Agomelatine, a Melatonin-Derived Drug, as a New Strategy for the Treatment of Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
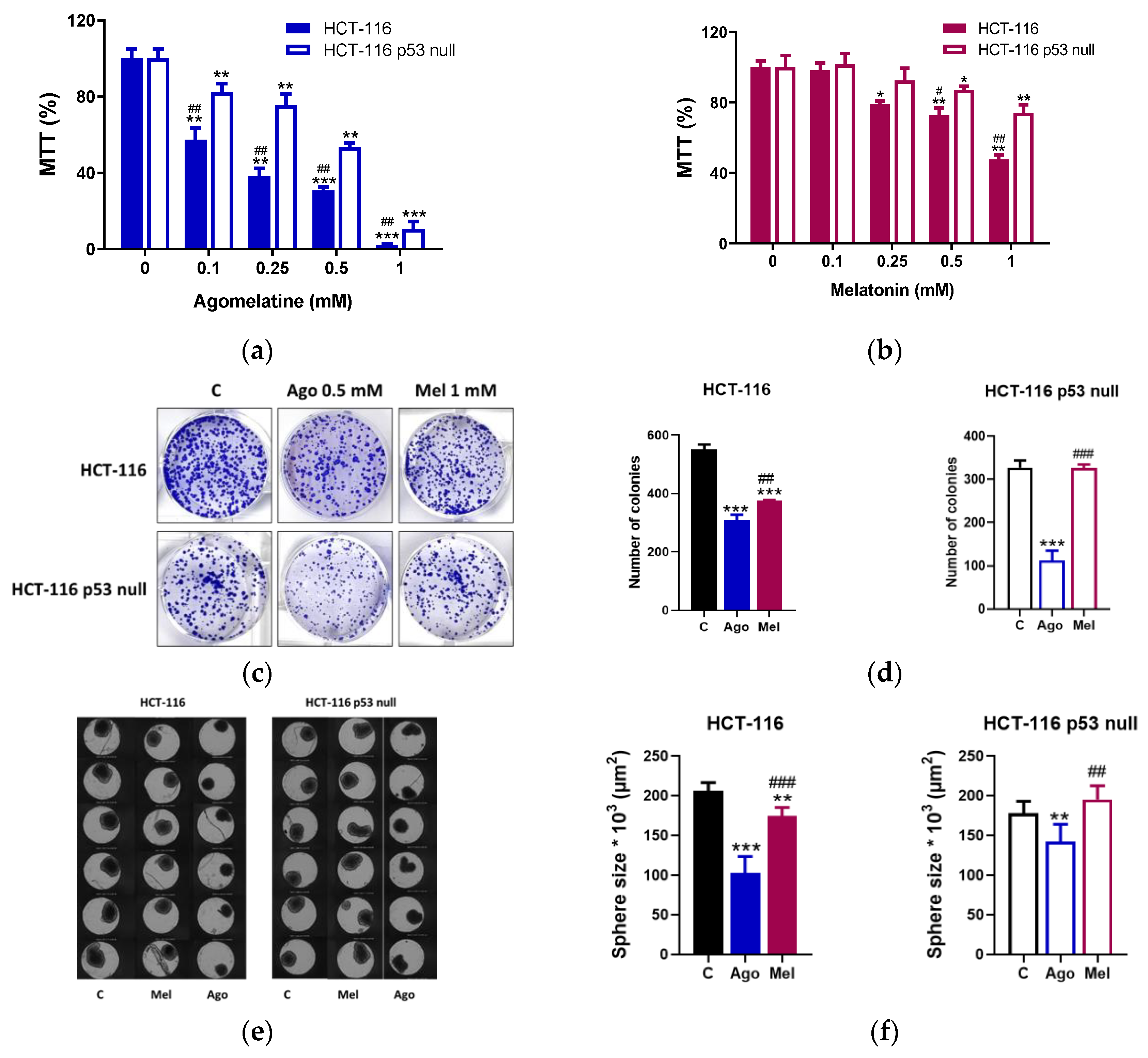
2.2. Cell Viability Assay
2.3. Sphere-Formation Assay
2.4. Clonogenic Assay
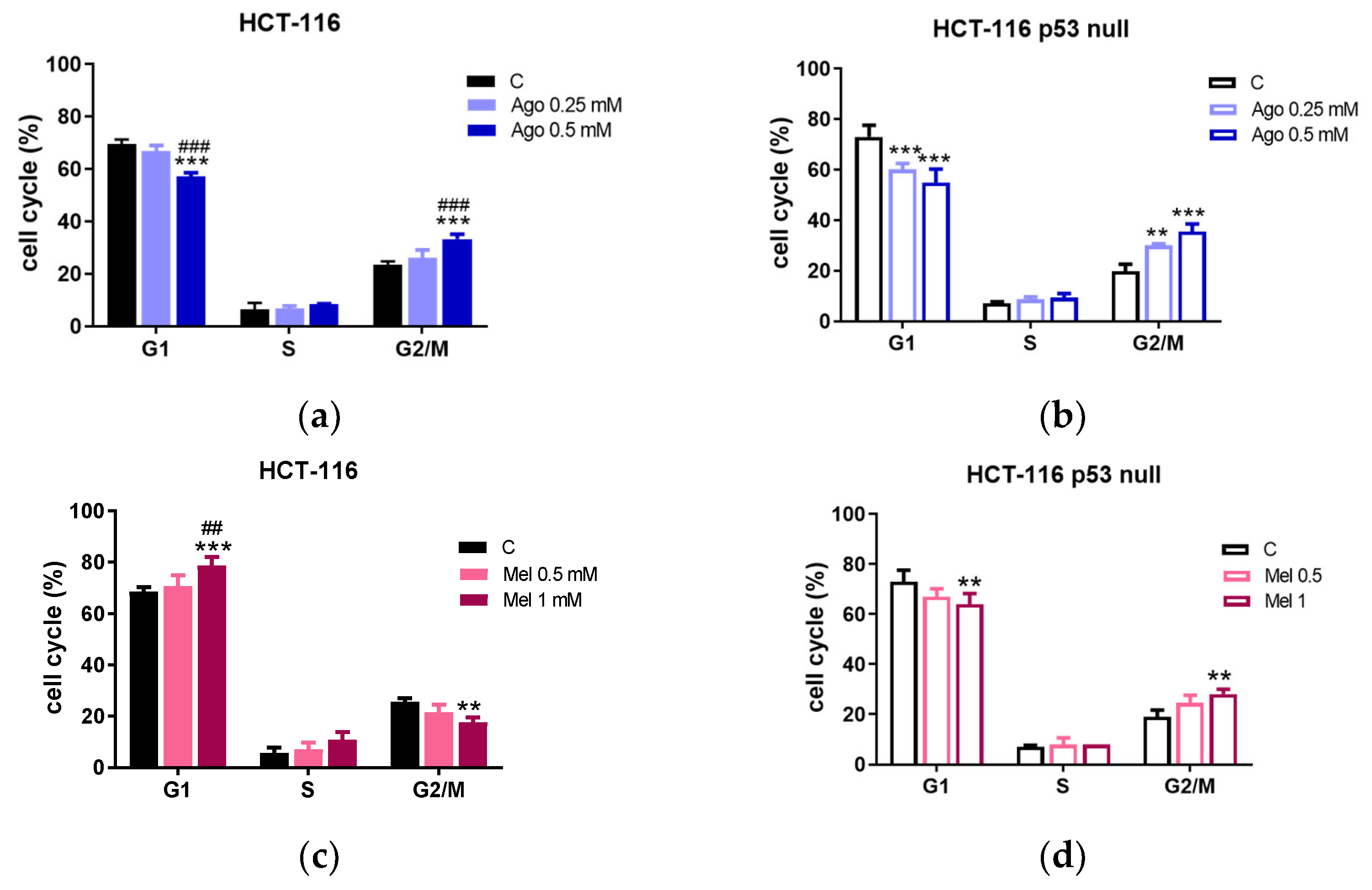
2.5. Cell-Cycle Analysis
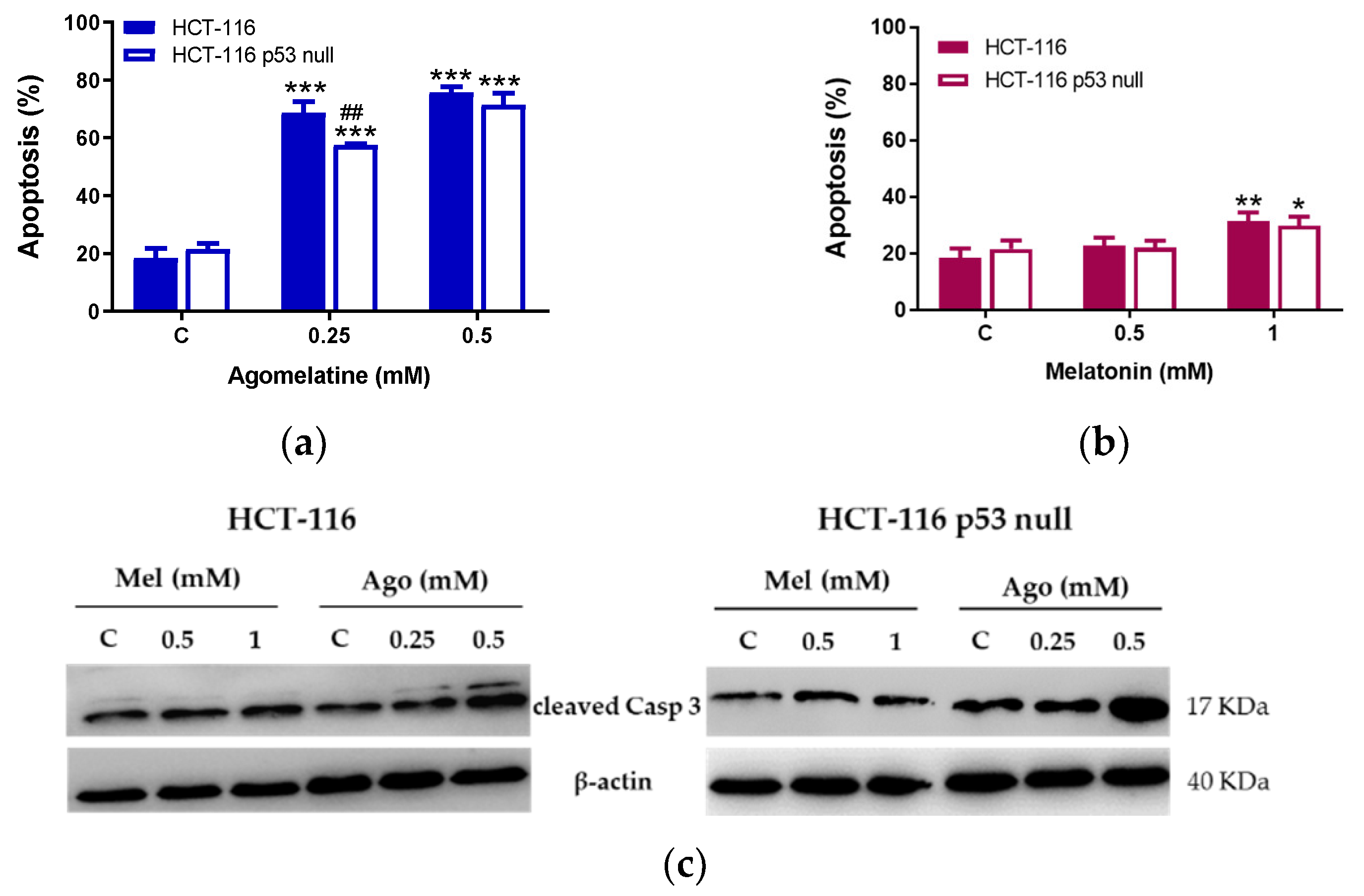
2.6. Apoptosis Assay
2.7. Real-Time PCR
2.8. Immunoblot
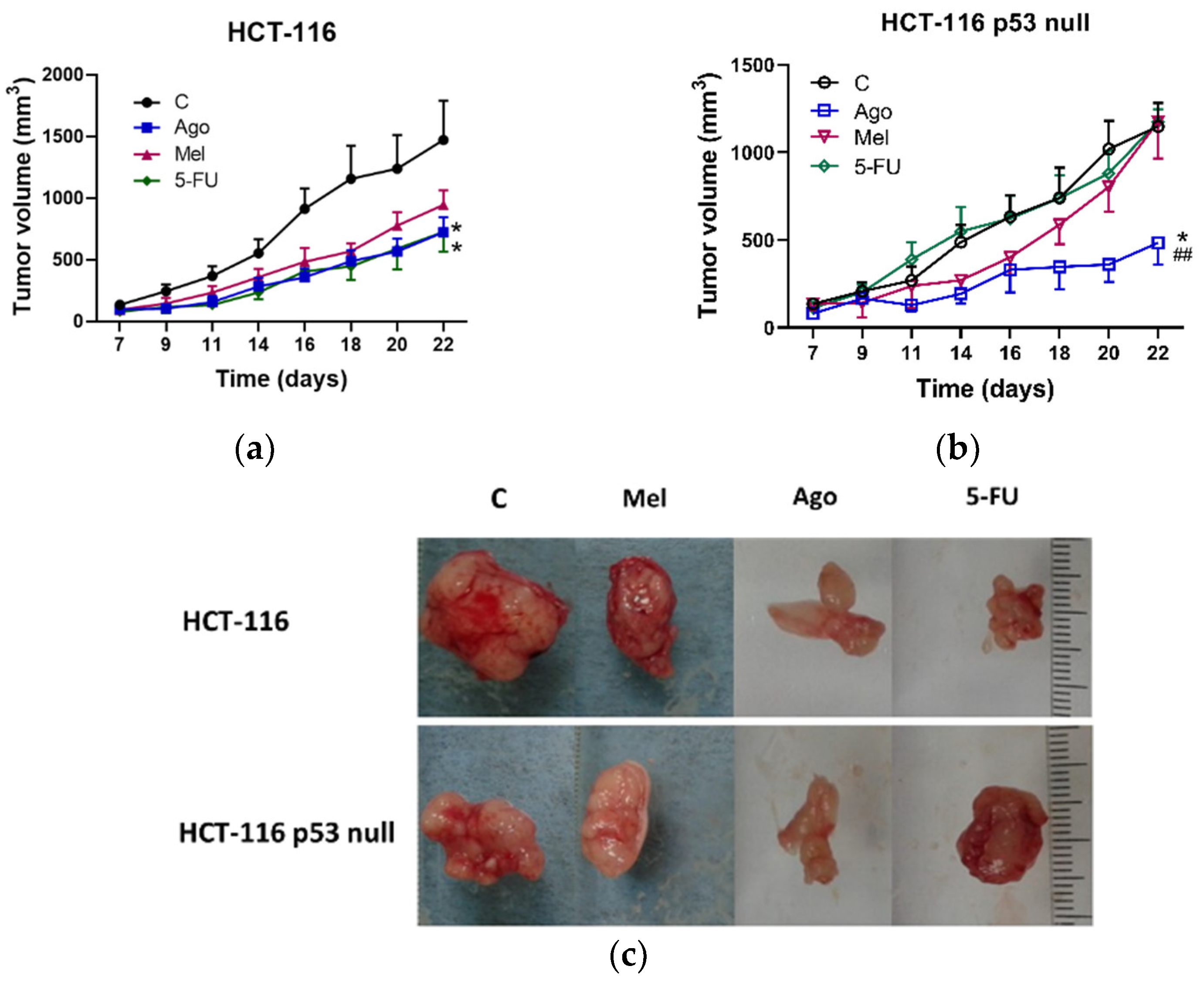
2.9. In Vivo Anti-Tumor Xenograft Studies
2.10. Statistical Analysis
3. Results
3.1. Agomelatine Inibits the Growth of Human CRC Cells in a p53-Dependent Manner
3.2. Agomelatine Induces Cell-Cycle Arrest and Caspase-Dependent Apoptosis in CRC Cells
3.3. Regulation of Tumor Growth In Vivo by Agomelatine
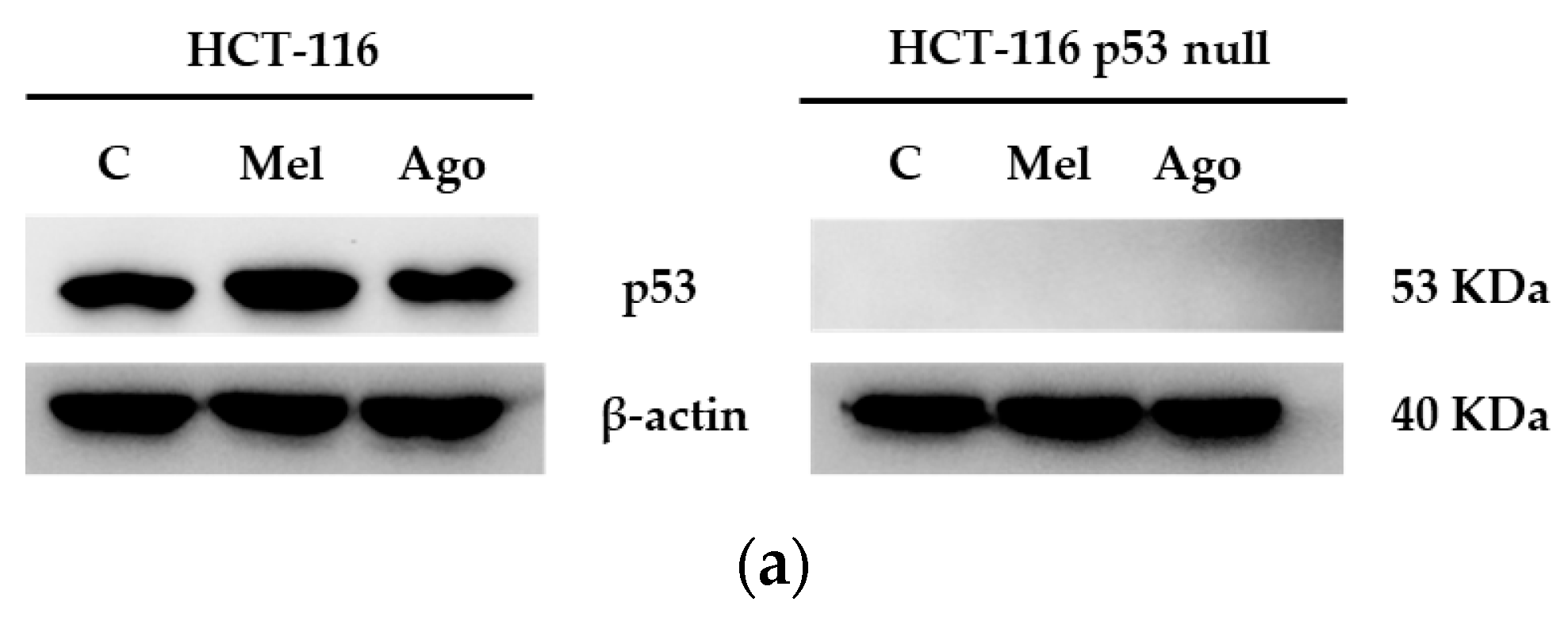
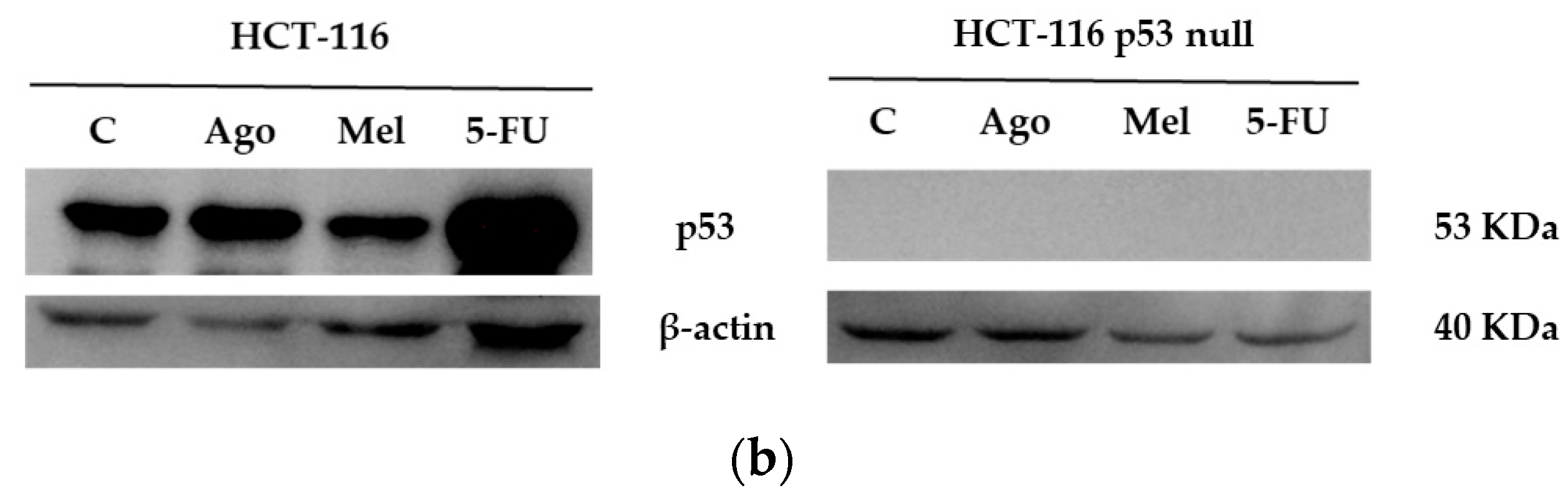
3.4. Agomelatine Regulates p53-Protein Levels in In Vitro and In Vivo Models of CRC
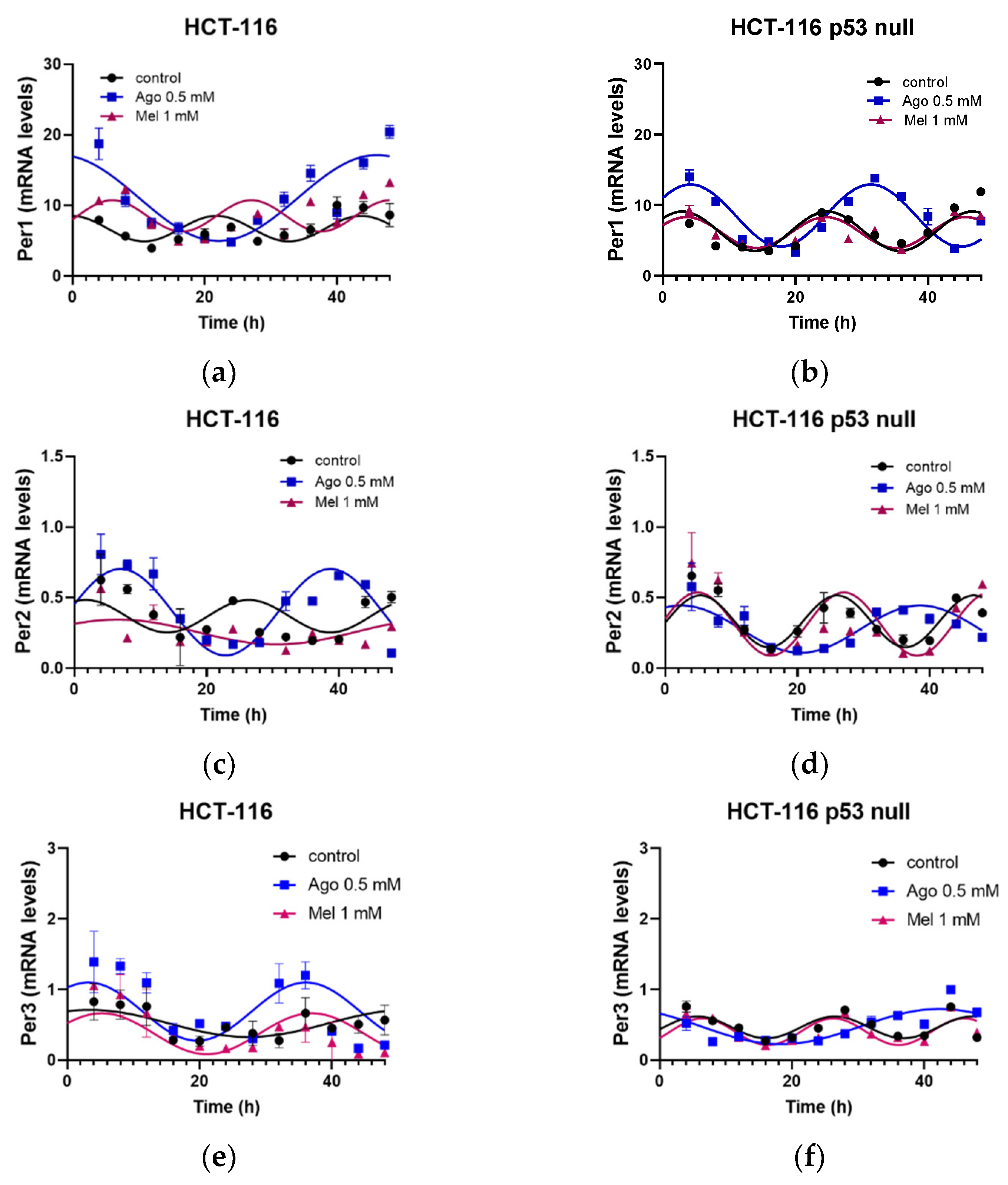
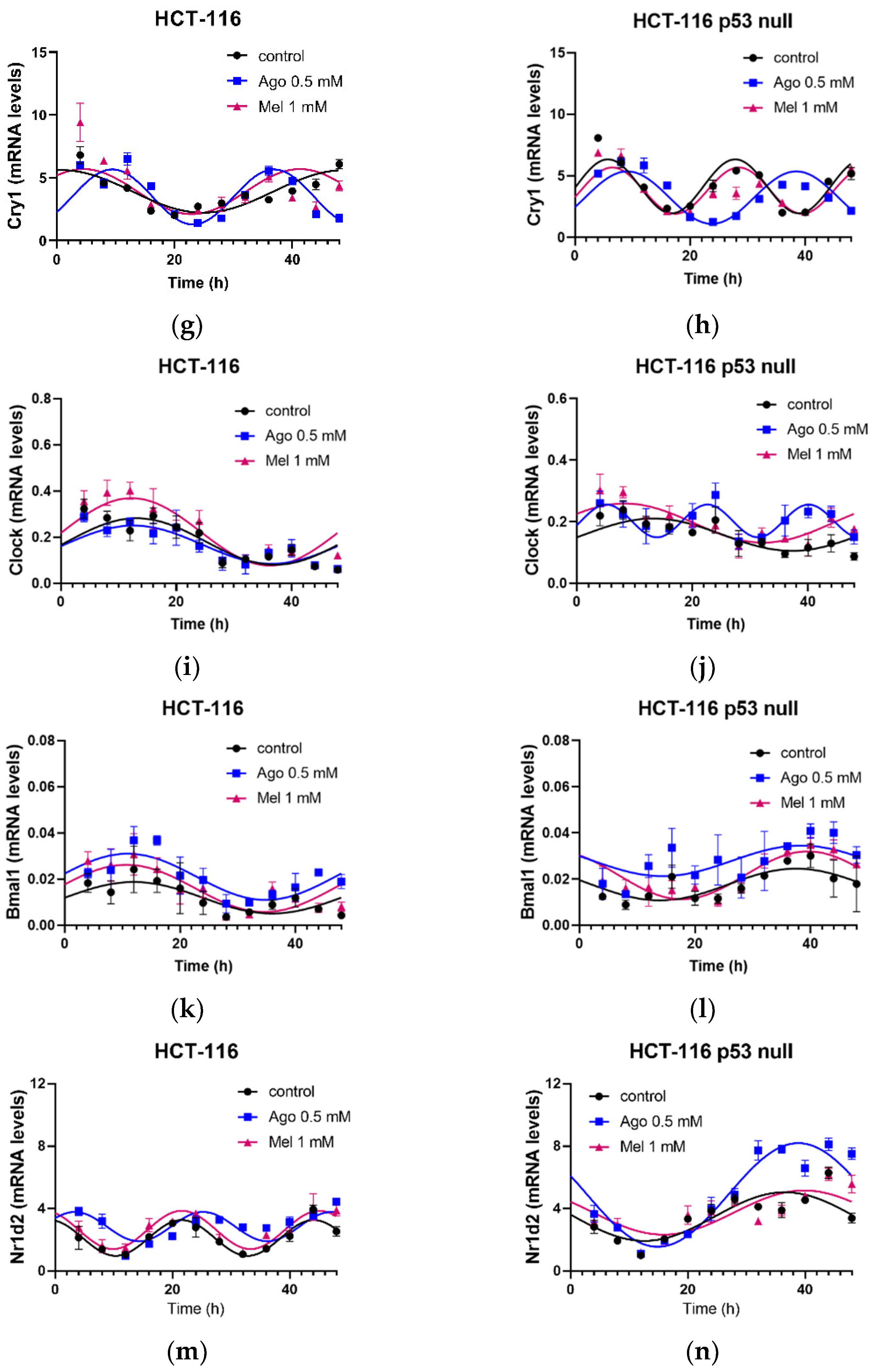
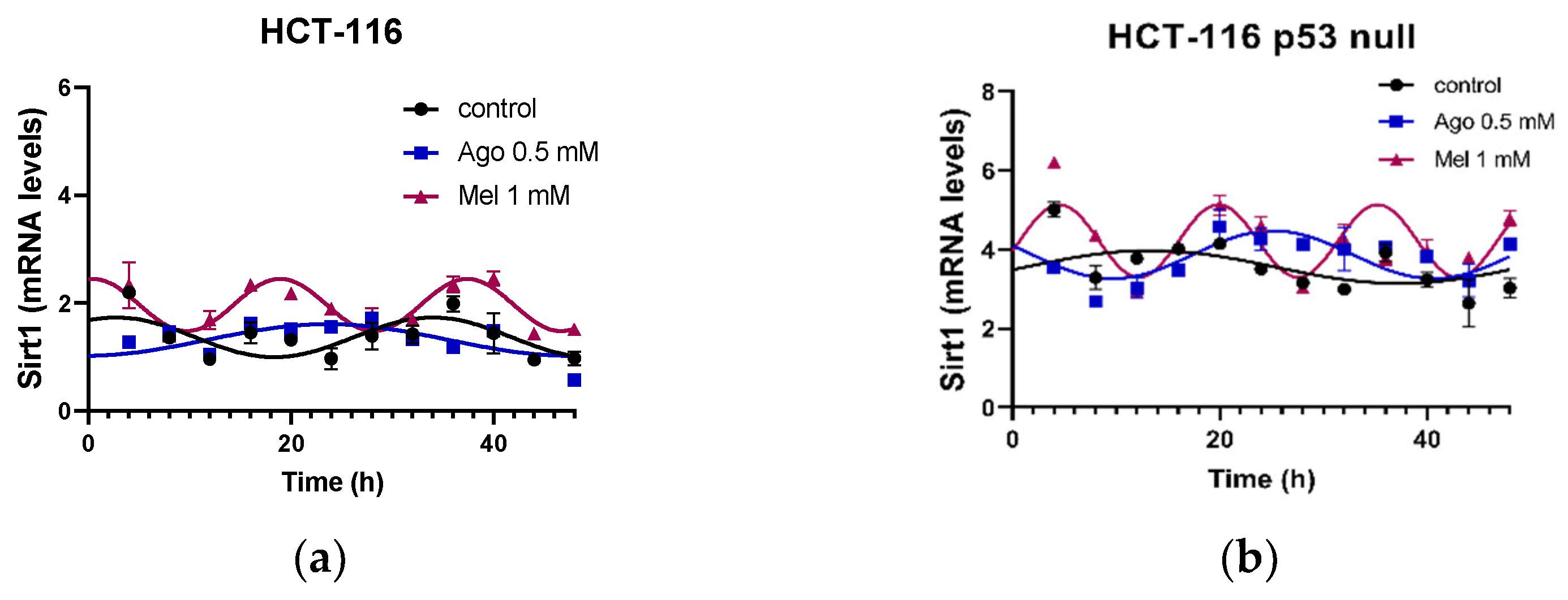
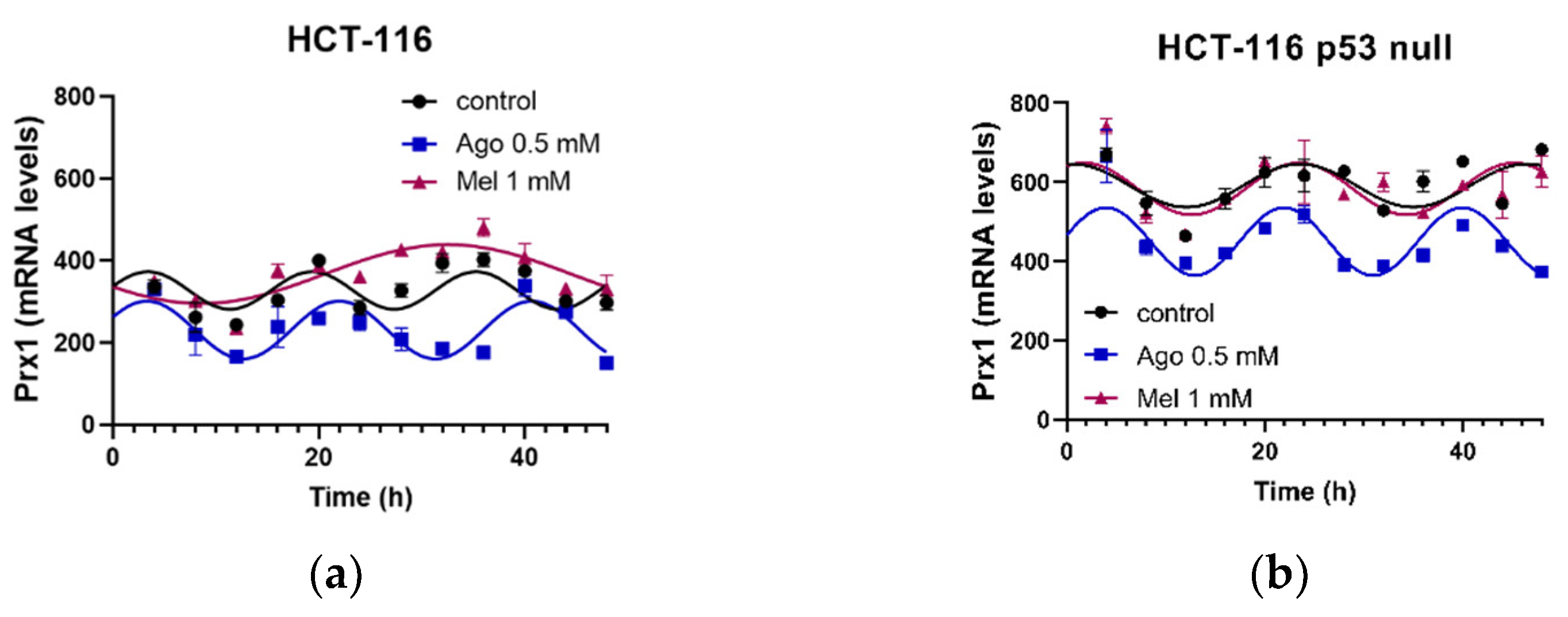
3.5. Agomelatine Regulates Circadian-Clock Genes’ Rhythmicity in CRC Cell Lines In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| Ago | agomelatine |
| ALT | alanine transaminase |
| ARE | anti-oxidant-response element |
| BMAL | brain and muscle ARNT-like |
| Clock | circadian locomotor cycles kaput |
| CRC | colorectal cancer |
| Cry | cryptocrome |
| CSCs | cancer stem cells |
| Cul3 | cullin 3 |
| Keap1 | kelch-like ECH-associated protein 1 |
| Mel | melatonin |
| MESOR | midline estimating statistic of rhythm |
| MT | melatonin receptor |
| NAD | nicotinamide adenine dinucleotide |
| Nr1d2 | nuclear receptor subfamily 1 group D member 2 |
| Nrf2 | nuclear factor erythroid 2 related factor-2 |
| Per | period |
| PR | percentage of rhythm |
| Prx-1 | peroxiredoxin-1 |
| RORα | RAR-related orphan receptor alpha |
| SIRT1 | sirtuin 1 |
| TTFL | transcription–translation feedback loops. |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive Review of Targeted Therapy for Colorectal Cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-Fluorouracil and Other Fluoropyrimidines in Colorectal Cancer: Past, Present and Future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.; Reddy, A.B. Molecular Mechanisms of the Circadian Clockwork in Mammals. FEBS Lett. 2014, 588, 2477–2483. [Google Scholar] [CrossRef]
