Influence of Redox and Dopamine Regulation in Cocaine-Induced Phenotypes Using Drosophila
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drosophila Strains and Culture
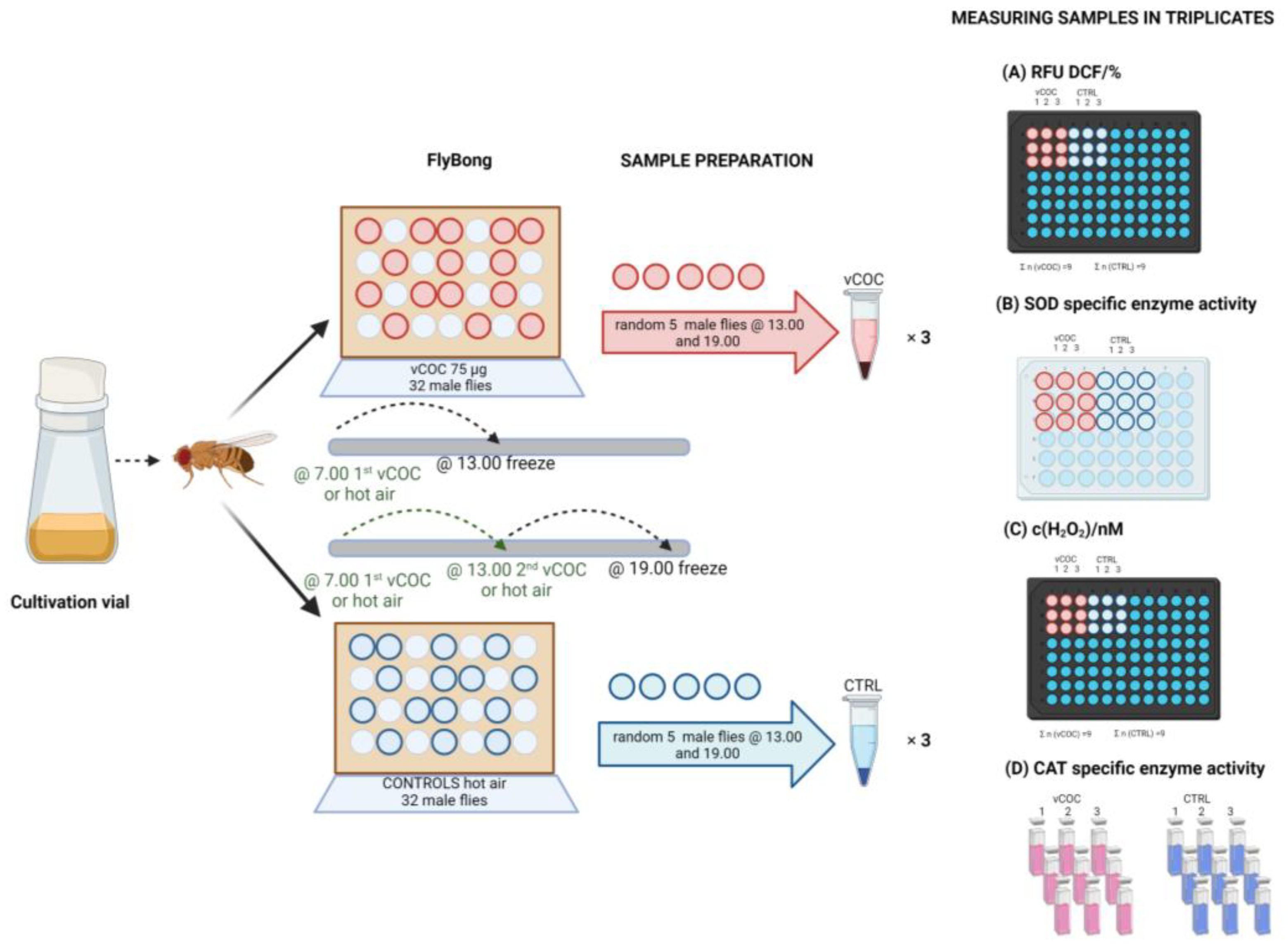
2.2. Oral Pretreatment of Flies
2.3. vCOC Administration
2.4. Sample Collection and Preparation
2.4.1. Protein Quantification
2.4.2. Specific Enzyme Activity
2.4.3. Reactive Oxygen Species
2.4.4. Hydrogen Peroxide Concentration Measurement
2.4.5. fAGEs Measurement
2.5. Data Analysis and Statistics
3. Results
3.1. vCOC Disrupts Redox Regulation
3.2. CAT Activity in Dopaminergic and Serotonergic Neurons Is Required for the Development of Locomotor Sensitization to vCOC
3.3. H2O2 Is Neccesary for The Development of Locomotor Sensitization to vCOC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Quantification of the Locomotor Activity and Sleep

Appendix B
Measurement of Monoamines

Appendix C
Oral Pretreatment of Flies

Appendix D
FlyBong Control Experiments

References
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxidative Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, J.-B.; Mangeol, A.; Revel, M.-O.; Burgun, C.; Aunis, D.; Zwiller, J. Acute or repeated cocaine administration generates reactive oxygen species and induces antioxidant enzyme activity in dopaminergic rat brain structures. Neuropharmacology 2005, 48, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Uys, J.D.; Knackstedt, L.; Hurt, P.; Tew, K.D.; Manevich, Y.; Hutchens, S.; Townsend, D.M.; Kalivas, P.W. Cocaine-Induced Adaptations in Cellular Redox Balance Contributes to Enduring Behavioral Plasticity. Neuropsychopharmacology 2011, 36, 2551–2560. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Aki, T.; Funakoshi, T.; Unuma, K.; Uemura, K. Role of Mitochondrial Dynamics in Cocaine’s Neurotoxicity. Int. J. Mol. Sci. 2022, 23, 5418. [Google Scholar] [CrossRef] [PubMed]
- Massaad, C.A.; Klann, E. Reactive Oxygen Species in the Regulation of Synaptic Plasticity and Memory. Antioxid. Redox Signal. 2011, 14, 2013–2054. [Google Scholar] [CrossRef] [Green Version]
- Nestler, E. The Neurobiology of Cocaine Addiction. Sci. Pract. Perspect. 2005, 3, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Bravo, R.R.; Faria, A.C.; Brito-Da-Costa, A.M.; Carmo, H.; Mladěnka, P.; da Silva, D.D.; Remião, F.; Researchers, O.B.O.T.O. Cocaine: An Updated Overview on Chemistry, Detection, Biokinetics, and Pharmacotoxicological Aspects including Abuse Pattern. Toxins 2022, 14, 278. [Google Scholar] [CrossRef]
