Impairment and Acute Kidney Injury after Major Vascular Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data and Sample Collection
2.3. Analytical Measurements
2.4. Statistical Analysis
3. Results
3.1. Cohort Characteristics
3.2. Quinolinate Measurements
3.3. Quinolinate to Tryptophan Ratios
3.4. Association between Quinolinate and Postoperative Creatinine
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hobson, C.; Lysak, N.; Huber, M.; Scali, S.; Bihorac, A. Epidemiology, outcomes, and management of acute kidney injury in the vascular surgery patient. J. Vasc. Surg. 2018, 68, 916–928. [Google Scholar] [CrossRef]
- Saratzis, A.; Melas, N.; Mahmood, A.; Sarafidis, P. Incidence of Acute Kidney Injury (AKI) after Endovascular Abdominal Aortic Aneurysm Repair (EVAR) and Impact on Outcome. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 534–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.E.; Muntner, P.; Chertow, G.M.; Warnock, D.G. Acute Kidney Injury and Mortality in Hospitalized Patients. Am. J. Nephrol. 2012, 35, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Harris, D.G.; Koo, G.; McCrone, M.P.; Weltz, A.S.; Chiu, W.C.; Sarkar, R.; Scalea, T.M.; Diaz, J.J.; Lissauer, M.E.; Crawford, R.S. Acute Kidney Injury in Critically Ill Vascular Surgery Patients is Common and Associated with Increased Mortality. Front. Surg. 2015, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tallgren, M.; Niemi, T.; Pöyhiä, R.; Raininko, E.; Railo, M.; Salmenperä, M.; Lepäntalo, M.; Hynninen, M. Acute Renal Injury and Dysfunction Following Elective Abdominal Aortic Surgery. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 550–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellenberger, C.; Schweizer, A.; Diaper, J.; Kalangos, A.; Murith, N.; Katchatourian, G.; Panos, A.; Licker, M. Incidence, risk factors and prognosis of changes in serum creatinine early after aortic abdominal surgery. Intensive Care Med. 2006, 32, 1808–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.; Angelini, G. Pharmacological Strategies for the Prevention of Acute Kidney Injury Following Cardiac Surgery: An Overview of Systematic Reviews. Curr. Pharm. Des. 2014, 20, 5484–5488. [Google Scholar] [CrossRef]
- Manrique-Caballero, C.L.; Kellum, J.A.; Gómez, H.; De Franco, F.; Giacchè, N.; Pellicciari, R. Innovations and Emerging Therapies to Combat Renal Cell Damage: NAD+ as a Drug Target. Antioxid. Redox. Signal. 2021, 35, 1449–1466. [Google Scholar] [CrossRef]
- Xie N, Zhang L, Gao W; et al NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Ralto, K.M.; Rhee, E.P.; Parikh, S.M. NAD+ homeostasis in renal health and disease. Nat. Rev. Nephrol. 2020, 16, 99–111. [Google Scholar] [CrossRef]
- Poyan Mehr, A.; Tran, M.T.; Ralto, K.M.; Leaf, D.E.; Washco, V.; Messmer, J.; Lerner, A.; Kher, A.; Kim, S.H.; Khoury, C.C.; et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat. Med. 2018, 24, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Devalaraja-Narashimha, K.; Singaravelu, K.; Padanilam, B.J. Poly(ADP-ribose) polymerase-mediated cell injury in acute renal failure. Pharmacol. Res. 2005, 52, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Kotlinska-Hasiec, E.; Nowicka-Stazka, P.; Parada-Turska, J.; Stazka, K.; Stazka, J.; Zadora, P.; Dabrowski, W. Plasma Kynurenic Acid Concentration in Patients Undergoing Cardiac Surgery: Effect of Anaesthesia. Arch. Immunol. Ther. Exp. (Warsz). 2015, 63, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bignon, Y.; Rinaldi, A.; Nadour, Z.; Poindessous, V.; Nemazanyy, I.; Lenoir, O.; Fohlen, B.; Weill-Raynal, P.; Hertig, A.; Karras, A.; et al. Cell stress response impairs de novo NAD+ biosynthesis in the kidney. JCI Insight 2022, 7, e153019. [Google Scholar] [CrossRef]
- Poyan Mehr, A.; Parikh, S.M. PPARγ-Coactivator-1α, Nicotinamide Adenine Dinucleotide and Renal Stress Resistance. Nephron 2017, 137, 253–255. [Google Scholar] [CrossRef]
- Parikh, S.M. Metabolic Stress Resistance in Acute Kidney Injury: Evidence for a PPAR-Gamma-Coactivator-1 Alpha-Nicotinamide Adenine Dinucleotide Pathway. Nephron 2019, 143, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Raines, N.; Cheung, M.; Wilson, L.; Edberg, J.; Erdmann, N.; Schmaier, A.; Berryhill, T.; Manickas-Hill, Z.; Li, J.; Yu, X.; et al. Nicotinamide Adenine Dinucleotide Biosynthetic Impairment and Urinary Metabolomic Alterations Observed in Hospitalized Adults with COVID-19-Related Acute Kidney Injury. Kidney Int. Rep. 2021, 6, 3002–3013. [Google Scholar] [CrossRef] [PubMed]
