The Antioxidant Properties of Lavandula multifida Extract Contribute to Its Beneficial Effects in High-Fat Diet-Induced Obesity in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Preparation of the Extract
2.3. Chemical Composition of LME Using UHPLC-MS Conditions
2.4. Total Phenolic Content and Antioxidant Capacity Assays
2.5. In Vitro Studies
2.6. Animals and Experimental Design
2.7. Glucose Tolerance Test
2.8. Biochemical Determinations
2.9. Thiobarbituric Acid Reactive Substance Assay
2.10. Histological Studies
2.11. Analysis of Gene Expression by RT-qPCR
2.12. Analysis of Protein Expression by Western Blot
2.13. Statistic
3. Results and Discussion
3.1. Chemical Characterization of LME
3.2. Antioxidant Capacity of LME
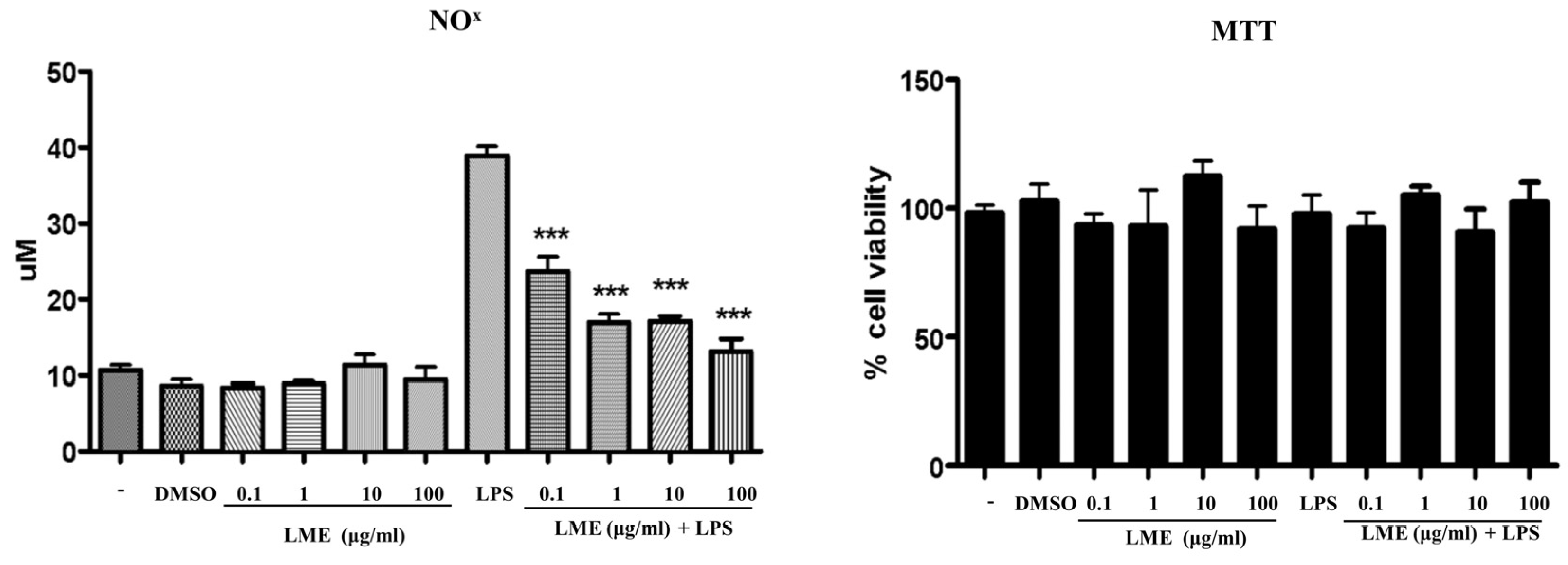
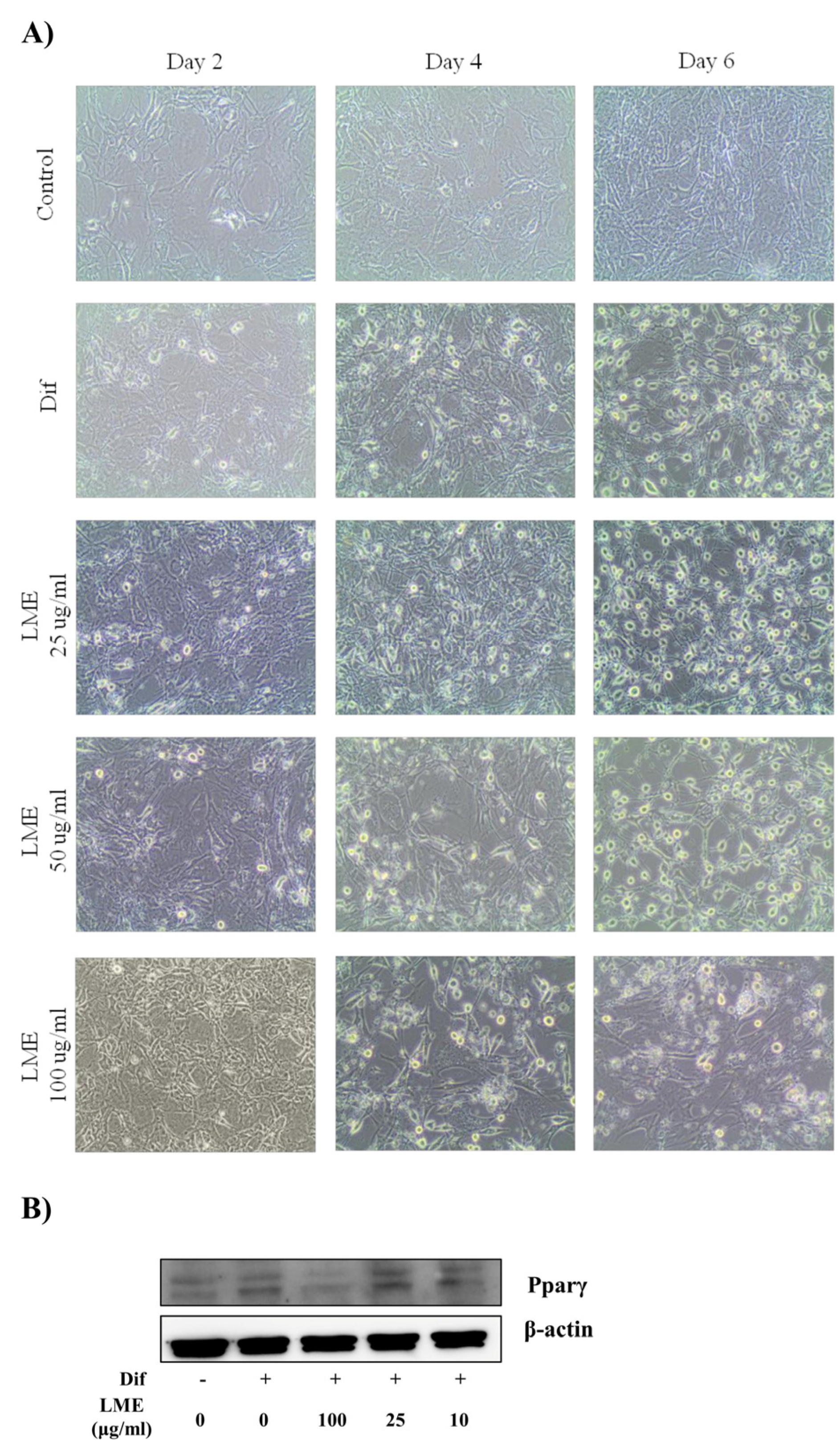