- Tang, B.L. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kleszcz, R.; Paluszczak, J.; Baer-Dubowska, W. Targeting Aberrant Cancer Metabolism—The Role of Sirtuins. Pharmacol. Rep. 2015, 67, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Bellet, M.M.; Orozco-Solis, R.; Sahar, S.; Eckel-Mahan, K.; Sassone-Corsi, P. The Time of Metabolism: NAD+, SIRT1, and the Circadian Clock. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 31–38. [Google Scholar] [CrossRef]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins Are Conserved Markers of Circadian Rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Peng, L.; An, L.; Sun, N.; Hu, X.; Zhou, P.; Xu, Y.; Li, P.; Chen, J. Tanshindiol C Inhibits Oxidized Low-Density Lipoprotein Induced Macrophage Foam Cell Formation via a Peroxiredoxin 1 Dependent Pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Ahn, J.-Y.; Liang, P.; Ip, C.; Zhang, Y.; Park, Y.-M. Human Prx1 Gene Is a Target of Nrf2 and Is Up-Regulated by Hypoxia/Reoxygenation: Implication to Tumor Biology. Cancer Res. 2007, 67, 546–554. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox. Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Khan, H.; Majumdar, A. Crosstalk between Sirtuins and Nrf2: SIRT1 Activators as Emerging Treatment for Diabetic Neuropathy. Metab. Brain Dis. 2022, 37, 2181–2195. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, F.C.; Rao, A.; Maguire, A. Circadian Molecular Clocks and Cancer. Cancer Lett. 2014, 342, 9–18. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Xu, H.-C.; Li, Y.-J.; Lu, X.-B.; Ji, G. Drug Resistance and New Therapies in Colorectal Cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Lee, Y. Roles of Circadian Clocks in Cancer Pathogenesis and Treatment. Exp. Mol. Med. 2021, 53, 1529–1538. [Google Scholar] [CrossRef]
- García-Costela, M.; Escudero-Feliú, J.; Puentes-Pardo, J.D.; San Juán, S.M.; Morales-Santana, S.; Ríos-Arrabal, S.; Carazo, Á.; León, J. Circadian Genes as Therapeutic Targets in Pancreatic Cancer. Front. Endocrinol. 2020, 11, 638. [Google Scholar] [CrossRef]
- León, J.; Casado, J.; Ruiz, S.M.J.; Zurita, M.S.; González-Puga, C.; Rejón, J.D.; Gila, A.; de Rueda, P.M.; Pavón, E.J.; Reiter, R.J.; et al. Melatonin Reduces Endothelin-1 Expression and Secretion in Colon Cancer Cells through the Inactivation of FoxO-1 and NF-κβ. J. Pineal Res. 2014, 56, 415–426. [Google Scholar] [CrossRef]
- Wei, J.-Y.; Li, W.-M.; Zhou, L.-L.; Lu, Q.-N.; He, W. Melatonin Induces Apoptosis of Colorectal Cancer Cells through HDAC4 Nuclear Import Mediated by CaMKII Inactivation. J. Pineal Res. 2015, 58, 429–438. [Google Scholar] [CrossRef]