- Welten, G.M.; Chonchol, M.; Schouten, O.; Hoeks, S.; Bax, J.J.; van Domburg, R.T.; van Sambeek, M.; Poldermans, D. Statin use is associated with early recovery of kidney injury after vascular surgery and improved long-term outcome. Nephrol. Dial. Transplant. 2008, 23, 3867–3873. [Google Scholar] [CrossRef] [Green Version]
- Argalious, M.Y.; Dalton, J.E.; Cywinski, J.B.; Seif, J.; Abdelmalak, M.; Sessler, D.I. Association between preoperative statin therapy and postoperative change in glomerular filtration rate in endovascular aortic surgery. Br. J. Anaesth. 2012, 109, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Welten, G.M.; Chonchol, M.; Hoeks, S.E.; Schouten, O.; Dunkelgrün, M.; van Gestel, Y.R.; Goei, D.; Bax, J.J.; van Domburg, R.T.; Poldermans, D. Statin therapy is associated with improved outcomes in vascular surgery patients with renal impairment. Am. Heart J. 2007, 154, 954–961. [Google Scholar] [CrossRef]
- Moulakakis, K.G.; Matoussevitch, V.; Borgonio, A.; Gawenda, M.; Brunkwall, J. Evidence that Statins Protect Renal Function during Endovascular Repair of AAAs. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Schouten, O.; Kok, N.F.; Boersma, E.; Bax, J.J.; Feringa, H.H.; Vidakovic, R.; van Eps, R.G.S.; van Sambeek, M.R.; Poldermans, D. Effects of Statins on Renal Function after Aortic cross Clamping during Major Vascular Surgery. Am. J. Cardiol. 2006, 97, 1383–1385. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Schwieler, L.; Trepci, A.; Krzyzanowski, S.; Hermansson, S.; Granqvist, M.; Piehl, F.; Venckunas, T.; Brazaitis, M.; Kamandulis, S.; Lindqvist, D.; et al. A novel, robust method for quantification of multiple kynurenine pathway metabolites in the cerebrospinal fluid. Bioanalysis 2020, 12, 379–392. [Google Scholar] [CrossRef] [Green Version]
- Lever, J.; Krzywinski, M.; Altman, N. Principal component analysis. Nat. Methods. 2017, 14, 641–642. [Google Scholar] [CrossRef] [Green Version]
- Simic, P.; Vela Parada, X.F.; Parikh, S.M.; Dellinger, R.; Guarente, L.P.; Rhee, E.P. Nicotinamide riboside with pterostilbene (NRPT) increases NAD+ in patients with acute kidney injury (AKI): A randomized, double-blind, placebo-controlled, stepwise safety study of escalating doses of NRPT in patients with AKI. BMC Nephrol. 2020, 21, 342. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, D.; Qui, Y.; Airhart, S.; Liu, Y.; Stempien-Otero, A.; O’Brien, K.; Tian, R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020, 130, 6054–6063. [Google Scholar] [CrossRef] [PubMed]




| Characteristic * | AKI (n = 8) | No AKI (n = 8) | p-Value |
|---|---|---|---|
| Age | 70 (55, 79) | 73 (58, 83) | 0.58 |
| Female | 4 (50%) | 3 (38%) | 0.61 |
| Body mass index (kg/m2) | 27 (34, 35) | 30 (23, 37) | 0.32 |
| Medical history | |||
| Hypertension | 8 (100%) | 8 (100%) | 1 |
| Type II diabetes | 2 (25%) | 2 (25%) | 1 |
| eGFR (mL/min/1.73 m2) | 47 (34, 56) | 49 (41, 58) | 0.36 |
| Procedure characteristics | |||
| IV contrast use | 3 (38%) | 3 (38%) | 1 |
| Volume of IV contrast (mL) | 0 (0, 132) | 0 (0, 122) | 0.72 |
| Characteristic * | AKI (n = 8) | No AKI (n = 8) | p-Value |
|---|---|---|---|
| Age | 72 (65, 79) | 74 (66, 83) | 0.58 |
| Female | 4 (50%) | 2 (25%) | 0.30 |
| Body mass index (kg/m2) | 26 (23, 31) | 29 (23, 34) | 0.31 |
| Medical history | |||
| Hypertension | 8 (100%) | 8 (100%) | 1 |
| Type II diabetes | 2 (25%) | 2 (25%) | 1 |
| eGFR (mL/min/1.73 m2) | 42 (29, 56) | 47 (38, 58) | 0.14 |
| Procedure characteristics | |||
| IV contrast use | 4 (50%) | 4 (50%) | 1 |
| Volume of IV contrast (mL) | 18 (0, 132) | 0 (0, 122) | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mede, A.I.; Milne, G.L.; Wei, D.; Smith, D.K.; Smith, L.E. NAD+ Biosynthesis Impairment and Acute Kidney Injury after Major Vascular Surgery. Antioxidants 2023, 12, 821. https://doi.org/10.3390/antiox12040821
Mede AI, Milne GL, Wei D, Smith DK, Smith LE. NAD+ Biosynthesis Impairment and Acute Kidney Injury after Major Vascular Surgery. Antioxidants. 2023; 12(4):821. https://doi.org/10.3390/antiox12040821
Chicago/Turabian StyleMede, Annmarie I., Ginger L. Milne, Dawei Wei, Derek K. Smith, and Loren E. Smith. 2023. "NAD+ Biosynthesis Impairment and Acute Kidney Injury after Major Vascular Surgery" Antioxidants 12, no. 4: 821. https://doi.org/10.3390/antiox12040821
APA StyleMede, A. I., Milne, G. L., Wei, D., Smith, D. K., & Smith, L. E. (2023). NAD+ Biosynthesis Impairment and Acute Kidney Injury after Major Vascular Surgery. Antioxidants, 12(4), 821. https://doi.org/10.3390/antiox12040821