3.3. Effects of LME on Nitrite Production in RAW 264 Cells and Adipogenesis in 3T3-L1 Cells
3.4. Effects of LME on Weight Evolution, Glucose Tolerance Test, and Plasma Biochemical Profile
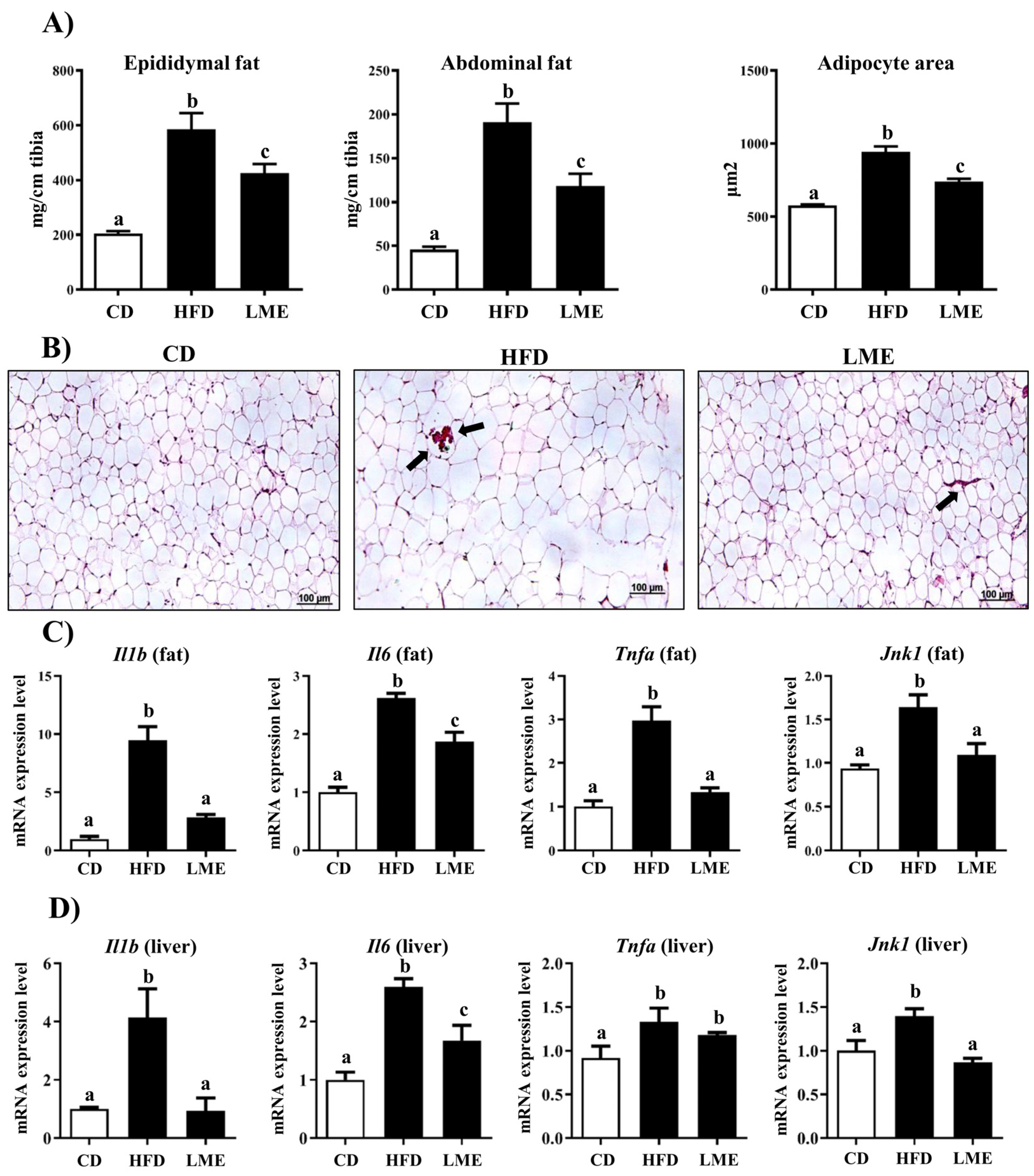
3.5. Effects of LME on the Systemic Inflammatory Status in Metabolic Tissues
3.6. Effects of LME Treatment on Intestinal Barrier Dysfunction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 February 2023).