- Boess, F.; Ndikum-Moffor, F.M.; A Boelsterli, U.; Roberts, S.M. Effects of cocaine and its oxidative metabolites on mitochondrial respiration and generation of reactive oxygen species. Biochem. Pharmacol. 2000, 60, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Vari, C.E. Psychoactive Drugs—From Chemical Structure to Oxidative Stress Related to Dopaminergic Neurotransmission. A Review. Antioxidants 2021, 10, 381. [Google Scholar] [CrossRef]
- Womersley, J.S.; Uys, J.D. S-Glutathionylation and Redox Protein Signaling in Drug Addiction. Prog. Mol. Biol. Transl. Sci. 2015, 137, 87–121. [Google Scholar] [CrossRef] [Green Version]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products. Dermato-Endocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treweek, J.B.; Dickerson, T.J.; Janda, K.D. Drugs of Abuse That Mediate Advanced Glycation End Product Formation: A Chemical Link to Disease Pathology. Acc. Chem. Res. 2009, 42, 659–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Münch, G.; Gerlach, M.; Sian, J.; Wong, A.; Riederer, P. Advanced glycation end products in neurodegeneration: More than early markers of oxidative stress? Ann. Neurol. 1998, 44, S85–S88. [Google Scholar] [CrossRef]
- Vujnović, A.F.; Jović, K.; Pištan, E.; Waldowski, R.A. Influence of Dopamine on Fluorescent Advanced Glycation End Products Formation Using Drosophila melanogaster. Biomolecules 2021, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Womersley, J.S.; Townsend, D.M.; Kalivas, P.W.; Uys, J.D. Targeting redox regulation to treat substance use disorder using N-acetylcysteine. Eur. J. Neurosci. 2018, 50, 2538–2551. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Delgado, J.; Blanlot, C.; Calderon, F.; Yarur, H.E.; Novoa, J.; Vega-Quiroga, I.; Bastias, C.P.; Gysling, K. Reactive oxygen species modulate locomotor activity and dopamine extracellular levels induced by amphetamine in rats. Behav. Brain Res. 2022, 427, 113857. [Google Scholar] [CrossRef]
- Jang, E.Y.; Yang, C.H.; Hedges, D.M.; Kim, S.P.; Lee, J.Y.; Ekins, T.G.; Garcia, B.T.; Kim, H.Y.; Nelson, A.C.; Kim, N.J.; et al. The role of reactive oxygen species in methamphetamine self-administration and dopamine release in the nucleus accumbens. Addict. Biol. 2016, 22, 1304–1315. [Google Scholar] [CrossRef]
- Involvement of Reactive Oxygen Species in Cocaine-Taking Behaviors in Rats-Jang-2015-Addiction Biology—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/adb.12159 (accessed on 22 February 2023).
