Herbal/Natural Compounds Resist Hallmarks of Brain Aging: From Molecular Mechanisms to Therapeutic Strategies
Abstract
1. Introduction
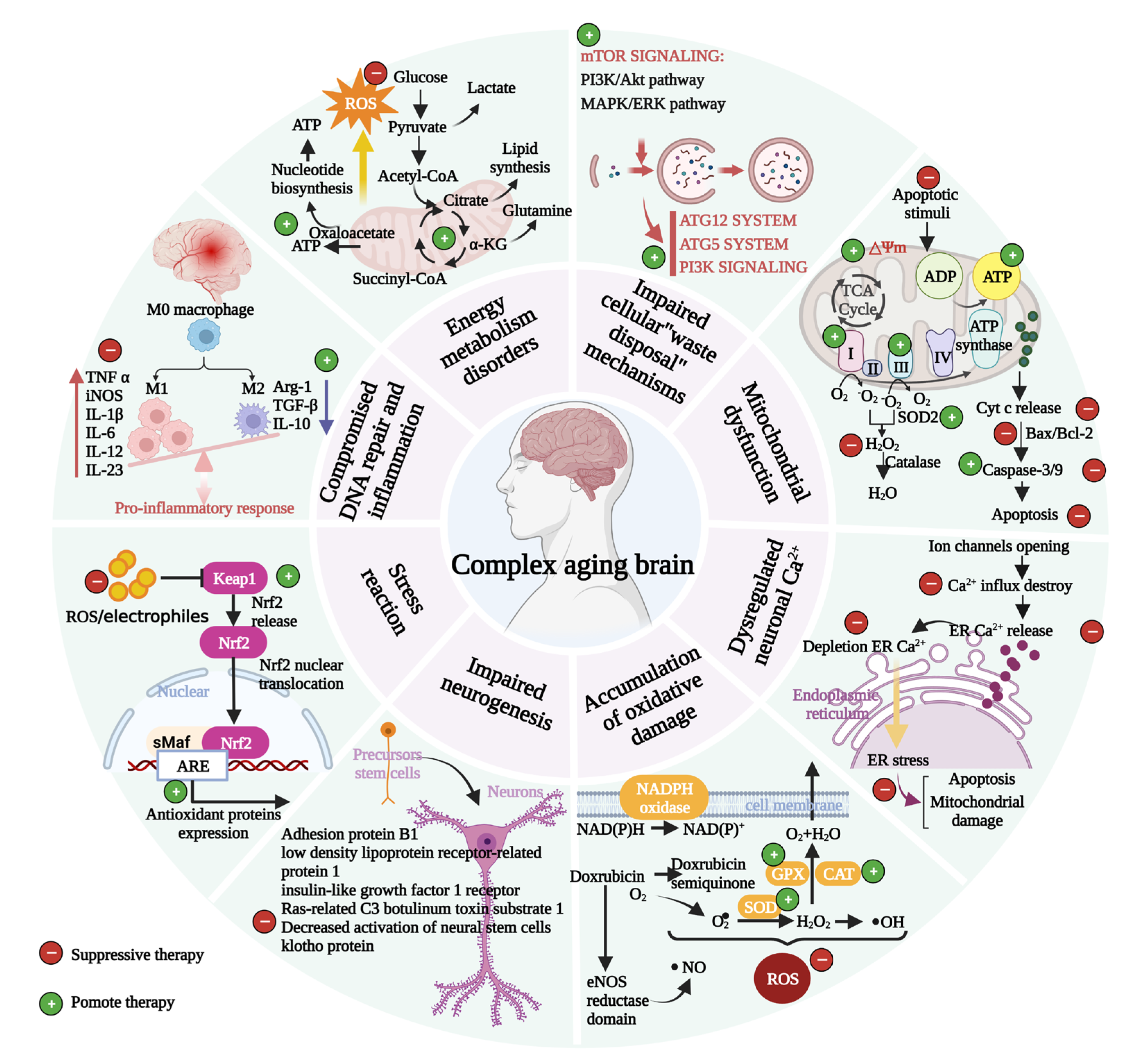
2. Structural and Functional Characteristics of Brain Aging
3. How do Herbal/Natural Compounds Respond to Molecular Changes during Brain Aging?
3.1. Mitochondrial Dysfunction
3.2. Accumulation of Oxidative Damage
3.3. Impaired Biological Function of Lysosomes and Proteasomes in Neurons
3.4. Dysregulated Neuronal Ca2+ Homeostasis
3.5. Stress Response
3.6. Compromised DNA Repair Disorders and Inflammation
3.7. Impaired Neurogenesis
3.8. Energy Metabolism Disorders
4. Herbal/Natural Compounds for the Treatment and Prevention of Neurological Disorders
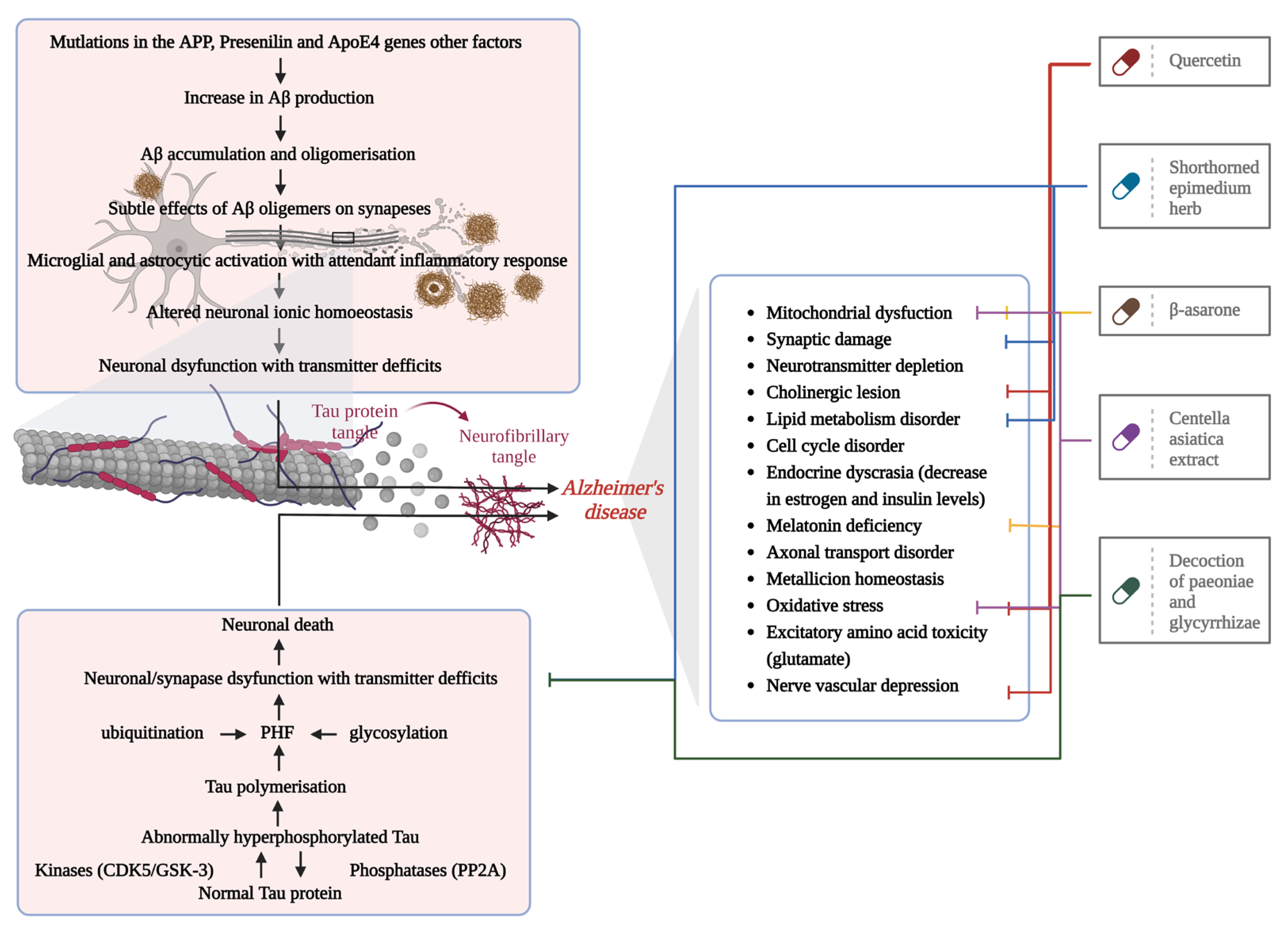
4.1. AD
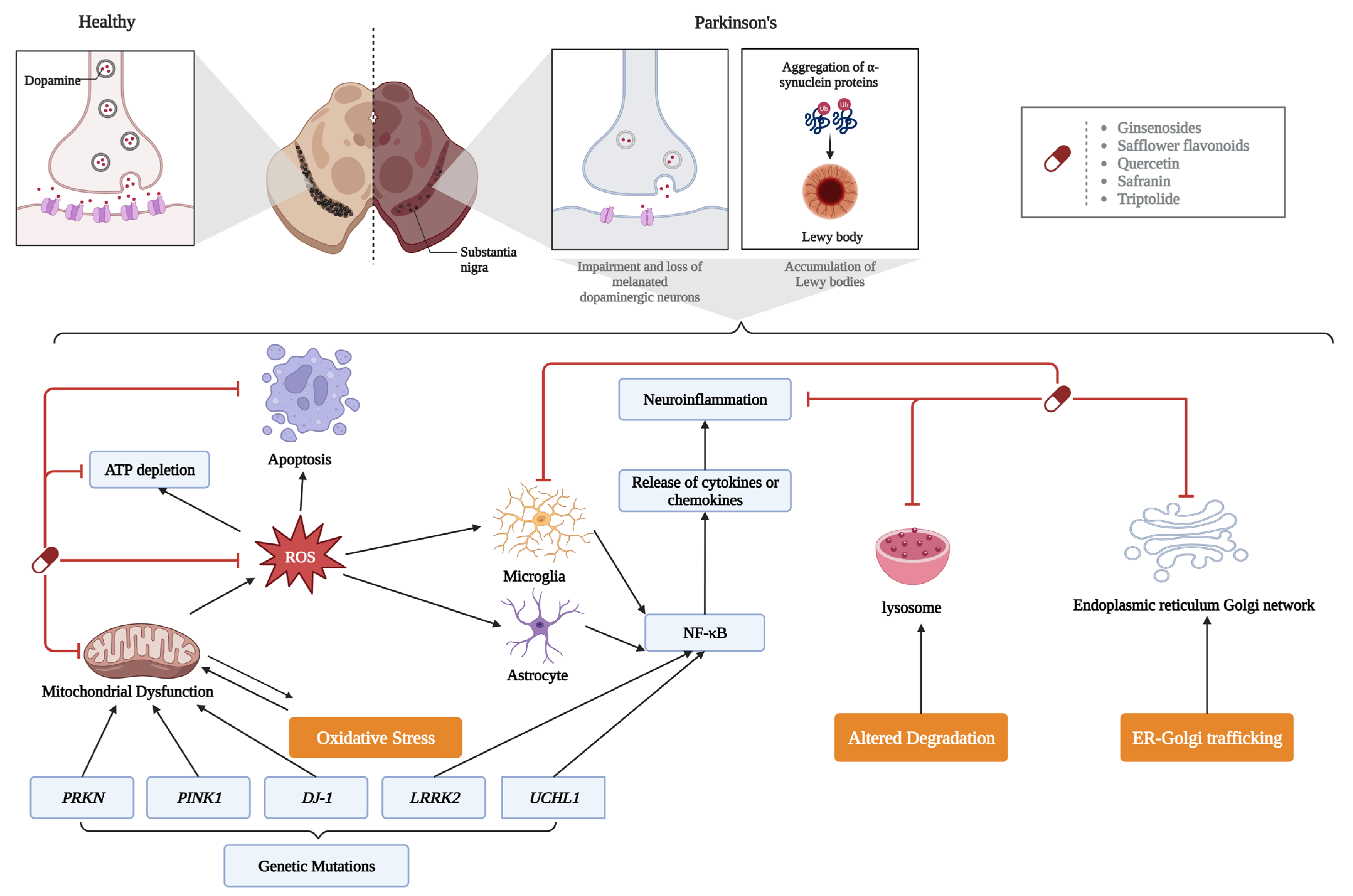
4.2. PD
5. Materials and Methods
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ATP | Adenosine triphosphate |
| Arg-1 | Arginase-1 |
| ARE | Antioxidant response element |
| ADP | Adenosine diphosphate |
| ATG 5 | Autophagy-related proteins 5 |
| ATG 12 | Autophagy-related proteins 12 |
| AKT | Protein kinase B |
| Arl8 | A small ADP-ribose-factor-like RAS family GTPase that mediates lysosomal transport driven by kinesin |
| AMPK | AMP-activated protein kinase |
| APP | Amyloid precursor protein |
| ApoE4 | ApolipoproteinE 4 |
| Bax | BCL2-Associated X |
| Bcl-2 | B-cell lymphoma-2 |
| BDNF | Brain-derived neurotrophic factor |
| BBB | Blood–brain barrier |
| CAT | Catalase |
| CREB | Cyclic AMP response element binding |
| CDK5 | Cyclin-dependent kinases-5 |
| DJ-1 | Parkinson disease protein 7, PARK7 |
| DA | Dopamine |
| ERK | Extracellular signal-regulated kinase |
| ERS | Endoplasmic reticulum stress |
| ER | Endoplasmic reticulum |
| FOXO | Fork head box class O |
| GPX | Glutathioneperoxidases |
| GSK-3 | Glycogen synthase kinase-3 |
| iNOS | Inducible nitric oxide synthase |
| IL-1β | Interleukins-1β |
| IL-6 | Interleukins-6 |
| IL-10 | Interleukins-10 |
| IL-12 | Interleukins-12 |
| IL-13 | Interleukins-13 |
| Keap1 | Kelch-like ECH-associated protein-1 |
| LRRK2 | Leucine-rich repeat kinase 2 |
| MAPK | Mitogen-activated protein kinase |
| mTOR | Mammalian target of rapamycin |
| Nrf2 | Nuclear regulatory factor 2 |
| NADPH | Reduced form of nicotinamide-adenine dinucleotide phosphate |
| NF-κB | Nuclear factor κB |
| NFP | Non-saponin component |
| NMN | Nicotinamide mononucleotide |
| NAD+ | Nicotinamide adenine dinucleotide |
| OGG1 | 8-hydroxyguanine glycosidase 1 |
| PD | Parkinson’s disease |
| PI3K | Phosphatidylinositol 3-kinase |
| PGC-1α | Peroxisome proliferator-activated receptor-γ coactivator-1α |
| PHF | Paired helical filament |
| PRKN | Parkin RBR E3 ubiquitin protein ligase |
| PINK1 | PTEN induced putative kinase 1 |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SPJ | Saponins of Panax japonicus |
| SKIP | Skeletal muscle and kidney-enriched inositol polyphosphate 5-phosphatase |
| STAT 3 | Signal transducer and activator of transcription 3 |
| STAT 6 | Signal transducer and activator of transcription 6 |
| SAFE | Saffron flavonoid extract |
| STAT6 | Signal transducer and activator of transcription 6 |
| TNF-α | Tumor necrosis factor α |
| TGF-β | Transforming growth factor-β |
| TCA | Tricarboxylic acid cycle |
| UCHL1 | Ubiquitin C-terminal hydrolase L1 |
| α-Syn | alpha-synuclein |
| α-KG | alpha-ketoglutarate |
| γ-H2AX | Histone H2AX phosphorylation |
References
- Isaev, N.K.; Stelmashook, E.V.; Genrikhs, E.E. Neurogenesis and brain aging. Rev. Neurosci. 2019, 30, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Blinkouskaya, Y.; Caçoilo, A.; Gollamudi, T.; Jalalian, S.; Weickenmeier, J. Brain aging mechanisms with mechanical manifestations. Mech. Ageing Dev. 2021, 200, 111575. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M. Aging, Cellular Senescence, and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 1989. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Rodriguez-Sabate, C.; Morales, I.; Sanchez, A.; Sabate, M. Parkinson’s disease as a result of aging. Aging Cell 2015, 14, 293–308. [Google Scholar] [CrossRef]
- Oliveira, Z.B.; Oliveira, A.R.; Miola, V.F.B.; Guissoni, L.M.; Spilla, C.S.G.; Barbalho, S.M. Panax ginseng and aging related disorders: A systematic review. Exp. Gerontol. 2022, 161, 111731. [Google Scholar]
- Lee, S.H.; Lee, H.Y.; Yu, M.; Yeom, E.; Lee, J.H.; Yoon, A.; Lee, K.S.; Min, K.J. Extension of Drosophila lifespan by Korean red ginseng through a mechanism dependent on dSir2 and insulin/IGF-1 signaling. Aging 2019, 11, 9369–9387. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Zhai, L.; Sun, L.; Zhao, D.; Wang, Z.; Li, X. Ginsenoside extract from ginseng extends lifespan and health span in Caenorhabditis elegans. Food Funct. 2021, 12, 6793–6808. [Google Scholar] [CrossRef]