- Mehrzad, R. Etiology of obesity. In Obesity; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Kachur, S.; Lavie, C.J.; Carbone, S.; Pandey, A.; Ortega, F.B.; Milani, R.V. An Overview and Update on Obesity and the Obesity Paradox in Cardiovascular Diseases. Prog. Cardiovasc. Dis. 2018, 61, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.H.; Umashanker, D.; Igel, L.I.; Kumar, R.B.; Aronne, L.J. Obesity Pharmacotherapy. Med. Clin. N. Am. 2018, 102, 135–148. [Google Scholar] [CrossRef]
- Bahmani, M.; Eftekhari, Z.; Saki, K.; Fazeli-Moghadam, E.; Jelodari, M.; Rafieian-Kopaei, M. Obesity Phytotherapy: Review of Native Herbs Used in Traditional Medicine for Obesity. J. Evid. Based Complement. Altern. Med. 2016, 21, 228–234. [Google Scholar] [CrossRef]
- Sebai, H.; Selmi, S.; Rtibi, K.; Souli, A.; Gharbi, N.; Sakly, M. Lavender (Lavandula stoechas L.) essential oils attenuate hyperglycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids Health Dis. 2013, 12, 189. [Google Scholar] [CrossRef] [Green Version]
- Dobros, N.; Zawada, K.; Paradowska, K. Phytochemical Profile and Antioxidant Activity of Lavandula angustifolia and Lavandula x intermedia Cultivars Extracted with Different Methods. Antioxidants 2022, 11, 711. [Google Scholar] [CrossRef]
- El-Hilaly, J.; Hmammouchi, M.; Lyoussi, B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco). J. Ethnopharmacol. 2003, 86, 149–158. [Google Scholar] [CrossRef]
- Sosa, S.; Altinier, G.; Politi, M.; Braca, A.; Morelli, I.; Della Loggia, R. Extracts and constituents of Lavandula multifida with topical anti-inflammatory activity. Phytomedicine 2005, 12, 271–277. [Google Scholar] [CrossRef]
- Al-Mijalli, S.; Elsharkawy, E.L.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Alshahrani, M.M.; Mrabti, H.N.; Bouyahya, A. Determination of Volatile Compounds of Mentha piperita and Lavandula multifida and Investigation of Their Antibacterial, Antioxidant, and Antidiabetic Properties. Evid. Based Complement. Altern. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef]
- Algieri, F.; Rodriguez-Nogales, A.; Vezza, T.; Garrido-Mesa, J.; Garrido-Mesa, N.; Utrilla, M.P.; Gonzalez-Tejero, M.R.; Casares-Porcel, M.; Molero-Mesa, J.; Del Mar Contreras, M.; et al. Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L. J. Ethnopharmacol. 2016, 190, 142–158. [Google Scholar] [CrossRef]
- Cadiz-Gurrea, M.L.; Pinto, D.; Delerue-Matos, C.; Rodrigues, F. Olive Fruit and Leaf Wastes as Bioactive Ingredients for Cosmetics-A Preliminary Study. Antioxidants 2021, 10, 245. [Google Scholar] [CrossRef]
- Malterud, K.E.; Farbrot, T.L.; Huse, A.E.; Sund, R.B. Antioxidant and radical scavenging effects of anthraquinones and anthrones. Pharmacology 1993, 47 (Suppl. S1), 77–85. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Malagon, A.J.; Rodriguez-Sojo, M.J.; Hidalgo-Garcia, L.; Molina-Tijeras, J.A.; Garcia, F.; Pischel, I.; Romero, M.; Duarte, J.; Diez-Echave, P.; Rodriguez-Cabezas, M.E.; et al. The Antioxidant Activity of Thymus serpyllum Extract Protects against the Inflammatory State and Modulates Gut Dysbiosis in Diet-Induced Obesity in Mice. Antioxidants 2022, 11, 1073. