The Influence of 5′,8-Cyclo-2′-Deoxyguanosine on ds-DNA Charge Transfer Depends on Its Diastereomeric Form: A Theoretical Study
Abstract
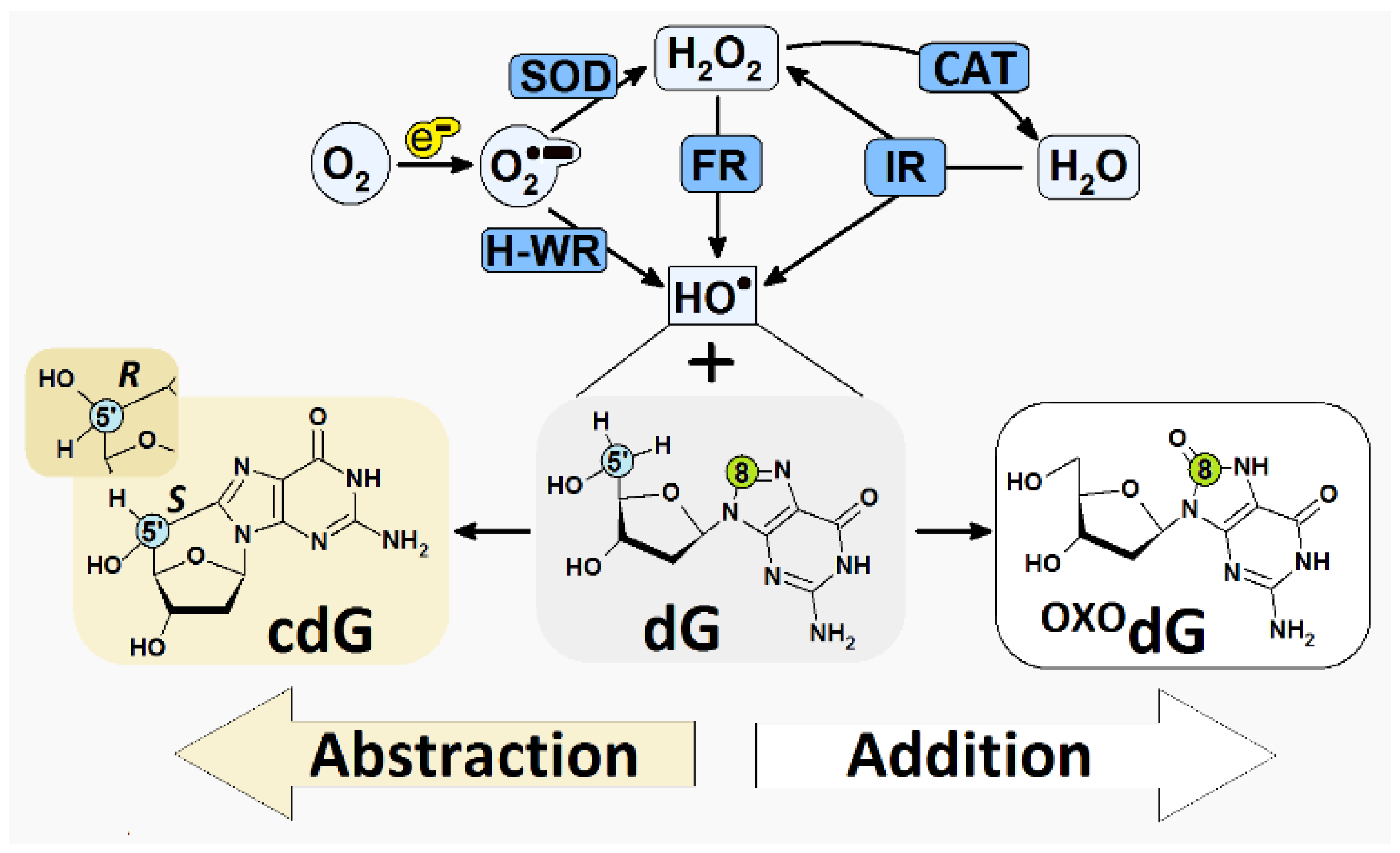
1. Introduction
2. Materials and Methods
2.1. Computation Methodology of ONIOM Studies
2.2. Computation Methodology of DFT Study
3. Results and Discussion
3.1. The Influence of (5′R/S)cdG on the Double Helix Structure in Comparison with OXOdG
3.2. The Influence of (5′R/S)cdG on Charge and Spin Distribution within the Double Helix
3.3. Electronic Properties of Oligo-RcdG and Oligo-ScdG
3.4. Charge Transfer through ds-DNA Containing (’’R/S)cdG and OXOdG
4. Conclusions
- A global structural analysis shows that oligo-ScdG containing (5′S)cdG had a greater geometry susceptibility than oligo-RcdG in which (5′R)cdG was present towards electron loss or adoption, which was shown by an RMSD value calculation.