- Kannen, V.; Marini, T.; Zanette, D.L.; Frajacomo, F.T.; Silva, G.E.B.; Silva, W.A.; Garcia, S.B. The Melatonin Action on Stromal Stem Cells within Pericryptal Area in Colon Cancer Model under Constant Light. Biochem. Biophys. Res. Commun. 2011, 405, 593–598. [Google Scholar] [CrossRef] [PubMed]
- León, J.; Casado, J.; Carazo, Á.; Sanjuán, L.; Maté, A.; Rueda, P.M.; de Cueva, P.; de la Quiles, R.; Ruíz, S.; Ruíz-Extremera, Á.; et al. Gender-Related Invasion Differences Associated with MRNA Expression Levels of Melatonin Membrane Receptors in Colorectal Cancer. Mol. Carcinog. 2012, 51, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Casado, J.; Iñigo-Chaves, A.; Jiménez-Ruiz, S.M.; Ríos-Arrabal, S.; Carazo-Gallego, Á.; González-Puga, C.; Núñez, M.I.; Ruíz-Extremera, Á.; Salmerón, J.; León, J. AA-NAT, MT1 and MT2 Correlates with Cancer Stem-Like Cell Markers in Colorectal Cancer: Study of the Influence of Stage and P53 Status of Tumors. Int. J. Mol. Sci. 2017, 18, 1251. [Google Scholar] [CrossRef] [PubMed]
- Guardiola-Lemaitre, B.; De Bodinat, C.; Delagrange, P.; Millan, M.J.; Munoz, C.; Mocaër, E. Agomelatine: Mechanism of Action and Pharmacological Profile in Relation to Antidepressant Properties. Br. J. Pharm. 2014, 171, 3604–3619. [Google Scholar] [CrossRef] [PubMed]
- Mairesse, J.; Silletti, V.; Laloux, C.; Zuena, A.R.; Giovine, A.; Consolazione, M.; Van Camp, G.; Malagodi, M.; Gaetani, S.; Cianci, S.; et al. Chronic Agomelatine Treatment Corrects the Abnormalities in the Circadian Rhythm of Motor Activity and Sleep/Wake Cycle Induced by Prenatal Restraint Stress in Adult Rats. Int. J. Neuropsychopharmacol. 2013, 16, 323–338. [Google Scholar] [CrossRef]
- Kannen, V.; Bader, M.; Sakita, J.Y.; Uyemura, S.A.; Squire, J.A. The Dual Role of Serotonin in Colorectal Cancer. Trends Endocrinol. Metab. 2020, 31, 611–625. [Google Scholar] [CrossRef]
- Soni, S.K.; Basu, P.; Singaravel, M.; Sharma, R.; Pandi-Perumal, S.R.; Cardinali, D.P.; Reiter, R.J. Sirtuins and the Circadian Clock Interplay in Cardioprotection: Focus on Sirtuin 1. Cell. Mol. Life Sci. 2021, 78, 2503–2515. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Abd El-Ghafar, O.A.M.; Alzoghaibi, M.A.; Hassanein, E.H.M. Agomelatine Prevents Gentamicin Nephrotoxicity by Attenuating Oxidative Stress and TLR-4 Signaling, and Upregulating PPARγ and SIRT1. Life Sci. 2021, 278, 119600. [Google Scholar] [CrossRef]
- Mahmoud, N.M.; Elshazly, S.M.; Hassan, A.A.; Soliman, E. Agomelatine Improves Streptozotocin-Induced Diabetic Nephropathy through Melatonin Receptors/SIRT1 Signaling Pathway. Int. Immunopharmacol. 2023, 115, 109646. [Google Scholar] [CrossRef]