- Panaro, M.A.; Corrado, A.; Benameur, T.; Paolo, C.F.; Cici, D.; Porro, C. The Emerging role of curcumin in the modulation of TLR-4 signaling pathway: Focus on neuroprotective and anti-rheumatic properties. Int. J. Mol. Sci. 2020, 21, 2299. [Google Scholar] [CrossRef]
- Porro, C.; Cianciulli, A.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Curcumin regulates anti-inflflammatory responses by JAK/STAT/SOCS signaling pathway in BV-2 microglial cells. Biology 2019, 8, 51. [Google Scholar] [CrossRef]
- Sahu, M.R.; Rani, L.; Subba, R.; Mondal, A.C. Cellular senescence in the aging brain: A promising target for neurodegenerative diseases. Mech. Ageing Dev. 2022, 204, 111675. [Google Scholar] [CrossRef]
- Yankner, B.A.; Lu, T.; Loerch, P. The aging brain. Annu. Rev. Pathol. 2008, 3, 41–66. [Google Scholar] [CrossRef]
- Sowell, E.R.; Peterson, B.S.; Thompson, P.M.; Welcome, S.E.; Henkenius, A.L.; Toga, A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003, 6, 309–315. [Google Scholar] [CrossRef]
- Liu, T.; Wen, W.; Zhu, W.; Trollor, J.; Reppermund, S.; Crawford, J.; Jin, J.S.; Luo, S.; Brodaty, H.; Sachdev, P. The effects of age and sex on cortical sulci in the elderly. Neuroimage 2010, 51, 19–27. [Google Scholar] [CrossRef]
- Liu, T.; Wen, W.; Zhu, W.; Kochan, N.A.; Trollor, J.N.; Reppermund, S.; Jin, J.S.; Luo, S.; Brodaty, H.; Sachdev, P.S. The relationship between cortical sulcal variability and cognitive performance in the elderly. Neuroimage 2011, 56, 865–873. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Jochems, A.C.C.; Muñoz Maniega, S.; Del C Valdés Hernández, M.; Barclay, G.; Anblagan, D.; Ballerini, L.; Meijboom, R.; Wiseman, S.; Taylor, A.M.; Corley, J.; et al. Contribution of white matter hyperintensities to ventricular enlargement in older adults. Neuroimage Clin. 2022, 34, 103019. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Compagnoni, G.M.; Fonzo, A.D.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The Role of Mitochondria in Neurodegenerative Diseases: The Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef]
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef]
- Olesen, M.A.; Toorres, A.K.; Jara, C.; Murphy, M.P.; Tapia, R.C. Premature synaptic mitochondrial dysfunction in the hippocampus during aging contributes to memory loss. Redox. Biol. 2020, 34, 101558. [Google Scholar] [CrossRef]
- de Sá-Nakanishi, A.B.; Soares, A.A.; de Oliveira, A.L.; Comar, J.F.; Peralta, R.M.; Bracht, A. Effects of treating old rats with an aqueous Agaricus blazei extract on oxidative and functional parameters of the brain tissue and brain mitochondria. Oxid. Med. Cell. Longev. 2014, 2014, 563179. [Google Scholar] [PubMed]
- Eckert, A. Mitochondrial effects of Ginkgo biloba extract. Int. Psychogeriatr. 2012, 24, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A.; Sudheesh, N.P.; Roshny, D.; Abishek, G.; Janardhanan, K.K. Effect of Ganoderma lucidum on the activities of mitochondrial dehydrogenases and complex I and II of electron transport chain in the brain of aged rats. Exp. Gerontol. 2009, 44, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, M.; Rudra, P.O.; Sagar, C.; Agrawal, A.; Dubey, G.P. Protective effect of curcuminoids on age-related mitochondrial impairment in female Wistar rat brain. Biogerontology 2014, 15, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Lee, J.H.; Lee, M.J.; Kim, S.J.; Ju, X.; Cui, J.; Zhu, J.; Lee, Y.L.; Namgung, E.; Sung, H.W.J.; et al. Schisandra Extract and Ascorbic Acid Synergistically Enhance Cognition in Mice Through Modulation of Mitochondrial Respiration. Nutrients 2020, 12, undefined. [Google Scholar] [CrossRef]
- Cheng, S.M.; Ho, Y.J.; Yu, S.H.; Liu, Y.F.; Lin, Y.Y.; Huang, C.Y.; Ou, H.C.; Huang, H.L.; Lee, S.D. Anti-Apoptotic Effects of Diosgenin in D-Galactose-Induced Aging Brain. Am. J. Chin. Med. 2020, 48, 391–406. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Neuroprotective and Cognitive Enhancement Potentials of Baicalin: A Review. Brain Sci. 2018, 8, 104. [Google Scholar] [CrossRef]
- Fan, Z.; Wen, H.; Zhang, X.; Li, J.; Zang, J. Cyanidin 3-O-β-Galactoside Alleviated Cognitive Impairment in Mice by Regulating Brain Energy Metabolism During Aging. J. Agric. Food Chem. 2022, 70, 1111–1121. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Xiao, J.; Song, M.; Cao, Y.; Xiao, H.; Liu, X. Astaxanthin attenuates d-galactose-induced brain aging in rats by ameliorating oxidative stress, mitochondrial dysfunction, and regulating metabolic markers. Food Funct. 2020, 11, 4103–4113. [Google Scholar] [CrossRef]
- Wang, D.; Cao, L.; Zhou, X.; Wang, G.; Ma, Y.; Hao, X.; Fan, H. Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1α/Sirt3. Hazard. Mater. 2022, 437, 129381. [Google Scholar] [CrossRef]