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5, 2048004016633371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanai, H.; Tomono, Y.; Ito, K.; Furutani, N.; Yoshida, H.; Tada, N. The underlying mechanisms for development of hypertension in the metabolic syndrome. Nutr. J. 2008, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Haddouchi, F.; Chaouche, T.M.; Saker, M.; Ghellai, I.; Boudjemai, O. Phytochemical screening, phenolic content and antioxidant activity of Lavandula species extracts from Algeria. J. Fac. Pharm. Istanb. Univ. 2021, 51, 111–118. [Google Scholar] [CrossRef]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pushp, P.; Sharma, N.; Joseph, G.S.; Singh, R.P. Antioxidant activity and detection of (-)epicatechin in the methanolic extract of stem of Tinospora cordifolia. J. Food Sci. Technol. 2013, 50, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Yesilbursa, D.; Serdar, Z.; Serdar, A.; Sarac, M.; Coskun, S.; Jale, C. Lipid peroxides in obese patients and effects of weight loss with orlistat on lipid peroxides levels. Int. J. Obes. Lond 2005, 29, 142–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 2015, 16, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Murugan, D.D.; Khan, H.; Huang, Y.; Cheang, W.S. Roles and Therapeutic Implications of Endoplasmic Reticulum Stress and Oxidative Stress in Cardiovascular Diseases. Antioxidants 2021, 10, 1167. [Google Scholar] [CrossRef]
- Diez-Echave, P.; Vezza, T.; Rodriguez-Nogales, A.; Ruiz-Malagon, A.J.; Hidalgo-Garcia, L.; Garrido-Mesa, J.; Molina-Tijeras, J.A.; Romero, M.; Robles-Vera, I.; Pimentel-Moral, S.; et al. The prebiotic properties of Hibiscus sabdariffa extract contribute to the beneficial effects in diet-induced obesity in mice. Food Res. Int. 2020, 127, 108722. [Google Scholar] [CrossRef]
- Tijani, R.O.; Molina-Tijeras, J.A.; Vezza, T.; Ruiz-Malagon, A.J.; Cadiz-Gurrea, M.L.; Segura-Carretero, A.; Abiodun, O.O.; Galvez, J. Myrianthus arboreus P. Beauv improves insulin sensitivity in high fat diet-induced obese mice by reducing inflammatory pathways activation. J. Ethnopharmacol. 2022, 282, 114651. [Google Scholar] [CrossRef]
- Suganami, T.; Tanaka, M.; Ogawa, Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocr. J. 2012, 59, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Tandon, P.; Wafer, R.; Minchin, J.E.N. Adipose morphology and metabolic disease. J. Exp. Biol. 2018, 221, 164970. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef] [Green Version]