- Refinetti, R. Non-Parametric Procedures for the Determination of Phase Markers of Circadian Rhythms. Int. J. Biomed. Comput. 1992, 30, 49–56. [Google Scholar] [CrossRef]
- Orozco-Solis, R.; Ramadori, G.; Coppari, R.; Sassone-Corsi, P. SIRT1 Relays Nutritional Inputs to the Circadian Clock through the Sf1 Neurons of the Ventromedial Hypothalamus. Endocrinology 2015, 156, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Violette, S.; Poulain, L.; Dussaulx, E.; Pepin, D.; Faussat, A.-M.; Chambaz, J.; Lacorte, J.-M.; Staedel, C.; Lesuffleur, T. Resistance of Colon Cancer Cells to Long-Term 5-Fluorouracil Exposure Is Correlated to the Relative Level of Bcl-2 and Bcl-X(L) in Addition to Bax and P53 Status. Int. J. Cancer 2002, 98, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Santoro, R.; Marani, M.; Blandino, G.; Muti, P.; Strano, S. Melatonin Triggers P53Ser Phosphorylation and Prevents DNA Damage Accumulation. Oncogene 2012, 31, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ma, S.-J.; Luo, J.-H.; Zhang, H.; Wang, R.-X.; Liu, H.; Li, L.; Zhang, Z.-G.; Zhou, R.-X. Melatonin Induces the Apoptosis and Inhibits the Proliferation of Human Gastric Cancer Cells via Blockade of the AKT/MDM2 Pathway. Oncol. Rep. 2018, 39, 1975–1983. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Zhai, K.; Varghese, E.; Samuel, S.M.; Šudomová, M.; Lucansky, V.; Kassayova, M.; Pec, M.; et al. Metabolic Anti-Cancer Effects of Melatonin: Clinically Relevant Prospects. Cancers 2021, 13, 3018. [Google Scholar] [CrossRef]
- Winczyk, K.; Fuss-Chmielewska, J.; Lawnicka, H.; Pawlikowski, M.; Karasek, M. Luzindole but Not 4-Phenyl-2- Propionamidotetralin (4P-PDOT) Diminishes the Inhibitory Effect of Melatonin on Murine Colon 38 Cancer Growth In Vitro. Neuro Endocrinol. Lett. 2009, 30, 657–662. [Google Scholar]
- Karasek, M.; Carrillo-Vico, A.; Guerrero, J.M.; Winczyk, K.; Pawlikowski, M. Expression of Melatonin MT(1) and MT(2) Receptors, and ROR Alpha(1) Receptor in Transplantable Murine Colon 38 Cancer. Neuro Endocrinol. Lett. 2002, 23, 55–60. [Google Scholar]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharm. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef]
- Diez-Echave, P.; Vezza, T.; Algieri, F.; Ruiz-Malagón, A.J.; Hidalgo-García, L.; García, F.; Morón, R.; Sánchez, M.; Toral, M.; Romero, M.; et al. The Melatonergic Agonist Agomelatine Ameliorates High Fat Diet-Induced Obesity in Mice through the Modulation of the Gut Microbiome. Biomed. Pharm. 2022, 153, 113445. [Google Scholar] [CrossRef]