- The electron loss causes Rise factor increases between cG2C4 and A3T3 base-pairs by 0.13/0.04 [Å] with subsequent distance shortening in the case of the A3T3; OG4C2 dimer by 0.34/0.24 [Å] for oligo-RcdG/oligo-ScdG, respectively.
- The excess electron adoption by oligo-RcdG leads to Rise parameter increases within [cG2A3]*[C4T3] and [A3OXOG4]*[C2T3] by 0.11 [Å] and 0.15 [Å], respectively. Conversely, the Rise parameter assigned for the corresponding BP-dimers isolated from oligo-ScdG showed more significant increases in both cases, i.e., 0.3 [Å] for the [cG2A3]*[T3C4] and 0.24 [Å] for the [A3OG4]*[C2T3] systems.
- The charge and spin distribution analysis in vertical and adiabatic modes showed that the radical cation is mainly located at the OXOdG4C2 base-pair, irrespective of the diastereomeric form of the second lesion. Contrary to the above, electron adoption by oligo-RcdG and oligo-ScdG leads to different consequences in the case of oligo-ScdG. The negative charge was mainly found at the (5′S)cdG2C4 base-pair (in vertical and adiabatic ds-oligo forms), while the presence of (5‘R)cdG leads to an initial negative charge appearing at (5′R)cdG2C4 with subsequent migration towards OXOdG4C2.
- The charge transfer investigation, according to Marcus theory, revealed that in the case of (5′R/S)cdG, the radical cation migration towards the OXOdG4C2 base pair is found to be privileged. However, in the case of excess electron transfer, differences were observed between (5′R) and (5′S)cdG when present in the ds-oligo structure. In the case of oligo-RcdG, the electron transfer towards the OXOdG4C2 moiety was noted as privileged, while in the case of oligo-ScdG, it was privileged towards (5′S)cdG2C4.