- Stienstra, R.; Duval, C.; Muller, M.; Kersten, S. PPARs, Obesity, and Inflammation. PPAR Res. 2007, 2007, 95974. [Google Scholar] [CrossRef] [Green Version]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Ricote, M.; Li, A.C.; Willson, T.M.; Kelly, C.J.; Glass, C.K. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998, 391, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, X.; Yu, S.; Zheng, R. The Leptin Resistance. Adv. Exp. Med. Biol. 2018, 1090, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Molina-Tijeras, J.A.; Diez-Echave, P.; Vezza, T.; Hidalgo-Garcia, L.; Ruiz-Malagon, A.J.; Rodriguez-Sojo, M.J.; Romero, M.; Robles-Vera, I.; Garcia, F.; Plaza-Diaz, J.; et al. Lactobacillus fermentum CECT5716 ameliorates high fat diet-induced obesity in mice through modulation of gut microbiota dysbiosis. Pharmacol. Res. 2021, 167, 105471. [Google Scholar] [CrossRef] [PubMed]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflug. Arch. 2020, 472, 1273–1298. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbrini, E.; Magkos, F. Hepatic Steatosis as a Marker of Metabolic Dysfunction. Nutrients 2015, 7, 4995–5019. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Kahn, B.B.; Shi, H.; Xue, B.Z. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 2010, 285, 19051–19059. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.J.; Gauthier, M.S.; Hess, D.T.; Apovian, C.M.; Cacicedo, J.M.; Gokce, N.; Farb, M.; Valentine, R.J.; Ruderman, N.B. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J. Lipid. Res. 2012, 53, 792–801. [Google Scholar] [CrossRef] [Green Version]
- Shuai, L.; Zhang, L.N.; Li, B.H.; Tang, C.L.; Wu, L.Y.; Li, J.; Li, J.Y. SIRT5 Regulates Brown Adipocyte Differentiation and Browning of Subcutaneous White Adipose Tissue. Diabetes 2019, 68, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.J.; Dai, Y.W.; Wang, C.L.; Fang, L.W.; Huang, W.C. Maslinic acid protects against obesity-induced nonalcoholic fatty liver disease in mice through regulation of the Sirt1/AMPK signaling pathway. FASEB J. 2019, 33, 11791–11803. [Google Scholar] [CrossRef] [Green Version]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Ott, B.; Skurk, T.; Hastreiter, L.; Lagkouvardos, I.; Fischer, S.; Buttner, J.; Kellerer, T.; Clavel, T.; Rychlik, M.; Haller, D.; et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci. Rep. 2017, 7, 11955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergstrom, K.S.; Guttman, J.A.; Rumi, M.; Ma, C.; Bouzari, S.; Khan, M.A.; Gibson, D.L.; Vogl, A.W.; Vallance, B.A. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen. Infect. Immun. 2008, 76, 796–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Rodriguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sanchez, M.; Toral, M.; Martin-Garcia, B.; Gomez-Caravaca, A.M.; Arraez-Roman, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar] [CrossRef]
- Sharifnia, T.; Antoun, J.; Verriere, T.G.; Suarez, G.; Wattacheril, J.; Wilson, K.T.; Peek, R.M., Jr.; Abumrad, N.N.; Flynn, C.R. Hepatic TLR4 signaling in obese NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G270–G278. [Google Scholar] [CrossRef] [Green Version]
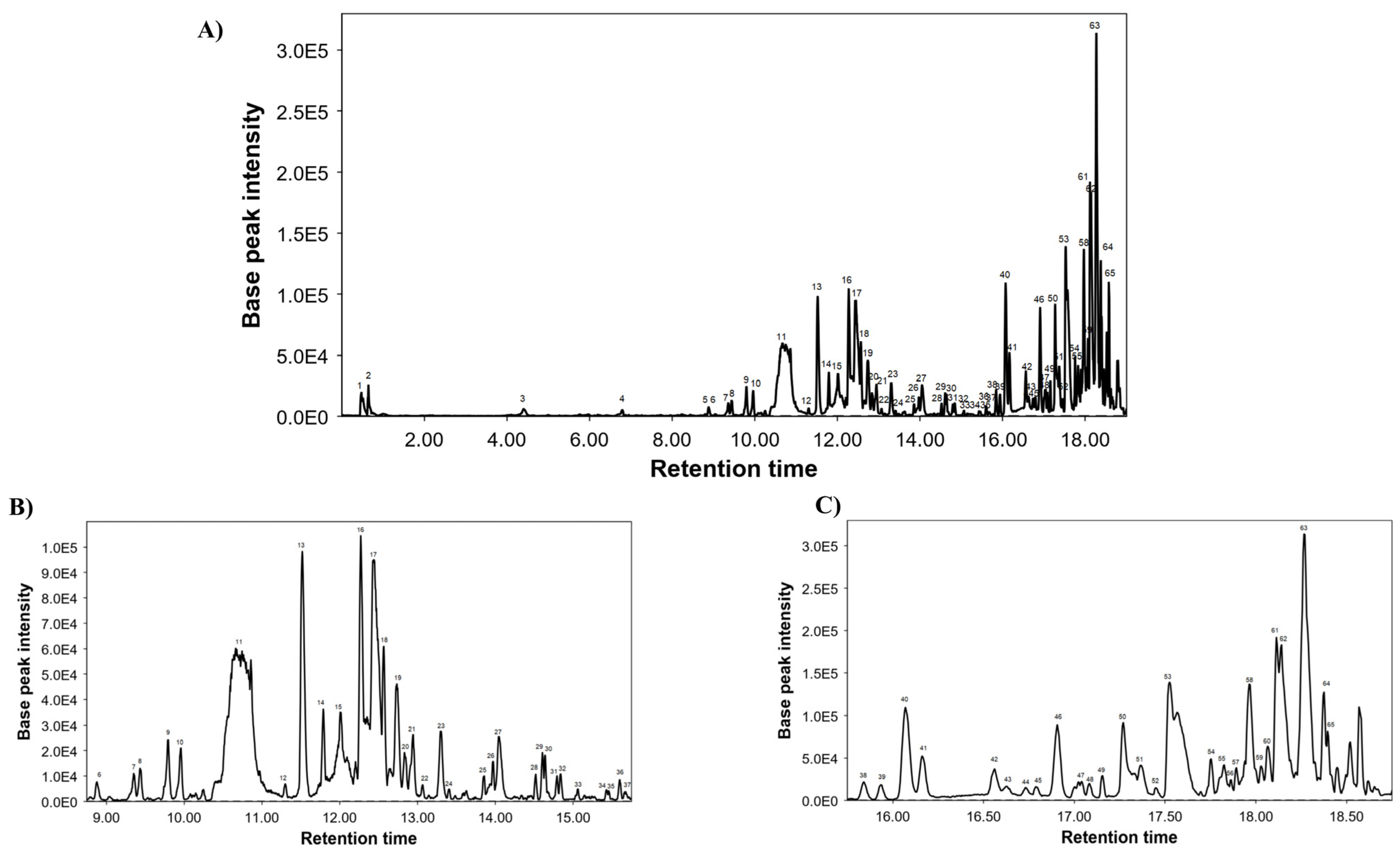





| Peak | RT | m/z | Molecular Formula | Proposed Compounds |
|---|---|---|---|---|
| 1 | 0.47 | 343.0356 | C13H12O11 | Mucic acid lactone gallate |
| 2 | 0.61 | 341.1075 | C12H22O11 | Sucrose |
| 3 | 4.42 | 133.0283 | C4H6O5 | Malic acid |