- Wiedenhoeft, T.; Tarantini, S.; Nyúl-Tóth, Á.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Kiss, T.; Csiszar, A.; Ungvari, Z. Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience 2019, 41, 711–725. [Google Scholar] [CrossRef]
- Mustafa, A.G.; Al-Shboul, O.; Alfaqih, M.A.; Al-Qudah, M.A.; Al-Dwairi, A.N. Phenelzine reduces the oxidative damage induced by peroxynitrite in plasma lipids and proteins. Arch. Physiol. Biochem. 2018, 124, 418–423. [Google Scholar] [CrossRef]
- Maiti, A.K.; Spoorthi, B.C.; Saha, N.C.; Panigrahi, A.K. Mitigating peroxynitrite mediated mitochondrial dysfunction in aged rat brain by mitochondria-targeted antioxidant MitoQ. Biogerontology 2018, 19, 271–286. [Google Scholar] [CrossRef]
- Wan, J.Z.; Wang, R.; Zhou, Z.Y.; Deng, L.L.; Zhang, C.C.; Liu, C.Q.; Zhao, H.X.; Yuan, C.F.; He, Y.M.; Dun, Y.Y.; et al. Saponins of Panax japonicus Confer Neuroprotection against Brain Aging through Mitochondrial Related Oxidative Stress and Autophagy in Rats. Curr. Pharm. Biotechnol. 2020, 21, 667–680. [Google Scholar] [CrossRef]
- Guix, F.X. The interplay between aging-associated loss of protein homeostasis and extracellular vesicles in neurodegeneration. J. Neurosci. Res. 2020, 98, 262–283. [Google Scholar] [CrossRef]
- Martinez, V.M.; Sovak, G.; Cuervo, A.M. Protein degradation and aging. Exp. Gerontol. 2005, 40, 622–633. [Google Scholar] [CrossRef]
- Truschel, S.T.; Clayton, D.R.; Beckel, J.M.; Yabes, J.G.; Yao, Y.; Wolf-Johnston, A.; Birder, L.A.; Apodaca, G. Age-related endolysosome dysfunction in the rat urothelium. PLoS ONE 2018, 13, e0198817. [Google Scholar] [CrossRef]
- Moon, J.H.; Lee, J.H.; Lee, Y.J.; Park, S.Y. Autophagy flux induced by ginsenoside-Rg3 attenuates human prion protein-mediated neurotoxicity and mitochondrial dysfunction. Oncotarget 2016, 7, 85697–85708. [Google Scholar] [CrossRef]
- Papp, D.; Kovács, T.; Billes, V.; Varga, M.; Tarnóci, A.; Hackler, L.; Puskás, L.G., Jr.; Liliom, H.; Tárnok, K.; Schlett, K.; et al. AUTEN-67, an autophagy-enhancing drug candidate with potent antiaging and neuroprotective effects. Autophagy 2016, 12, 273–286. [Google Scholar] [CrossRef]
- Kwon, Y.; Bang, Y.; Moon, S.H.; Kim, A.; Choi, H.J. Amitriptyline interferes with autophagy-mediated clearance of protein aggregates via inhibiting autophagosome maturation in neuronal cells. Cell Death Dis. 2020, 11, 874. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, S.; Wang, J.; Zhang, X.; Yuan, D.; Zhang, C.; Liu, C.; Wang, T.; Zhou, Z. Icariin improves brain function decline in aging rats by enhancing neuronal autophagy through the AMPK/mTOR/ULK1 pathway. Pharm. Biol. 2021, 59, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Minakaki, G.; Nguyen, M.; Krainc, D. Preserving Lysosomal Function in the Aging Brain: Insights from Neurodegeneration. Neurotherapeutics 2019, 16, 611–634. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, A.; Mattie, S.; Prudent, J.; McBride, H.M. Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 2017, 542, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.S.; Cho, D.H. Peroxisomal dysfunction in neurodegenerative diseases. Arch. Pharm. Res. 2019, 42, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Movahedpour, A.; Vakili, O.; Khalifeh, M.; Mousavi, P.; Mahmoodzadeh, A.; Taheri-Anganeh, M.; Razmeh, S.; Shabaninejad, Z.; Yousefi, F.; Behrouj, H.; et al. Mammalian target of rapamycin (mTOR) signaling pathway and traumatic brain injury: A novel insight into targeted therapy. Cell Biochem. Funct. 2022, 40, 232–247. [Google Scholar] [CrossRef]
- Masini, D.; Bonito-Oliva, A.; Bertho, M.; Fisone, G. Inhibition of mTORC1 Signaling Reverts Cognitive and Affective Deficits in a Mouse Model of Parkinson’s Disease. Front. Neurol. 2018, 9, 208. [Google Scholar] [CrossRef]
- Suresh, S.N.; Chavalmane, A.K.; Dj, V.; Yarreiphang, H.; Rai, S.; Paul, A.; Manjithaya, R. A novel autophagy modulator 6-Bio ameliorates SNCA/alpha-synuclein toxicity. Autophagy 2017, 13, 1221–1234. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, J.A.; Rodriguez, L.; Casarejos, M.J.; Solano, R.M.; Gomez, A.; Perucho, J.; Cuervo, A.M.; García, Y.J.d.; Mena, M.A. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol. Dis. 2010, 39, 423–438. [Google Scholar] [CrossRef]
- Lv, J.; Xiao, X.; Bi, M.; Tang, T.; Kong, D.; Diao, M.; Jiao, Q.; Chen, X.; Yan, C.; Du, X. ATP-sensitive potassium channels: A double-edged sword in neurodegenerative diseases. Ageing Res. Rev. 2022, 80, 101676. [Google Scholar] [CrossRef]
- Jeong, S.; Cho, H.; Kim, Y.J.; Ma, H.I.; Jang, S. Drug-induced Parkinsonism: A strong predictor of idiopathic Parkinson’s disease. PLoS ONE 2021, 16, e0247354. [Google Scholar] [CrossRef]
- Bourdenx, M.; Daniel, J.; Genin, E.; Soria, F.N.; Blanchard-Desce, M.; Bezard, E. Nanoparticles restore lysosomal acidification defects: Implications for Parkinson and other lysosomal-related diseases. Autophagy 2016, 12, 472–483. [Google Scholar] [CrossRef]