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Sudhir Ambekar, S. DNA: Damage and Repair Mechanisms in Humans. Glob. J. Pharm. Pharm. Sci. 2017, 3, 7. [Google Scholar] [CrossRef]
- Powell, S.N.; Bindra, R.S. Targeting the DNA damage response for cancer therapy. DNA Repair (Amst) 2009, 8, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid Redox Signal 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Pryor, W.A.; Houk, K.N.; Foote, C.S.; Fukuto, J.M.; Ignarro, L.J.; Squadrito, G.L.; Davies, K.J.A. Free radical biology and medicine: It’s a gas, man! Am. J. Physiol.–Regul. Integr. Comp. Physiol. 2006, 291, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxygen Radicals in Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 1989; Volume 256, ISBN 9781468455700. [Google Scholar]
- Kuznetsova, A.A.; Knorre, D.G.; Fedorova, O.S. Oxidation of DNA and its components with reactive oxygen species. Russ. Chem. Rev. 2009, 78, 659–678. [Google Scholar] [CrossRef]
- Bell, M.; Kumar, A.; Sevilla, M.D. Electron-Induced Repair of 2 -Deoxyribose Sugar Radicals in DNA: A Density Functional Theory (DFT) Study. Int. J. Mol. Sci. 2021, 22, 1736. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, B.; Pogozelski, W.K.; Tullius, T.D. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc. Natl. Acad. Sci. USA 1998, 95, 9738–9743. [Google Scholar] [CrossRef]
- Miaskiewicz, K.; Miller, J.H.; Fuciarelli, A.F. Theoretical analysis of DNA intrastrand cross linking by formation of 8,5′-cyclodeoxyadenosine. Nucleic Acids Res. 1995, 23, 515–521. [Google Scholar] [CrossRef]
- Krokidis, M.G.; Terzidis, M.A.; Efthimiadou, E.; Zervou, S.K.; Kordas, G.; Papadopoulos, K.; Hiskia, A.; Kletsas, D.; Chatgilialoglu, C. Purine 5′,8-cyclo-2′-deoxynucleoside lesions: Formation by radical stress and repair in human breast epithelial cancer cells. Free Radic. Res. 2017, 51, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Candeais, L.P.; Steenken, S. Reaction of HO• with guanine derivatives in aqueous solution: Formation of two different redox-active OH-adduct radicals and their unimolecular transformation reactions. Properties of G(-H)•. Chem. A Eur. J. 2000, 6, 475–484. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Kirkali, G.; Jaruga, P. Formamidopyrimidines in DNA: Mechanisms of formation, repair, and biological effects. Free Radic. Biol. Med. 2008, 45, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Wagner, R.J. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef]
- Scanlan, L.D.; Coskun, S.H.; Jaruga, P.; Hanna, S.K.; Sims, C.M.; Almeida, J.L.; Catoe, D.; Coskun, E.; Golan, R.; Dizdaroglu, M.; et al. Measurement of Oxidatively Induced DNA Damage in Caenorhabditis elegans with High-Salt DNA Extraction and Isotope-Dilution Mass Spectrometry. Anal. Chem. 2019, 91, 12149–12155. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, S.K.; Boguszewska, K.; Szewczuk, M.; Kázmierczak-Barańska, J.; Karwowski, B.T. 8-Oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) as a potential biomarker for gestational diabetes mellitus (GDM) development. Molecules 2020, 25, 202. [Google Scholar] [CrossRef] [PubMed]