- Baev, A.Y.; Vinokurov, A.Y.; Novikova, I.N.; Dremin, V.V.; Potapova, E.V.; Abramov, A.Y. Interaction of Mitochondrial Calcium and ROS in Neurodegeneration. Cells 2022, 11, 706. [Google Scholar] [CrossRef]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J. Exp. Neurosci. 2016, 10 (Suppl. 1), 23–48. [Google Scholar] [CrossRef] [Green Version]
- Kalinichenko, A.L.; Jappy, D.; Solius, G.M.; Maltsev, D.I.; Bogdanova, Y.A.; Mukhametshina, L.F.; Sokolov, R.A.; Moshchenko, A.A.; Shaydurov, V.A.; Rozov, A.V.; et al. Chemogenetic emulation of intraneuronal oxidative stress affects synaptic plasticity. Redox Biol. 2023, 60, 102604. [Google Scholar] [CrossRef]
- Kishida, K.T.; Klann, E. Sources and Targets of Reactive Oxygen Species in Synaptic Plasticity and Memory. Antioxid. Redox Signal. 2007, 9, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamlser, A.; Segal, M. Hydrogen Peroxide Modulation of Synaptic Plasticity. J. Neurosci. 2003, 23, 269–276. [Google Scholar] [CrossRef]
- Kamsler, A.; Segal, M. Paradoxical Actions of Hydrogen Peroxide on Long-Term Potentiation in Transgenic Superoxide Dismutase-1 Mice. J. Neurosci. 2003, 23, 10359–10367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobrido-Cameán, D.; Oswald, M.C.W.; Bailey, D.M.D.; Mukherjee, A.; Landgraf, M. Activity-regulated growth of motoneurons at the neuromuscular junction is mediated by NADPH oxidases. Front. Cell. Neurosci. 2023, 16, 1106593. [Google Scholar] [CrossRef]
- Oswald, M.C.; Brooks, P.S.; Zwart, M.F.; Mukherjee, A.; West, R.J.; Giachello, C.N.; Morarach, K.; A Baines, R.; Sweeney, S.T.; Landgraf, M. Reactive oxygen species regulate activity-dependent neuronal plasticity in Drosophila. eLife 2018, 7, 39393. [Google Scholar] [CrossRef]
- Dhawan, S.; Myers, P.; Bailey, D.M.D.; Ostrovsky, A.D.; Evers, J.F.; Landgraf, M. Reactive Oxygen Species Mediate Activity-Regulated Dendritic Plasticity Through NADPH Oxidase and Aquaporin Regulation. Front. Cell. Neurosci. 2021, 15, 641802. [Google Scholar] [CrossRef]
- Hong, G.; Pachter, R.; Ritz, T. Coupling Drosophila melanogaster Cryptochrome Light Activation and Oxidation of the Kvβ Subunit Hyperkinetic NADPH Cofactor. J. Phys. Chem. B 2018, 122, 6503–6510. [Google Scholar] [CrossRef]
- Kempf, A.; Song, S.M.; Talbot, C.B.; Miesenböck, G. A potassium channel β-subunit couples mitochondrial electron transport to sleep. Nature 2019, 568, 230–234. [Google Scholar] [CrossRef]
- Lathen, D.R.; Merrill, C.B.; Rothenfluh, A. Flying Together: Drosophila as a Tool to Understand the Genetics of Human Alcoholism. Int. J. Mol. Sci. 2020, 21, 6649. [Google Scholar] [CrossRef]