- Migdalska-Richards, A.; Daly, L.; Bezard, E.; Schapira, A.H. Ambroxol effects in glucocerebrosidase and alpha-synuclein transgenic mice. Ann. Neurol. 2016, 80, 766–775. [Google Scholar] [CrossRef]
- Migdalska-Richards, A.; Ko, W.K.D.; Li, Q.; Bezard, E.; Schapira, A.H.V. Oral ambroxol increases brain glucocerebrosidase activity in a nonhuman primate. Synapse 2017, 71, e21967. [Google Scholar] [CrossRef]
- Richter, F.; Fleming, S.M.; Watson, M.; Lemesre, V.; Pellegrino, L.; Ranes, B.; Zhu, C.; Mortazavi, F.; Mulligan, C.K.; Sioshansi, P.C. A GCase chaperone improves motor function in a mouse model of synucleinopathy. Neurotherapeutics 2014, 11, 840–856. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Beavan, M.; Gegg, M.E.; Chau, K.Y.; Whitworth, A.J.; Schapira, A.H. Parkinson disease-linked GBA mutation effects reversed by molecular chaperones in human cell and fly models. Sci. Rep. 2016, 6, 31380. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, J.T.; Zhu, X.C.; Zhang, Q.Q.; Cao, L.; Wang, H.F.; Tan, M.S.; Gao, Q.; Qin, H.; Zhang, Y.D.; et al. Temsirolimus attenuates tauopathy in vitro and in vivo by targeting tau hyperphosphorylation and autophagic clearance. Neuropharmacology 2014, 85, 121–130. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Zhang, X.; Li, T.; Tang, Y.; Liu, H.; Yang, W.; Le, W. Autophagy enhancer carbamazepine alleviates memory deficits and cerebral amyloid-beta pathology in a mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2013, 10, 433–441. [Google Scholar] [CrossRef]
- Steele, J.W.; Kim, S.H.; Cirrito, J.R.; Verges, D.K.; Restivo, J.L.; Westaway, D.; Fraser, P.; Hyslop, P.S.; Sano, M.; Bezprozvanny, I.; et al. Acute dosing of latrepirdine (Dimebon), a possible Alzheimer therapeutic, elevates extracellular amyloid-beta levels in vitro and in vivo. Mol. Neurodegener. 2009, 4, 51. [Google Scholar] [CrossRef]
- Kou, X.; Chen, N. Resveratrol as a Natural Autophagy Regulator for Prevention and Treatment of Alzheimer’s Disease. Nutrients 2017, 9, 927. [Google Scholar] [CrossRef]
- Matsunaga, S.; Kishi, T.; Annas, P.; Basun, H.; Hampel, H.; Iwata, N. Lithium as a treatment for Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2015, 48, 403–410. [Google Scholar] [CrossRef]
- Robinson, D.M.; Keating, G.M. Memantine: A review of its use in Alzheimer’s disease. Drugs 2006, 66, 1515–1534. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, Y.; Cen, X.; Shan, B.; Zhao, Q.; Xie, T.; Wang, Z.; Hou, T.; Xue, Y.; Zhang, M.; et al. Metformin activates chaperone-mediated autophagy and improves disease pathologies in an Alzheimer disease mouse model. Protein Cell 2021, 12, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Raffaello, A.; Mammucari, C.; Gherardi, G.; Rizzuto, R. Calcium at the center of cell signaling: Interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem. Sci. 2016, 41, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Boubakri, A.; Leri, M.; Bucciantini, M.; Najjaa, H.; Ben, A.A.; Stefani, M.; Neffati, M. Allium roseum L. extract inhibits amyloid beta aggregation and toxicity involved in Alzheimer’s disease. PLoS ONE 2020, 15, e0223815. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Qin, Y.; Gao, H.; Wang, G.; Song, H.; Wang, Y.; Cai, B. Chrysophanol exerts neuroprotective effects via interfering with endoplasmic reticulum stress apoptotic pathways in cell and animal models of Alzheimer’s disease. J. Pharm. Pharmacol. 2022, 74, 32–40. [Google Scholar] [CrossRef]
- Nikoletopoulou, V.; Tavernarakis, N. Calcium homeostasis in aging neurons. Front. Genet. 2012, 3, 200. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, P.; Xie, Y.; Chen, X.; Solowij, N.; Green, K.; Chew, Y.L.; Huang, X.F. Cannabidiol regulates CB1-pSTAT3 signaling for neurite outgrowth, prolongs lifespan, and improves health span in Caenorhabditis elegans of Aβ pathology models. FASEB J. 2021, 35, e21537. [Google Scholar] [CrossRef]
- Gant, J.C.; Sama, M.M.; Landfield, P.W.; Thibault, O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J. Neurosci. 2006, 26, 3482–3490. [Google Scholar] [CrossRef]
- Xiong, J.; Verkhratsky, A.; Toescu, E.C. Changes in mitochondrial status associated with altered Ca2+ homeostasis in aged cerebellar granule neurons in brain slices. J. Neurosci. 2002, 22, 10761–10771. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Du, Y.; Fu, M.; Wang, Y.T.; Dong, Z. Neuroprotective Effects of Ginsenoside Rf on Amyloid-β-Induced Neurotoxicity in vitro and in vivo. J. Alzheimers Dis. 2018, 64, 309–322. [Google Scholar] [CrossRef]
- Abe, C.; Inoue, T.; Inglis, M.A.; Viar, K.E.; Huang, L.; Ye, H.; Rosin, D.L.; Stornetta, R.L.; Okusa, M.D.; Guyenet, P.G. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci. 2017, 20, 700–707. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic dietary phytochemicals. Neuromolecular Med. 2008, 10, 236–246. [Google Scholar] [CrossRef]