- Steenken, S.; Jovanovic, S.V. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997, 119, 617–618. [Google Scholar] [CrossRef]
- Sancar, A.; Lindsey-Boltz, L.A.; Ünsal-Kaçmaz, K.; Linn, S. Molecular Mechanisms of Mammalian DNA Repair and the DNA Damage Checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.C.; Corbett, A.H.; Doetsch, P.W. The current state of eukaryotic DNA base damage and repair. Nucleic Acids Res. 2015, 43, 10083–10101. [Google Scholar] [CrossRef] [PubMed]
- Farnell, D.A. Nucleotide Excision Repair in the Three Domains of Life. West. Undergrad. Res. J. Health Nat. Sci. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Geacintov, N.E.; Krokidis, M.G.; Liu, Y.; Masi, A.; Shafirovich, V.; Terzidis, M.A.; Tsegay, P.S. 5′,8-Cyclopurine Lesions in DNA Damage: Chemical, Analytical, Biological, and Diagnostic Significance. Cells 2019, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.J. The cyclopurine deoxynucleosides: DNA repair, biological effects, mechanistic insights, and unanswered questions. Free. Radic. Biol. Med. 2016, 107, 90–100. [Google Scholar] [CrossRef]
- Kraemer, K.H.; Patronas, N.J.; Schiffmann, R.; Brooks, B.P.; Tamura, D.; DiGiovanna, J.J. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: A complex genotype-phenotype relationship. Neuroscience 2007, 145, 1388–1396. [Google Scholar] [CrossRef]
- Brooks, P.J. The case for 8,5′-cyclopurine-2′-deoxynucleosides as endogenous DNA lesions that cause neurodegeneration in xeroderma pigmentosum. Neuroscience 2007, 145, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Boal, A.K.; Yavin, E.; Lukianova, O.A.; O’Shea, V.L.; David, S.S.; Barton, J.K. DNA-bound redox activity of DNA repair glycosylases containing [4Fe-4S] clusters. Biochemistry 2005, 44, 8397–8407. [Google Scholar] [CrossRef]
- Sontz, P.A.; Muren, N.B.; Barton, J.K. DNA charge transport for sensing and signaling. Acc. Chem. Res. 2012, 45, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Singh, R.R.P.; Cox, D.L. Theoretical study of DNA damage recognition via electron transfer from the [4Fe-4S] complex of MutY. Biophys. J. 2008, 95, 3259–3268. [Google Scholar] [CrossRef] [PubMed]
- Fuss, J.O.; Tsai, C.; Ishida, J.P.; Tainer, J.A. Biochimica et Biophysica Acta Emerging critical roles of Fe–S clusters in DNA replication and repair. BBA Mol. Cell Res. 2015, 1853, 1253–1271. [Google Scholar] [CrossRef]
- Nano, A.; Furst, A.L.; Hill, M.G.; Barton, J.K. DNA Electrochemistry: Charge-Transport Pathways through DNA Films on Gold. J. Am. Chem. Soc. 2021, 143, 11631–11640. [Google Scholar] [CrossRef]
- Pheeney, C.G.; Barton, J.K. DNA Electrochemistry with Tethered Methylene Blue. Langmuir 2012, 28, 7063–7070. [Google Scholar] [CrossRef]
- Rajski, S.R.; Jackson, B.A.; Barton, J.K. DNA repair: Models for damage and mismatch recognition. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2000, 447, 49–72. [Google Scholar] [CrossRef]