| 4 | 6.79 | 371.0964 | C16H20O10 | Dihydroferulic acid glucuronide |
| 5 | 8.87 | 567.0776 | C26H32O14 | Phloretin xyloglucoside |
| 6 | 8.89 | 301.0705 | C16H14O6 | Hesperetin |
| 7 | 9.35 | 463.0876 | C21H20O12 | Quercetin glucoside |
| 8 | 9.43 | 593.0958 | C29H22O14 | (Epi)catechin digallate |
| 9 | 9.79 | 447.0922 | C21H20O11 | Luteolin 7-O-glucoside |
| 10 | 9.96 | 609.1448 | C27H30O16 | Rutin |
| 11 | 10.71 | 473.0711 | C22H18O12 | Chicoric acid |
| 12 | 11.3 | 489.1024 | C23H22O12 | Kaempferol acetyl-glucopyranoside |
| 13 | 11.52 | 477.0664 | C21H18O13 | Quercetin glucuronide |
| 14 | 11.79 | 461.0717 | C21H18O12 | Isoscutellarin 8-O-glucoronide |
| 15 | 12.01 | 503.3370 | C30H48O6 | Madecassic acid or its isomer |
| 16 | 12.27 | 491.0823 | C22H20O13 | Isorhamnetin 3-O-glucuronide |
| 17 | 12.44 | 307.0446 | C14H12O8 | Fulvic acid analogue 1 |
| 18 | 12.57 | 839.4089 | C42H64O17 | Yunganoside G2 or its isomer |
| 19 | 12.74 | 519.0928 | C27H20O11 | Citreaglycon A |
| 20 | 12.83 | 533.1661 | C26H30O12 | Amurensin |
| 21 | 12.94 | 839.4052 | C42H64O17 | Yunganoside G2 or its isomer |
| 22 | 13.07 | 545.3464 | C32H50O7 | Hovenidulcigenin B or its isomer |
| 23 | 13.29 | 839.4059 | C42H64O17 | Yunganoside G2 or its isomer |
| 24 | 13.41 | 545.3454 | C32H50O7 | Hovenidulcigenin B or its isomer |
| 25 | 13.86 | 939.3139 | C50H52O18 | Unknown |
| 26 | 13.89 | 327.2169 | C18H32O5 | Fatty acid |
| 27 | 14.07 | 307.0446 | C14H12O8 | Fulvic acid analogue 2 |
| 28 | 14.52 | 503.3365 | C30H48O6 | Madecassic acid or its isomer |
| 29 | 14.60 | 823.4134 | C42H64O16 | Licoricesaponin J2 or its isomer |
| 30 | 14.64 | 329.2487 | C30H48O7 | Fatty acid |
| 31 | 14.79 | 823.4134 | C42H64O16 | Licoricesaponin J2 or its isomer |
| 32 | 14.82 | 519.3374 | C30H48O7 | Hydroxyecdysone monoacetonide |
| 33 | 15.06 | 287.2228 | C16H32O4 | Fatty acid |
| 34 | 15.43 | 501.3208 | C30H46O6 | Medicagenic acid or its isomer |
| 35 | 15.46 | 777.2611 | C41H46O15 | Guaiacylglycerol buddlenol A |
| 36 | 15.6 | 501.3208 | C30H46O6 | Medicagenic acid or its isomer |
| 37 | 15.66 | 501.3304 | C30H46O6 | Medicagenic acid or its isomer |
| 38 | 15.84 | 503.3363 | C30H48O6 | Madecassic acid or its isomer |
| 39 | 15.93 | 503.3361 | C30H48O6 | Madecassic acid or its isomer |
| 40 | 16.07 | 503.3358 | C30H48O6 | Madecassic acid or its isomer |
| 41 | 16.16 | 503.3359 | C30H48O6 | Madecassic acid or its isomer |
| 42 | 16.56 | 503.3352 | C30H48O6 | Madecassic acid or its isomer |
| 43 | 16.63 | 503.3351 | C30H48O6 | Madecassic acid or its isomer |
| 44 | 16.73 | 503.3347 | C30H48O6 | Madecassic acid or its isomer |
| 45 | 16.76 | 677.3508 | C36H54O12 | Bryoamaride or its isomer |
| 46 | 16.9 | 677.353 | C36H54O12 | Bryoamaride or its isomer |
| 47 | 17.04 | 485.3261 | C30H46O5 | Quillaic acid or its isomer |
| 48 | 17.08 | 485.3249 | C30H46O5 | Quillaic acid or its isomer |
| 49 | 17.16 | 441.3369 | C29H46O3 | Camellenodiol |
| 50 | 17.24 | 487.3403 | C30H48O5 | Asiatic acid or its isomer |