- Kruman, I.I.; Kumaravel, T.S.; Lohani, A.; Pedersen, W.A.; Cutler, R.G.; Kruman, Y.; Haughey, N.; Lee, J.; Evans, M.; Mattson, M.P. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J. Neurosci. 2002, 22, 1752–1762. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Xu, H.; Shi, Q.; Chen, L.H.; Pedrini, S.; Pechman, D.; Baker, H.; Beal, M.F.; Gandy, S.E.; Gibson, G.E. Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer’s mouse model. Ageing 2009, 30, 1587–1600. [Google Scholar] [CrossRef]
- Ma, Q.L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Chen, P.P.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. β-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: Suppression by omega-3 fatty acids and curcumin. J. Neurosci. 2009, 29, 9078–9089. [Google Scholar] [CrossRef]
- Obregon, D.F.; Rezai-Zadeh, K.; Bai, Y.; Sun, N.; Hou, H.; Ehrhart, J.; Zeng, J.; Mori, T.; Arendash, G.W.; Shytle, D.; et al. ADAM10 activation is required for green tea -epigallocatechin-3-gallate-induced α-secretase cleavage of amyloid precursor protein. Biol. Chem. 2006, 281, 16419–16427. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Arumugam, T.V.; Cutler, R.G.; Telljohann, R.S.; Mughal, M.R.; Moore, T.A.; Luo, W.; Yu, Q.S.; Johnson, D.A.; et al. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J. Neurochem. 2010, 112, 1316–1326. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef]
- Gopinath, K.; Sudhandiran, G. Naringin modulates oxidative stress and inflflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 2012, 227, 134e143. [Google Scholar] [CrossRef]
- Olanow, C.W.; Kordower, J.H.; Lang, A.E.; Obeso, J.A. Dopaminergic transplantation for Parkinson’s disease: Current status and future prospects. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2009, 66, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Lee, K.; Martin, B.; Maudsley, S.; Golden, E.; Cutler, R.G.; Mattson, M.P. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 2009, 19, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Fu, H.; Liu, G.X. Effect of wuzi yanzong pill and its disassembled prescription on mitochondrial DNA deletion, respiratory chain complexes and ATP synthesis in aged rats. Zhong Guo Zhong Xi Yi Jie He Za Zhi 2001, 21, 437–440. [Google Scholar]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Raghuram, G.V.; Dsouza, J.; Shinde, S.; Jadhav, V.; Shaikh, A.; Rane, B.; Tandel, H.; Kondhalkar, D.; Chaudhary, S.; et al. A pro-oxidant combination of resveratrol and copper down-regulates multiple biological hallmarks of ageing and neurodegeneration in mice. Sci. Rep. 2022, 12, 17209. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhong, Y.; Peng, W.; Sun, Y.; Hu, Y.J.; Yang, Y.; Kong, W.J. Increased mitochondrial DNA damage and decreased base excision repair in the auditory cortex of D-galactose-induced aging rats. Mol. Biol. Rep. 2011, 38, 3635–3642. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.; Gomes, A.C.; Proença, F.; Coutinho, O.P. Novel nitrogen compounds enhance protection and repair of oxidative DNA damage in a neuronal cell model: Comparison with quercetin. Chem. Biol. Interact. 2009, 181, 328–337. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflflammation. Ageing Res. 2017, 36, 11e19. [Google Scholar] [CrossRef]
- Yang, X.; Xu, S.; Qian, Y.; Xiao, Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1alpha in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017, 64, 162–172. [Google Scholar] [CrossRef]
- Ano, Y.; Ohya, R.; Kita, M.; Taniguchi, Y.; Kondo, K. Theaflflavins improve memory impairment and depression-like behavior by regulating microglial activation. Molecules 2019, 24, 467. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Gentile, M.T.; Reccia, M.G.; Sorrentino, P.P.; Vitale, E.; Sorrentino, G.; Puca, A.A.; Colucci-D’Amato, L. Role of cytosolic calcium-dependent phospholipase A2 in Alzheimer’s disease pathogenesis. Mol. Neurobiol. 2012, 45, 596–604. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer disease—A brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012, 2, a006346. [Google Scholar] [CrossRef]
- Howcroft, T.K.; Campisi, J.; Louis, G.B.; Smith, M.T.; Wise, B.; Wyss-Coray, T.; Augustine, A.D.; McElhaney, J.E.; Kohanski, R.; Sierra, F. The role of inflammation in age-related disease. Aging 2013, 5, 84–93. [Google Scholar] [CrossRef]
- Wang, G.; Li, D.; Huang, C.; Lu, D.; Zhang, C.; Zhou, S.; Liu, J.; Zhang, F. Icariin reduces dopaminergic neuronal loss and microglia-mediated inflammation In Vivo and In Vitro. Front. Mol. Neurosci. 2018, 10, 441. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, G.; He, J.; Yang, Q.; Li, D.; Li, J.; Zhang, F. Icariin attenuates neuroinflammation and exerts dopamine neuroprotection via an Nrf2-dependent manner. J. Neuroinflamm. 2019, 16, 11–92. [Google Scholar] [CrossRef]