- Boal, A.K.; Barton, J.K. Electrochemical Detection of Lesions in DNA. Bioconjug Chem. 2005, 16, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. Free Radical Biology and Medicine Formation and processing of DNA damage substrates for the hNEIL enzymes. Free Radic. Biol. Med. 2017, 107, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.M. The formamidopyrimidines: Purine lesions formed in competition with 8-oxopurines from oxidative stress. Acc. Chem. Res. 2012, 45, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Dapprich, S.; Komáromi, I.; Byun, K.S.; Morokuma, K.; Frisch, M.J. A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. THEOCHEM 1999, 461–462, 1–21. [Google Scholar] [CrossRef]
- Zhao, Y.; Pu, J.; Lynch, B.J.; Truhlar, D.G. Tests of second-generation and third-generation density functionals for thermochemical kinetics. Phys. Chem. Chem. Phys. 2004, 6, 673. [Google Scholar] [CrossRef]
- Gu, J.; Xie, Y.; Schaefer, H.F. Electron attachment to nucleotides in aqueous solution. ChemPhysChem 2006, 7, 1885–1887. [Google Scholar] [CrossRef]
- Gu, J.; Xie, Y.; Schaefer, H.F. Electron attachment to DNA single strands: Gas phase and aqueous solution. Nucleic Acids Res. 2007, 35, 5165–5172. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, J.; Leszczynski, J. Electron Attachment-Induced DNA Single Strand Breaks: C3′–O3′ σ-Bond Breaking of Pyrimidine Nucleotides Predominates. J. Am. Chem. Soc. 2006, 128, 9322–9323. [Google Scholar] [CrossRef]
- Hehre, W.J.; Radom, L.; Schleyer, P.R.; Pople, J.; Wiley, J.; Wiberg, K.B.; Clark, T.; York, N. Ab Initio Molecular Orbital Theory. J. Comput. Chem. 1986, 7, 379–383. [Google Scholar] [CrossRef]
- Karwowski, B.T. The 2Ih and OXOG Proximity Consequences on Charge Transfer through ds-DNA: Theoretical Studies of Clustered DNA Damage. Molecules 2023, 28, 2180. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Tong, P.Q. Time-dependent density-functional theory for multicomponent systems. Phys. Rev. A 1986, 34, 529–532. [Google Scholar] [CrossRef]
- Lange, A.W.; Herbert, J.M. Both intra- and interstrand charge-transfer excited states in aqueous B-DNA are present at energies comparable to, or just above, the 1ππ* excitonic bright states. J. Am. Chem. Soc. 2009, 131, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Miertus, S.; Tomasi, J. Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem. Phys. 1982, 65, 239–245. [Google Scholar] [CrossRef]
- Kumar, A.; Adhikary, A.; Sevilla, M.D.; Close, D.M. One-electron oxidation of ds(5′-GGG-3′) and ds(5′-G(8OG)G-3′) and the nature of hole distribution: A density functional theory (DFT) study. Phys. Chem. Chem. Phys. 2020, 22, 5078–5089. [Google Scholar] [CrossRef] [PubMed]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Cave, R.J.; Newton, M.D. Generalization of the Mulliken-Hush treatment for the calculation of electron transfer matrix elements. Chem. Phys. Lett. 1996, 249, 15–19. [Google Scholar] [CrossRef]