- Kim, H.K.; Yang, K.I. Melatonin and Melatonergic Drugs in Sleep Disorders. Transl. Clin. Pharm. 2022, 30, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zou, D.; Yang, X.; Xue, X.; Zuo, L.; Zhou, Q.; Hu, R.; Wang, Y. Melatonin Inhibits Colon Cancer RKO Cell Migration by Downregulating Rho-associated Protein Kinase Expression via the P38/MAPK Signaling Pathway. Mol. Med. Rep. 2017, 16, 9383–9392. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. Agomelatine or Ramelteon as Treatment Adjuncts in Glioblastoma and Other M1- or M2-Expressing Cancers. Contemp. Oncol. Współczesna Onkol. 2015, 2, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, E.J.; Shabbir, M.A.; Mikhailidis, D.P.; Mumtaz, F.H.; Thompson, C.S. The Effect of Serotonin and Serotonin Antagonists on Bladder Cancer Cell Proliferation. BJU Int. 2006, 97, 634–639. [Google Scholar] [CrossRef]
- Pantaleo, M.A.; Palassini, E.; Labianca, R.; Biasco, G. Targeted Therapy in Colorectal Cancer: Do We Know Enough? Dig. Liver Dis. 2006, 38, 71–77. [Google Scholar] [CrossRef]
- Hill, S.M.; Belancio, V.P.; Dauchy, R.T.; Xiang, S.; Brimer, S.; Mao, L.; Hauch, A.; Lundberg, P.W.; Summers, W.; Yuan, L.; et al. Melatonin: An Inhibitor of Breast Cancer. Endocr. Relat. Cancer 2015, 22, R183–R204. [Google Scholar] [CrossRef]
- de Bodinat, C.; Guardiola-Lemaitre, B.; Mocaër, E.; Renard, P.; Muñoz, C.; Millan, M.J. Agomelatine, the First Melatonergic Antidepressant: Discovery, Characterization and Development. Nat. Rev. Drug Discov. 2010, 9, 628–642. [Google Scholar] [CrossRef]
- Tardito, D.; Molteni, R.; Popoli, M.; Racagni, G. Synergistic Mechanisms Involved in the Antidepressant Effects of Agomelatine. Eur. Neuropsychopharmacol. 2012, 22, S482–S486. [Google Scholar] [CrossRef]
- Jung-Hynes, B.; Reiter, R.J.; Ahmad, N. Sirtuins, Melatonin and Circadian Rhythms: Building a Bridge between Aging and Cancer. J. Pineal Res. 2010, 48, 9–19. [Google Scholar] [CrossRef]
- Ríos-Arrabal, S.; Muñoz-Gámez, J.A.; Jiménez-Ruíz, S.M.; Casado-Ruíz, J.; Artacho-Cordón, F.; León-López, J. Circadian Regulation of Colon Cancer Stem Cells: Implications for Therapy; IntechOpen: London, UK, 2016; ISBN 978-953-51-2545-7. [Google Scholar]
- Mayo, J.C.; Sainz, R.M.; González Menéndez, P.; Cepas, V.; Tan, D.-X.; Reiter, R.J. Melatonin and Sirtuins: A “Not-so Unexpected” Relationship. J. Pineal Res. 2017, 62, e12391. [Google Scholar] [CrossRef]