- Culig, L.; Chu, X.; Bohr, V.A. Neurogenesis in aging and age-related neurodegenerative diseases. Ageing Res. Rev. 2022, 78, 101636. [Google Scholar] [CrossRef]
- Shin, S.J.; Nam, Y.; Park, Y.H.; Kim, M.J.; Lee, E.; Jeon, S.G.; Bae, B.S.; Seo, J.; Shim, S.L.; Kim, J.S.; et al. Therapeutic effects of non-saponin fraction with rich polysaccharide from Korean red ginseng on aging and Alzheimer’s disease. Free Radic. Biol. Med. 2021, 20, 233–248. [Google Scholar] [CrossRef]
- Santos, R.; Ruiz, A.C.; Bulteau, A.L.; Gomes, C.M. Neurodegeneration, neurogenesis, and oxidative stress. Oxid. Med. Cell. Longev. 2013, 2013, 730581. [Google Scholar] [CrossRef]
- Hsu, W.H.; Huang, N.K.; Shiao, Y.J.; Lu, C.K.; Chao, Y.M.; Huang, Y.J.; Yeh, C.H.; Lin, Y.L. Gastrodiae rhizoma attenuates brain aging via promoting neuritogenesis and neurodifferentiation. Phytomedicine 2021, 87, 153576. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.; Fão, L.; Mota, S.; Rego, A.C. Mitochondrial function and dynamics in neural stem cells and neurogenesis: Implications for neurodegenerative diseases. Ageing Res. Rev. 2022, 80, 101667. [Google Scholar] [CrossRef] [PubMed]
- Miao, B.B.; Gao, D.; Hao, J.P.; Li, Y.L.; Li, L.; Wang, J.B.; Xiao, X.H.; Yang, C.C.; Zhang, L. Tetrahydroxy stilbene glucoside alters neurogenesis and neuroinflammation to ameliorate radiation-associated cognitive disability via AMPK/Tet2. Int. Immunopharmacol. 2022, 110, 108928. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Suzuki, E.; Asada, A.; Saito, T.; Iijima, K.M.; Ando, K. Increasing neuronal glucose uptake attenuates brain aging and promotes life span under dietary restriction in Drosophila. Iscience 2021, 24, 101979. [Google Scholar] [CrossRef]
- Baron, D.C.; Marko, D.M.; Tsiani, E.; MacPherson, R.E.K. Rosemary extract increases neuronal cell glucose uptake and activates AMPK. Appl. Physiol. Nutr. Metab. 2021, 46, 141–147. [Google Scholar] [CrossRef]
- Yan, F.; Liu, J.; Chen, M.X.; Zhang, Y.; Wei, S.J.; Jin, H.; Nie, J.; Fu, X.L.; Shi, J.S.; Zhou, S.Y.; et al. Icariin ameliorates memory deficits through regulating brain insulin signaling and glucose transporters in 3×Tg-AD mice. Neural. Regen. Res. 2023, 18, 183–188. [Google Scholar]
- Qin, G.; Dong, Y.; Liu, Z.; Gong, Z.; Gao, C.; Zheng, M.; Tian, M.; He, Y.; Zhong, L.; Wang, P. Shen-Zhi-Ling oral liquid ameliorates cerebral glucose metabolism disorder in early AD via insulin signal transduction pathway in vivo and in vitro. Chin. Med. 2021, 16, 128. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut. Microbes. 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Zou, X.; Feng, X.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C.; Zhang, Y. Icariin attenuates amyloid-β (Aβ)-induced neuronal insulin resistance through PTEN downregulation. Front. Pharmacol. 2020, 11, 880. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, W.; Zhan, L.; Sui, H.; Zhang, L.; Zhao, C.; Lu, X. The ZiBuPiYin recipe regulates proteomic alterations in brain mitochondria-associated ER membranes caused by chronic psychological stress exposure: Implications for cognitive decline in Zucker diabetic fatty rats. Aging 2020, 12, 23698–23726. [Google Scholar] [CrossRef]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell. 2019, 18, e13048. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Lin, D.; Guo, W.; Xu, Z.; Wang, L.; Guan, S. Ginsenoside Prolongs the Lifespan of C. elegans via Lipid Metabolism and Activating the Stress Response Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 9668. [Google Scholar] [CrossRef]
- Gray, N.E.; Zweig, J.A.; Caruso, M.; Zhu, J.Y.; Wright, K.M.; Quinn, J.F.; Soumyanath, A. Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in β-amyloid overexpressing mice. Mol. Cell. Neurosci. 2018, 93, 1–9. [Google Scholar] [CrossRef]
- Yang, S.; Xie, Z.; Pei, T.; Zeng, Y.; Xiong, Q.; Wei, H.; Wang, Y.; Cheng, W. Salidroside attenuates neuronal ferroptosis by activating the Nrf2/HO1 signaling pathway in Aβ1-42-induced Alzheimer’s disease mice and glutamate-injured HT22 cells. Chin. Med. 2022, 17, 82. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.; Zhou, D.; Bai, X.Y.; Zhou, W.L.; Huang, C.; Song, J.; Meng, F.; Wu, C.; Li, L. Quercetin protects against the Aβ(25-35)-induced amnesic injury: Involvement of inactivation of rage-mediated pathway and conservation of the NVU. Neuropharmacology 2013, 67, 419–431. [Google Scholar] [CrossRef]
- Urano, T.; Tohda, C. Icariin improves memory impairment in Alzheimer’s disease model mice (5xFAD) and attenuates amyloid β-induced neurite atrophy. Phytother. Res. 2010, 24, 1658–1663. [Google Scholar] [CrossRef]