- Karwowski, B.T. The influence of phosphorothioate on charge migration in single and double stranded DNA: A theoretical approach. Phys. Chem. Chem. Phys. 2015, 17, 21507–21516. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- BIOVIA. Discovery Studio Visualizer; v16.1.0.15350; BIOVIA: San Diego, CA, USA, 2015. [Google Scholar]
- Li, S.; Olson, W.K.; Lu, X.J. Web 3DNA 2.0 for the analysis, visualization, and modeling of 3D nucleic acid structures. Nucleic Acids Res. 2019, 47, W26–W34. [Google Scholar] [CrossRef] [PubMed]
- Jasti, V.P.; Das, R.S.; Hilton, B.A.; Weerasooriya, S.; Zou, Y.; Basu, A.K. (5′ S)-8,5′-Cyclo-2′-Deoxyguanosine is a strong block to replication, a potent pol V-dependent mutagenic lesion, and is inefficiently repaired in Escherichia coli. Biochemistry 2011, 50, 3862–3865. [Google Scholar] [CrossRef] [PubMed]
- Olson, W.K.; Bansal, M.; Burley, S.K.; Dickerson, R.E.; Gerstein, M.; Harvey, S.C.; Heinemann, U.; Lu, X.; Neidle, S.; Shakked, Z.; et al. A Standard Reference Frame for the Description of Nucleic Acid Base-pair Geometry. J. Mol. Biol. 2001, 313, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Zaliznyak, T.; Lukin, M.; De Los Santos, C. Structure and stability of duplex DNA containing (5′ S)-5′,8-cyclo-2′-deoxyadenosine: An oxidatively generated lesion repaired by NER. Chem. Res. Toxicol. 2012, 25, 2103–2111. [Google Scholar] [CrossRef]
- Kneller, G.R. Comment on “Using quaternions to calculate RMSD” [J. Comp. Chem. 25, 1849 (2004)]. J. Comput. Chem. 2005, 26, 1660–1662. [Google Scholar] [CrossRef]
- Shukla, L.I.; Adhikary, A.; Pazdro, R.; Becker, D.; Sevilla, M.D. Formation of 8-oxo-7,8-dihydroguanine-radicals in γ-irradiated DNA by multiple one-electron oxidations. Nucleic Acids Res. 2004, 32, 6565–6574. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, A.; Khanduri, D.; Sevilla, M.D. Direct Observation of the Hole Protonation State and Hole Localization Site in DNA-Oligomers. J. Am. Chem. Soc. 2009, 9, 8614–8619. [Google Scholar] [CrossRef]
- Mabesoone, M.F.J.; Palmans, A.R.A.; Meijer, E.W. Solute-Solvent Interactions in Modern Physical Organic Chemistry: Supramolecular Polymers as a Muse. J. Am. Chem. Soc. 2020, 142, 19781–19798. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, T.; Jagtap, N.S.; Erbe, A. Review of the electrical characterization of metallic nanowires on dna templates. Int. J. Mol. Sci. 2018, 19, 3019. [Google Scholar] [CrossRef]
- Jortner, J.; Bixon, M.; Langenbacher, T.; Michel-Beyerle, M.E. Charge transfer and transport in DNA. Proc. Natl. Acad. Sci. USA 1998, 95, 12759–12765. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.D.; Liu, J.; Weigel, W.; Rettig, W.; Kurnikov, I.V.; Beratan, D.N. Donor-bridge-acceptor energetics determine the distance dependence of electron tunneling in DNA. Proc. Natl. Acad. Sci. USA 2002, 99, 12536–12541. [Google Scholar] [CrossRef] [PubMed]
- Genereux, J.C.; Barton, J.K. Mechanisms for DNA charge transport. Chem. Rev. 2010, 110, 1642–1662. [Google Scholar] [CrossRef]