- Ray, S.; Valekunja, U.K.; Stangherlin, A.; Howell, S.A.; Snijders, A.P.; Damodaran, G.; Reddy, A.B. Circadian Rhythms in the Absence of the Clock Gene Bmal1. Science 2020, 367, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Matsumoto, T.; Zhao, Z.; Lee, C.C. P53 Regulates Period2 Expression and the Circadian Clock. Nat. Commun. 2013, 4, 2444. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-Dependent Deacetylase SIRT1 Modulates CLOCK-Mediated Chromatin Remodeling and Circadian Control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.F.; Bernardo, V.S.; de Paula, C.P.; da Silva, J.P.M.D.O.; de Almeida, E.A.; da Cunha, A.F.; da Silva, D.G.H. Influence of Melatonin Treatment on Cellular Mechanisms of Redox Adaptation in K562 Erythroleukemic Cells. Genes 2022, 13, 2337. [Google Scholar] [CrossRef]
- Sun, X.-X.; Dai, M.-S.; Lu, H. 5-Fluorouracil Activation of P53 Involves an MDM2-Ribosomal Protein Interaction. J. Biol. Chem. 2007, 282, 8052–8059. [Google Scholar] [CrossRef]
- Bunz, F.; Hwang, P.M.; Torrance, C.; Waldman, T.; Zhang, Y.; Dillehay, L.; Williams, J.; Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Disruption of P53 in Human Cancer Cells Alters the Responses to Therapeutic Agents. J. Clin. Investig. 1999, 104, 263–269. [Google Scholar] [CrossRef]
- Stopper, H.; Garcia, S.B.; Waaga-Gasser, A.M.; Kannen, V. Antidepressant Fluoxetine and Its Potential against Colon Tumors. World J. Gastrointest. Oncol. 2014, 6, 11–21. [Google Scholar] [CrossRef]
- Zheng, Y.; Chang, X.; Huang, Y.; He, D. The Application of Antidepressant Drugs in Cancer Treatment. Biomed. Pharm. 2023, 157, 113985. [Google Scholar] [CrossRef]
| Gene | Treatment | PR 1 | p-Value 2 | Amplitude (A.U.) 3 | Acrophase (h) 4 | MESOR (A.U.) |
|---|---|---|---|---|---|---|
| Per1 | Control | 29.41 | 0.03 | 5.17 ± 1.45 | 3.06 ± 1.91 | 9.77 ± 0.99 |
| Ago 0.5 | 42.85 | 0.003 | 6.06 ± 1.22 | 45.30 ± 1.53 *** | 10.40 ± 0.86 | |
| Mel 1 | 34.77 | 0.008 | 2.24 ± 0.53 # | 32.83 ± 2.00 ***,### | 8.25 ± 0.39 | |
| Per2 | Control | 31.66 | 0.006 | 0.076 ± 0.018 | 2.76 ± 1.19 | 0.28 ± 0.01 |
| Ago 0.5 | 42.02 | 0.001 | 0.140 ± 0.003 * | 9.35 ± 0.08 *** | 0.25 ± 0.02 | |
| Mel 1 | 27.81 | 0.003 | 0.088 ± 0.024 | 6.58 ± 2.09 ***,### | 0.26 ± 0.02 | |
| Per3 | Control | 25.36 | 0.008 | 0.33 ± 0.09 | 17.47 ± 1.15 | 0.72 ± 0.07 |
| Ago 0.5 | 60.62 | 0.0001 | 0.91 ± 0.14 * | 6.06 ± 0.68 *** | 0.94 ± 0.09 | |
| Mel 1 | 31.33 | 0.002 | 0.96 ± 0.25 * | 5.55 ± 1.44 *** | 0.83 ± 0.18 | |
| Cry1 | Control | 63.01 | <0.0001 | 1.62 ± 0.22 | 1.79 ± 1.02 | 3.81 ± 0.15 |
| Ago 0.5 | 73.64 | <0.0001 | 2.19 ± 0.21 | 9.47 ± 0.48 *** | 3.48 ± 0.16 | |