- Chiu, Y.J.; Lin, C.H.; Lee, M.C.; Hsieh-Li, H.M.; Chen, C.M.; Wu, Y.R.; Chang, K.H.; Lee-Chen, G.J. Formulated Chinese medicine Shaoyao Gancao Tang reduces NLRP1 and NLRP3 in Alzheimer’s disease cell and mouse models for neuroprotection and cognitive improvement. Aging 2021, 13, 15620–15637. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, H.; Huang, X.; Han, S.; Zhang, D.; Ni, J.; He, X. Neuroprotective effects of icariin on brain metabolism, mitochondrial functions, and cognition in triple-transgenic Alzheimer’s disease mice. CNS Neurosci. Ther. 2016, 22, 63–73. [Google Scholar] [CrossRef]
- Sheng, C.; Xu, P.; Zhou, K.; Deng, D.; Zhang, C.; Wang, Z. Icariin attenuates synaptic and cognitive deficits in an Aβ1-42-induced rat model of Alzheimer’s disease. Biomed. Res. Int. 2017, 2017, 7464872. [Google Scholar] [CrossRef]
- Wang, N.; Wang, H.; Pan, Q.; Kang, J.; Liang, Z.; Zhang, R. The Combination of β-Asarone and Icariin Inhibits Amyloid-β and Reverses Cognitive Deficits by Promoting Mitophagy in Models of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2021, 2021, 7158444. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012, 16, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Varamini, B.; Sikalidis, A.K.; Bradford, K. Resveratrol increases cerebral glycogen synthase kinase phosphorylation as well as protein levels of drebrin and transthyretin in mice: An exploratory study. Int. J. Food Sci. Nutr. 2014, 65, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.A.; Obeso, J.A. Clinical and pathological features of Parkinson’s disease. Curr. Top. Behav. Neurosci. 2015, 22, 205–220. [Google Scholar]
- Gonzalez-Latapi, P.; Bhowmick, S.S.; Saranza, G.; Fox, S.H. Non-Dopaminergic Treatments for Motor Control in Parkinson’s Disease: An Update. CNS Drugs 2020, 34, 1025–1044. [Google Scholar] [CrossRef]
- Kwon, I.H.; Choi, H.S.; Shin, K.S.; Lee, B.K.; Lee, C.K.; Hwang, B.Y.; Lim, S.C.; Lee, M.K. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson’s disease. Neurosci. Lett. 2010, 1, 29–33. [Google Scholar] [CrossRef]
- Wu, C.R.; Tsai, C.W.; Chang, S.W.; Lin, C.Y.; Huang, L.C.; Tsai, C.W. Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: Involvement of antioxidative enzymes induction. Chem.-Biol. Interact. 2015, 225, 40–46. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Farbood, Y.; Sameri, M.J.; Sarkaki, A.; Naghizadeh, B.; Rafeirad, M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 2013, 2, 1028–1033. [Google Scholar] [CrossRef]
- Farbood, Y.; Sarkaki, A.; Dolatshahi, M.; Mansouri, S.M.T.; Khodadadi, A. Ellagic acid protects the brain against 6-hydroxydopamine induced neuroinflammation in a rat model of Parkinson’s disease. Basic Clin. Neurosci. 2015, 2, 83. [Google Scholar]
- Hong, Z.; Wang, G.; Gu, J.; Pan, J.; Bai, L.; Zhang, S.; Chen, S.D. Tripchlorolide protects against MPTP-induced neurotoxicity in C57BL/6 mice. Eur. J. Neurosci. 2007, 6, 1500–1508. [Google Scholar] [CrossRef]
- Ren, R.; Shi, C.; Cao, J.; Sun, Y.; Zhao, X.; Guo, Y.; Wang, C.; Lei, H.; Jiang, H.; Ablat, N.; et al. Neuroprotective Effects of a Standardized Flavonoid Extract of Safflower Against Neurotoxin-Induced Cellular and Animal Models of Parkinson’s Disease. Sci. Rep. 2016, 6, 22135. [Google Scholar] [CrossRef]
- Hu, S.; Han, R.; Mak, S.; Han, Y. Protection against 1-methyl-4-phenylpyridinium ion (MPP+)-induced apoptosis by water extract of ginseng (Panax ginseng C.A. Meyer) in SH-SY5Y cells. J. Ethnopharmacol. 2011, 135, 34–42. [Google Scholar] [CrossRef]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson’s Disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef]
- González-Burgos, E.; Fernandez-Moriano, C.; Gómez-Serranillos, M.P. Potential neuroprotective activity of Ginseng in Parkinson’s disease: A review. Neuroimmune Pharmacol. 2015, 10, 14–29. [Google Scholar] [CrossRef]
- Sonntag, K.C.; Song, B.; Lee, N.; Jung, J.H.; Cha, Y.; Leblanc, P.; Neff, C.; Kong, S.W.; Carter, B.S.; Schweitzer, J.; et al. Pluripotent stem cell-based therapy for Parkinson’s disease: Current status and future prospects. Prog. Neurobiol. 2018, 168, 1–20. [Google Scholar] [CrossRef]
- Ho, Y.S.; So, K.F.; Chang, R.C. Anti-aging herbal medicine--how and why can they be used in aging-associated neurodegenerative diseases? Ageing Res. Rev. 2010, 9, 354–362. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- Ng, G.Y.; Sheng, D.P.L.K.; Bae, H.G.; Kang, S.W.; Fann, D.Y.; Park, J.; Kim, J.; Alli-Shaik, A.; Lee, J.; Kim, E.; et al. Integrative epigenomic and transcriptomic analyses reveal metabolic switching by intermittent fasting in brain. Geroscience 2022, 44, 2171–2194. [Google Scholar] [CrossRef]
- Soma, M.; Lalam, S.K. The role of nicotinamide mononucleotide (NMN) in anti-aging, longevity, and its potential for treating chronic conditions. Mol. Biol. Rep. 2022, 49, 9737–9748. [Google Scholar] [CrossRef]