| 51 | 17.37 | 677.3522 | C36H54O12 | Bryoamaride or its isomer |
| 52 | 17.45 | 487.3406 | C30H48O5 | Asiatic acid or its isomer |
| 53 | 17.52 | 487.3409 | C30H48O5 | Asiatic acid or its isomer |
| 54 | 17.75 | 295.2265 | C18H32O3 | Fatty acid |
| 55 | 17.81 | 471.3475 | C30H48O4 | Maslinic acid or its isomer |
| 56 | 17.86 | 293.2109 | C18H30O3 | Fatty acid |
| 57 | 17.89 | 425.3413 | C29H46O2 | Stigmastene dione |
| 58 | 17.96 | 469.3408 | C30H46O4 | Glycyrrhetinic acid |
| 59 | 18.03 | 471.3473 | C30H48O4 | Maslinic acid or its isomer |
| 60 | 18.06 | 471.3464 | C30H48O4 | Maslinic acid or its isomer |
| 61 | 18.11 | 471.347 | C30H48O4 | Maslinic acid or its isomer |
| 62 | 18.14 | 471.3461 | C30H48O4 | Maslinic acid or its isomer |
| 63 | 18.27 | 471.3476 | C30H48O4 | Maslinic acid or its isomer |
| 64 | 18.38 | 277.2156 | C18H30O2 | Fatty acid |
| 65 | 18.4 | 467.3159 | C27H48O6 | Fatty acid |
| Method | Value |
|---|---|
| Folin-Ciocalteu (mg GAE/g d.e.) | 179 ± 1 |
| FRAP (mmol eq. FeSO4/g d.e.) | 2.576 ± 0.002 |
| TEAC (mmol eq. Trolox/g d.e.) | 1.30 ± 0.02 |
| ORAC (mmol eq. Trolox/g d.e.) | 2.08 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Tijeras, J.A.; Ruiz-Malagón, A.J.; Hidalgo-García, L.; Diez-Echave, P.; Rodríguez-Sojo, M.J.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A.; del Palacio, J.P.; González-Tejero, M.R.; Rodríguez-Cabezas, M.E.; et al. The Antioxidant Properties of Lavandula multifida Extract Contribute to Its Beneficial Effects in High-Fat Diet-Induced Obesity in Mice. Antioxidants 2023, 12, 832. https://doi.org/10.3390/antiox12040832
Molina-Tijeras JA, Ruiz-Malagón AJ, Hidalgo-García L, Diez-Echave P, Rodríguez-Sojo MJ, Cádiz-Gurrea MdlL, Segura-Carretero A, del Palacio JP, González-Tejero MR, Rodríguez-Cabezas ME, et al. The Antioxidant Properties of Lavandula multifida Extract Contribute to Its Beneficial Effects in High-Fat Diet-Induced Obesity in Mice. Antioxidants. 2023; 12(4):832. https://doi.org/10.3390/antiox12040832
Chicago/Turabian StyleMolina-Tijeras, Jose Alberto, Antonio Jesús Ruiz-Malagón, Laura Hidalgo-García, Patricia Diez-Echave, María Jesús Rodríguez-Sojo, María de la Luz Cádiz-Gurrea, Antonio Segura-Carretero, José Pérez del Palacio, María Reyes González-Tejero, María Elena Rodríguez-Cabezas, and et al. 2023. "The Antioxidant Properties of Lavandula multifida Extract Contribute to Its Beneficial Effects in High-Fat Diet-Induced Obesity in Mice" Antioxidants 12, no. 4: 832. https://doi.org/10.3390/antiox12040832
APA StyleMolina-Tijeras, J. A., Ruiz-Malagón, A. J., Hidalgo-García, L., Diez-Echave, P., Rodríguez-Sojo, M. J., Cádiz-Gurrea, M. d. l. L., Segura-Carretero, A., del Palacio, J. P., González-Tejero, M. R., Rodríguez-Cabezas, M. E., Gálvez, J., Rodríguez-Nogales, A., Vezza, T., & Algieri, F. (2023). The Antioxidant Properties of Lavandula multifida Extract Contribute to Its Beneficial Effects in High-Fat Diet-Induced Obesity in Mice. Antioxidants, 12(4), 832. https://doi.org/10.3390/antiox12040832