| Mel 1 | 37.72 | 0.0004 | 1.79 ± 0.42 | 4.55 ± 1.31 **,### | 3.90 ± 0.29 | |
| Clock | Control | 57.99 | <0.0001 | 0.101 ± 0.015 | 13.14 ± 1.13 | 0.18 ± 0.01 |
| Ago 0.5 | 56.69 | 0.001 | 0.111 ± 0.019 | 11.76 ± 2.97 | 0.16 ± 0.01 | |
| Mel 1 | 56.75 | <0.0001 | 0.189 ± 0.029 **,### | 10.77 ± 1.16 | 0.26 ± 0.02 ***,### | |
| Bmal1 | Control | 61.01 | <0.0001 | 0.0088 ± 0.0012 | 12.99 ± 1.06 | 0.014 ± 0.001 |
| Ago 0.5 | 71.30 | <0.0001 | 0.0138 ± 0.0015 | 10.47 ± 0.84 | 0.025 ± 0.001 *** | |
| Mel 1 | 38.63 | 0.0003 | 0.0092 ± 0.0020 | 10.85 ± 1.67 | 0.016 ± 0.001 ### | |
| Nr1d2 | Control | 48.84 | <0.0001 | 1.73 ± 0.32 | 12.19 ± 0.63 | 3.79 ± 0.22 |
| Ago 0.5 | 55.56 | <0.0001 | 0.94 ± 0.15 ** | 3.32 ± 0.55 *** | 2.86 ± 0.11 *** | |
| Mel 1 | 57.84 | <0.0001 | 1.89 ± 0.28 ## | 11.55 ± 0.56 ### | 3.74 ± 0.20 ### |
| Gene | Treatment | PR 1 | p-Value 2 | Amplitude (A.U.) 3 | Acrophase (h) 4 | MESOR (A.U.) |
|---|---|---|---|---|---|---|
| Per1 | Control | 47.47 | 0.0001 | 2.42 ± 0.45 | 3.01 ± 0.65 | 6.11 ± 0.33 † |
| Ago 0.5 | 51.58 | 0.003 | 2.54 ± 1.22 | 4.80 ± 3.68 *** | 8.36 ± 0.86 ** | |
| Mel1 | 68.24 | <0.0001 | 2.2 ± 0.26 | 2.61 ± 0.41 ### | 6.13 ± 0.19 ## | |
| Per2 | Control | 61.90 | <0.0001 | 0.22 ± 0.03† | 4.89 ± 0.48 ††† | 0.34 ± 0.02 |
| Ago 0.5 | 44.57 | 0.0001 | 0.19 ± 0.04 | 1.29 ± 1.16 *** | 0.29 ± 0.03 | |
| Mel 1 | 56.40 | <0.0001 | 0.25 ± 0.04 | 5.44 ± 0.56 ### | 0.32 ± 0.03 | |
| Per3 | Control | 40.65 | 0.0002 | 0.13 ± 0.03 | 6.02 ± 0.71 | 0.37 ± 0.02 †† |
| Ago 0.5 | 65.87 | <0.0001 | 0.21 ± 0.03 * | 42.48 ± 0.95 *** | 0.37 ± 0.02 | |
| Mel 1 | 56.89 | <0.0001 | 0.17 ± 0.02 | 6.41 ± 1.16 ### | 0.34 ± 0.02 | |
| Cry1 | Control | 78.47 | <0.0001 | 2.19 ± 0.20 | 5.73 ± 0.34 ††† | 4.16 ± 0.14 |
| Ago 0.5 | 79.43 | <0.0001 | 2.13 ± 0.17 | 9.11 ± 0.42 * | 3.25 ± 0.13 *** | |
| Mel 1 | 67.89 | <0.0001 | 1.88 ± 0.22 | 6.51 ± 0.44 | 3.82 ± 0.16 ## | |
| Clock | Control | 41.13 | 0.0002 | 0.05 ± 0.01 † | 11.99 ± 1.59 | 0.16 ± 0.01 |
| Ago 0.5 | 73.01 | 0.0001 | 0.04 ± 0.01 | 4.57 ± 0.76 *** | 0.19 ± 0.01 ** | |
| Mel 1 | 56.47 | <0.0001 | 0.06 ± 0.01 | 8.86 ± 1.17 ## | 0.19 ± 0.01 ** | |
| Bmal1 | Control | 58.43 | <0.0001 | 0.018 ± 0.003 ††† | 35.12 ± 1.09 ††† | 0.025 ± 0.002 ††† |
| Ago 0.5 | 50.78 | <0.0001 | 0.011 ± 0.002 | 39.95 ± 1.31 | 0.022 ± 0.001 | |
| Mel 1 | 37.40 | 0.0004 | 0.010 ± 0.002 | 37.92 ± 1.56 | 0.020 ± 0.002 * | |
| Nr1d2 | Control | 45.84 | <0.0001 | 1.41 ± 0.27 †† | 35.29 ± 1.44 ††† | 3.25 ± 0.19 |
| Ago 0.5 | 71.46 | <0.0001 | 3.78 ± 0.41 *** | 37.66 ± 0.84 | 5.54 ± 0.29 *** | |