- Voityuk, A.A.; Rösch, N.; Bixon, M.; Jortner, J. Electronic coupling for charge transfer and transport in DNA. J. Phys. Chem. B 2000, 104, 9740–9745. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron Transfer Reactions in Chemistry: Theory and Experiment (Nobel Lecture). Angew. Chemie Int. Ed. Engl. 1993, 32, 1111–1121. [Google Scholar] [CrossRef]
- Rösch, N.; Voityuk, A.A. Quantum Chemical Calculation of Donor–Acceptor Coupling for Charge Transfer in DNA. In Long-Range Charge Transfer in DNA II; Springer: Berlin/Heidelberg, Germany, 2012; Volume 237, pp. 37–72. [Google Scholar] [CrossRef]
- Karwowski, B.T. The AT Interstrand Cross-Link: Structure, Electronic Properties, and Influence on Charge Transfer in dsDNA. Mol. Ther. Nucleic Acids 2018, 13, 665–685. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, B.T. FapydG in the Shadow of OXOdG—A Theoretical Study of Clustered DNA Lesions. Int. J. Mol. Sci. 2023, 24, 5361. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, B. How Clustered DNA Damage Can Change the Electronic Properties of ds-DNA, Differences between GAG, GAOXOG, and OXOGAOXOG. Biomolecules 2023, 13, 517. [Google Scholar] [CrossRef] [PubMed]


| Base-Pair Dimer | Oligo-RcdG | ||
|---|---|---|---|
| BPO [Å2] | Rise [Å] | Tilt [O] | |
| A1T5|cG2C4 | 3.90N/3.80C/3.89A | 2.82/2.78/2.78 | 1.30/2.17/1.29 |
| cG2C4|A3T3 | 3.55/2.80/3.54 | 2.97/3.10/3.08 | −3.06/−0.6/−2.7 |
| A3T3|OG4C2 | 3.71/4.51/3.73 | 3.29/2.95/3.44 | 1.56/1.62/−0.4 |
| OG4C2|A5T1 | 4.04/3.96/4.03 | 3.15/3.01/3.10 | −1.12/−0.5/−1.7 |
| oligo-ScdG | |||
| A1T5|cG2C4 | 3.29/3.26/3.55 | 3.17/3.24/3.11 | 12.0/11.1/11.2 |
| cG2C4|A3T3 | 4.21/3.38/4.12 | 2.90/2.94/3.20 | −1.51/−3.6/−2.3 |
| A3T3|OG4C2 | 3.79/4.15/3.91 | 3.25/3.01/3.01 | −1.33/−1.2/−0.3 |
| OG4C2|A5T1 | 4.19/4.08/4.16 | 3.16/3.02/3.31 | −1.70/0.2/−1.7 |
| Distance [Å] | Base-Pair | oligo-RcdG | oligo-ScdG |
| Pu C8-C6Py | A1T5 | 9.8/9.8/9.8 | 9.7/9.8/9.8 |
| cG2C4 | 9.9/9.9/9.9 | 9.9/9.9/10 | |
| A3T3 | 9.8/10/9.8 | 9.8/9.8/9.8 | |
| OG4C2 | 9.9/9.9/10 | 10/9.8/9.9 | |
| A5T1 | 9.8/9.8/9.8 | 9.8/9.7/9.7 | |
| RMSD [Å2] | Anion versus Neutral | ||
| ds-DNA | Base-Pair Ladder | Sugar-Phpsph. Frame | |
| oligo-RcdG | 0.22 | 0.20 | 0.24 |
| oligo-ScdG | 0.36 | 0.29 | 0.42 |
| Cation versus Neutral | |||
| oligo-RcdG | 0.54 | 0.44 | 0.62 |
| oligo-ScdG | 0.66 | 0.51 | 0.77 |
| System | VIPNE | VIPEQ | AIP | VEANE | VEAEQ | AEA |
|---|---|---|---|---|---|---|
| oligo-RdcG | (A) 6.51 | 5.89 | 5.47 | −0.99 | −1.33 | −2.11 |
| (B) 6.32 | 5.82 | 5.40 | −0.69 | −1.42 | −1.90 | |
| Base Pair | ||||||
| A1T5 | cG2C4 | A3T3 | OXOG4C2 | A5T1 | ||
| VIP | 6.69 | 6.15 | 6.58 | 5.93 | 6.73 | |
| AIP | 6.71 | 6.19 | 6.54 | 5.56 | 6.67 | |
| VEA | −1.38 | −1.49 | −1.45 | −1.53 | −1.42 | |
| AEA | −1.38 | −1.48 | −1.44 | −1.97 | −1.40 | |
| oligo-SdcG | Electronic properties in [eV] | |||||
| (A) 6.55 | 5.92 | 5.47 | −1.01 | −1.60 | −2.12 | |
| (B) 6.37 | 5.86 | 5.39 | −0.68 | −1.44 | −1.88 | |
| Base Pair | ||||||
| A1T5 | cG2C4 | A3T3 | OXOG4C2 | A5T1 | ||