- Stefano, A.D.; Sozio, P.; Cerasa, L.S.; Iannitelli, A. L-Dopa prodrugs: An overview of trends for improving Parkinson’s disease treatment. Curr. Pharm. Des. 2011, 17, 3482–3493. [Google Scholar] [CrossRef]
- Bastide, M.F.; Meissner, W.G.; Picconi, B.; Fasano, S.; Fernagut, P.O.; Feyder, M.; Francardo, V.; Alcacer, C.; Ding, Y.; Brambilla, R.; et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog. Neurobiol. 2015, 132, 96–168. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.J.; Yang, J. Translational opportunities for amyloid-targeting fluorophores. Chem. Commun. 2018, 54, 9107–9118. [Google Scholar] [CrossRef] [PubMed]
- Cichon, N.; Saluk-Bijak, J.; Gorniak, L.; Przyslo, L.; Bijak, M. Flavonoids as a Natural Enhancer of Neuroplasticity-An Overview of the Mechanism of Neurorestorative Action. Antioxidants 2020, 9, 1035. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, F.M.; Frota, A.F.; de Jesus, L.B.; Cuenca-Bermejo, L.; Ferreira, K.M.S.; Santos, C.C.; Soares, E.N.; Souza, J.T.; Sanches, F.S.; Costa, A.C.S.; et al. Protective Effects of Flavonoid Rutin Against Aminochrome Neurotoxicity. Neurotox. Res. 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Poovaiah, N.; Davoudi, Z.; Peng, H.; Schlichtmann, B.; Mallapragada, S.; Narasimhan, B.; Wang, Q. Treatment of neurodegenerative disorders through the blood-brain barrier using nanocarriers. Nanoscale 2018, 10, 16962–16983. [Google Scholar] [CrossRef]
- Trotta, T.; Panaro, M.A.; Prifti, E.; Porro, C. Modulation of biological activities in glioblastoma mediated by curcumin. Cancer 2019, 71, 1241–1253. [Google Scholar] [CrossRef]

| Drugs | Mechanism | Outcomes | Reference(s) |
|---|---|---|---|
| Agaricus blazei extract | Regulate mitochondrial respiration | Increased mitochondrial respiratory enzyme activity (NADH: ubiquinone oxidoreductase, succinate dehydrogenase, cytochrome c oxidase) | [21] |
| Ginkgo biloba extract | [22] | ||
| Ganoderma lucidum | [23] | ||
| Curcuminoids | [24] | ||
| Schisandra Extract | [25] | ||
| Diosgenin | Prevent the release of apoptotic factors | Inhibited senescence-induced mitochondria-dependent apoptotic pathway (Bax, cytochrome c, active caspase-9 and active caspase-3) and IGF-1-PI3K-AKT survival pathway | [26] |
| Baicalin | [27] | ||
| Baicalein | [27] | ||
| Cyanidin 3- O-β-Galactoside | Energy metabolism | Increased the levels of N-acetyl-l-leucine, N-acetyl-l-tyrosine, and methionine sulfoxide; reduced the levels of both hyodeoxycholic acid and chenodeoxycholic acid | [28] |
| Astaxanthin | [29] | ||
| Honokiol | Mitochondrial oxidative stress | Increased activity and expression of SOD2 and activated Sirt3 to promote mtROS clearance | [30] |
| Disease | Drugs | Mechanism | Outcomes | Reference(s) |
|---|---|---|---|---|
| PD | Rapamycin | mTOR-related | Inhibited mTORC1 and S6K; mediated degradation of SNCA/α-synuclein aggregates by GSK3 | [45,46,47] |
| Temsirolimus | ||||
| 6-Bio | ||||
| Trehalose | mTOR-unrelated | Maintained glucose transporter-1 and promoted protein stability; inhibited Ca2+ overload | [48,49,50] | |
| Minoxidil | ||||
| Verapamil | ||||
| Acidic nanoparticles | Lysosomal | Restored lysosomal acidification; increased glucosylcerebrosidase; inhibited glucosylceramide synthase | [51,52,53,54,55] | |
| Ambroxol | ||||
| Isofagomine | ||||
| Venglustat | ||||
| AD | Carbamazepine | mTOR-related | Inhibition of mTOR activity | [56,57,58] |
| Latrepirdine | ||||
| Temsirolimus | ||||
| Lithium | mTOR-unrelated | Involved in IP3; as an activator of SIRT1; antagonized NMDAR | [59,60,61] | |
| Resveratrol | ||||
| Memantine | ||||
| Metformin | Lysosomal | Induced CMA, activation of TAK1-IKK α/β signaling | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, J.; Wang, C.; Chen, Y.; Yu, S.; Liu, Y.; Yu, S.; Jiang, L.; Jin, C.; Wang, X.; Zhang, P.; et al. Herbal/Natural Compounds Resist Hallmarks of Brain Aging: From Molecular Mechanisms to Therapeutic Strategies. Antioxidants 2023, 12, 920. https://doi.org/10.3390/antiox12040920
Qiao J, Wang C, Chen Y, Yu S, Liu Y, Yu S, Jiang L, Jin C, Wang X, Zhang P, et al. Herbal/Natural Compounds Resist Hallmarks of Brain Aging: From Molecular Mechanisms to Therapeutic Strategies. Antioxidants. 2023; 12(4):920. https://doi.org/10.3390/antiox12040920
Chicago/Turabian StyleQiao, Juhui, Chenxi Wang, Yu Chen, Shuang Yu, Ying Liu, Shiting Yu, Leilei Jiang, Chenrong Jin, Xinran Wang, Peiguang Zhang, and et al. 2023. "Herbal/Natural Compounds Resist Hallmarks of Brain Aging: From Molecular Mechanisms to Therapeutic Strategies" Antioxidants 12, no. 4: 920. https://doi.org/10.3390/antiox12040920
APA StyleQiao, J., Wang, C., Chen, Y., Yu, S., Liu, Y., Yu, S., Jiang, L., Jin, C., Wang, X., Zhang, P., Zhao, D., Wang, J., & Liu, M. (2023). Herbal/Natural Compounds Resist Hallmarks of Brain Aging: From Molecular Mechanisms to Therapeutic Strategies. Antioxidants, 12(4), 920. https://doi.org/10.3390/antiox12040920