| Mel 1 | 44.92 | 0.0001 | 2.08 ± 0.40 ## | 36.94 ± 1.47 | 4.07 ± 0.28 ### |
| Cell Line | Treatment | PR 1 | p-Value 2 | Amplitude (A.U.) 3 | Acrophase (h) 4 | MESOR (A.U.) |
|---|---|---|---|---|---|---|
| HCT-116 | Control | 47.58 | <0.0001 | 0.367 ± 0.071 | 2.80 ± 0.92 | 1.37 ± 0.15 |
| Ago 0.5 | 47.05 | <0.0001 | 0.456 ± 0.084 | 22.31 ± 1.41 *** | 1.23 ± 0.16 | |
| Mel 1 | 80.65 | <0.0001 | 0.483 ± 0.042 | 0.52 ± 0.24 ***,### | 1.96 ± 0.13 | |
| HCT-116 p53 null | Control | 34.8 | 0.0009 | 0.392 ± 0.093 | 16.42 ± 1.82 ††† | 3.48 ± 0.17 ††† |
| Ago 0.5 | 35.92 | 0.0006 | 0.518 ± 0.114 | 25.48 ± 1.12 *** | 3.91 ± 0.18 | |
| Mel 1 | 62.29 | <0.0001 | 0.766 ± 0.102 ** | 4.59 ± 0.34 ***,### | 4.14 ± 0.17 |
| Cell Line | Treatment | PR 1 | p-Value 2 | Amplitude (A.U) 3 | Acrophase (h) 4 | MESOR (A.U.) |
|---|---|---|---|---|---|---|
| HCT-116 | Control | 38.04 | 0.0004 | 45.7 ± 10.2 | 3.38 ± 0.56 | 329 ± 7 |
| Ago 0.5 | 74.50 | <0.0001 | 70.4 ± 7.4 | 3.27 ± 0.31 | 232 ± 5 *** | |
| Mel 1 | 67.01 | <0.0001 | 71.6 ± 8.7 | 32.54 ± 0.93 ***,### | 368 ± 6 ***,### | |
| HCT-116 p53 null | Control | 38.90 | 0.0003 | 54 ± 12 | 0.63 ± 0.79 †† | 591 ± 8 ††† |
| Ago 0.5 | 64.23 | <0.0001 | 81 ± 10 | 3.83 ± 0.39 *** | 447 ± 8 *** | |
| Mel 1 | 46.83 | <0.0001 | 65 ± 12 | 1.71 ± 0.62 ## | 583 ± 9 ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-SanJuan, S.; Puentes-Pardo, J.D.; Casado, J.; Escudero-Feliu, J.; Khaldy, H.; Arnedo, J.; Carazo, Á.; León, J. Agomelatine, a Melatonin-Derived Drug, as a New Strategy for the Treatment of Colorectal Cancer. Antioxidants 2023, 12, 926. https://doi.org/10.3390/antiox12040926
Moreno-SanJuan S, Puentes-Pardo JD, Casado J, Escudero-Feliu J, Khaldy H, Arnedo J, Carazo Á, León J. Agomelatine, a Melatonin-Derived Drug, as a New Strategy for the Treatment of Colorectal Cancer. Antioxidants. 2023; 12(4):926. https://doi.org/10.3390/antiox12040926
Chicago/Turabian StyleMoreno-SanJuan, Sara, Jose D. Puentes-Pardo, Jorge Casado, Julia Escudero-Feliu, Huda Khaldy, Javier Arnedo, Ángel Carazo, and Josefa León. 2023. "Agomelatine, a Melatonin-Derived Drug, as a New Strategy for the Treatment of Colorectal Cancer" Antioxidants 12, no. 4: 926. https://doi.org/10.3390/antiox12040926
APA StyleMoreno-SanJuan, S., Puentes-Pardo, J. D., Casado, J., Escudero-Feliu, J., Khaldy, H., Arnedo, J., Carazo, Á., & León, J. (2023). Agomelatine, a Melatonin-Derived Drug, as a New Strategy for the Treatment of Colorectal Cancer. Antioxidants, 12(4), 926. https://doi.org/10.3390/antiox12040926