| VIP | 6.67 | 6.14 | 6.64 | 5.93 | 6.72 | |
| AIP | 6.66 | 6.18 | 6.67 | 5.57 | 6.68 | |
| VEA | −1.41 | −1.55 | −1.40 | −1.52 | −1.42 | |
| AEA | −1.42 | −1.94 | −1.39 | −1.52 | −1.42 | |
| System | Electron-Hole Transfer | ||||
|---|---|---|---|---|---|
| oligo-RcdG | λ | ΔG | Ea | V12 | kHT (s−1) |
| A1T1→cG2C2 | −0.04 | −0.52 | −1.94 | 0.30 | ND |
| cG2C2←A3T3 | −0.02 | −0.35 | −2.04 | 0.22 | ND |
| A3T3→OXOG4C4 | 0.40 | −0.99 | 0.22 | 0.41 | 1.04 × 1012 |
| OXOG4C4←A5T5 | 0.38 | −1.11 | 0.36 | 0.42 | 3.83 × 109 |
| A1T1→A3T3 | 0.04 | −0.17 | 0.08 | 0.05 | 8.19 × 1012 |
| cG2C2→OXOG4C4 | 0.39 | −0.64 | 0.04 | 0.10 | 5.92 × 1013 |
| A3T5←A5T5 | 0.04 | −0.13 | 0.04 | 0.72 | 8.36 × 1013 |
| oligo-RcdG | Excess Electron Transfer | ||||
| A1T1→cG2C2 | 0.00 | −0.10 | −0.80 | 0.09 | ND |
| cG2C2←A3T3 | 0.02 | −0.05 | 0.01 | 0.04 | 1.22 × 1014 |
| A3T3→OXOG4C4 | 0.47 | −0.54 | 0.00 | 0.08 | 1.46 × 1014 |
| OXOG4C4←A5T5 | 0.49 | −0.57 | 0.00 | 0.05 | 4.74 × 1013 |
| A1T1→A3T3 | −0.02 | −0.06 | −0.08 | 0.08 | ND |
| cG2C2→OXOG4C4 | 0.45 | −0.49 | 0.001 | 0.46 | 5.06 × 1015 |
| A3T5←A5T5 | −0.04 | −0.04 | −0.04 | 0.08 | ND |
| System | Electron-Hole Transfer | ||||
| oligo-ScdG | λ | ΔG | Ea | V12 | kHT(s−1) |
| A1T1→cG2C2 | −0.04 | −0.47 | −1.52 | 0.29 | ND |
| cG2C2←A3T3 | −0.02 | −0.49 | −3.48 | 0.37 | ND |
| A3T3→OXOG4C4 | 0.39 | −1.11 | 0.33 | 0.39 | 9.39 × 109 |
| OXOG 4C4←A5T5 | 0.41 | −1.11 | 0.29 | 0.37 | 3.96 × 1010 |
| A1T1←A3T3 | −0.02 | −0.02 | −0.02 | 0.17 | ND |
| cG2C2→OXOG4C4 | 0.39 | −0.62 | 0.03 | 0.10 | 7.10 × 1013 |
| A3T5←A5T5 | 0.07 | −0.001 | 0.02 | 0.04 | 5.80 × 1013 |
| oligo-ScdG | Excess Electron Transfer | ||||
| A1T1→cG2C2 | 0.50 | −0.52 | 0.00 | 0.06 | 7.83 × 1013 |
| cG2C2←A3T3 | 0.49 | −0.56 | 0.00 | 0.04 | 4.34 × 1013 |
| A3T3→OXOG4C4 | 0.01 | −0.13 | 0.31 | 0.11 | 1.04 × 1010 |
| OXOG 4C4←A5T5 | 0.00 | −0.10 | 7.44 | 0.05 | 0.00 |
| A1T1←A3T3 | 0.00 | −0.03 | −0.56 | 0.07 | ND |
| cG2C2←OXOG4C4 | 0.48 | −0.42 | 0.002 | 0.05 | 5.89 × 1013 |
| A3T5→A5T5 | 0.02 | −0.03 | 0.01 | 0.07 | 5.98 × 1014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwowski, B.T. The Influence of 5′,8-Cyclo-2′-Deoxyguanosine on ds-DNA Charge Transfer Depends on Its Diastereomeric Form: A Theoretical Study. Antioxidants 2023, 12, 881. https://doi.org/10.3390/antiox12040881
Karwowski BT. The Influence of 5′,8-Cyclo-2′-Deoxyguanosine on ds-DNA Charge Transfer Depends on Its Diastereomeric Form: A Theoretical Study. Antioxidants. 2023; 12(4):881. https://doi.org/10.3390/antiox12040881
Chicago/Turabian StyleKarwowski, Bolesław T. 2023. "The Influence of 5′,8-Cyclo-2′-Deoxyguanosine on ds-DNA Charge Transfer Depends on Its Diastereomeric Form: A Theoretical Study" Antioxidants 12, no. 4: 881. https://doi.org/10.3390/antiox12040881
APA StyleKarwowski, B. T. (2023). The Influence of 5′,8-Cyclo-2′-Deoxyguanosine on ds-DNA Charge Transfer Depends on Its Diastereomeric Form: A Theoretical Study. Antioxidants, 12(4), 881. https://doi.org/10.3390/antiox12040881