- Filošević, A.; Al-Samarai, S.; Waldowski, R.A. High Throughput Measurement of Locomotor Sensitization to Volatilized Cocaine in Drosophila melanogaster. Front. Mol. Neurosci. 2018, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Trawczyńska, I. New Method of Determining Kinetic Parameters for Decomposition of Hydrogen Peroxide by Catalase. Catalysts 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Kostyuk, V.A.; I Potapovich, A. Superoxide—Driven oxidation of quercetin and a simple sensitive assay for determination of superoxide dismutase. Biochem. Int. 1989, 19, 1117–1124. [Google Scholar] [PubMed]
- Wang, Y.-C.; Lee, C.-M.; Lee, L.-C.; Tung, L.-C.; Hsieh-Li, H.-M.; Lee-Chen, G.-J.; Su, M.-T. Mitochondrial Dysfunction and Oxidative Stress Contribute to the Pathogenesis of Spinocerebellar Ataxia Type 12 (SCA12). J. Biol. Chem. 2011, 286, 21742–21754. [Google Scholar] [CrossRef] [Green Version]
- Rigo, F.; Filošević, A.; Petrović, M.; Jović, K.; Waldowski, R.A. Locomotor sensitization modulates voluntary self-administration of methamphetamine in Drosophila melanogaster. Addict. Biol. 2020, 26, 12963. [Google Scholar] [CrossRef] [PubMed]
- Bainton, R.J.; Tsai, L.T.-Y.; Singh, C.M.; Moore, M.S.; Neckameyer, W.S.; Heberlein, U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. 2000, 10, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Birhman, K.; Raheja, I.; Sharma, S.K.; Kar, H.K. Quercetin: A wonder bioflavonoid with therapeutic potential in disease management. Asian Pac. J. Trop. Dis. 2016, 6, 248–252. [Google Scholar] [CrossRef]
- Scofield, M.D.; Heinsbroek, J.A.; Gipson, C.D.; Kupchik, Y.M.; Spencer, S.; Smith, A.C.W.; Roberts-Wolfe, D.; Kalivas, P.W. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol. Rev. 2016, 68, 816–871. [Google Scholar] [CrossRef] [Green Version]
- Philyaw, T.J.; Rothenfluh, A.; Titos, I. The Use of Drosophila to Understand Psychostimulant Responses. Biomedicines 2022, 10, 119. [Google Scholar] [CrossRef]
- Fernandez-Chiappe, F.; Hermann-Luibl, C.; Peteranderl, A.; Reinhard, N.; Senthilan, P.R.; Hieke, M.; Selcho, M.; Yoshii, T.; Shafer, O.T.; Muraro, N.I.; et al. Dopamine Signaling in Wake-Promoting Clock Neurons Is Not Required for the Normal Regulation of Sleep in Drosophila. J. Neurosci. 2020, 40, 9617–9633. [Google Scholar] [CrossRef]
- Andretic, R.; van Swinderen, B.; Greenspan, R.J. Dopaminergic Modulation of Arousal in Drosophila. Curr. Biol. 2005, 15, 1165–1175. [Google Scholar] [CrossRef] [Green Version]
- Grover, D.; Ford, D.; Brown, C.; Hoe, N.; Erdem, A.; Tavaré, S.; Tower, J. Hydrogen Peroxide Stimulates Activity and Alters Behavior in Drosophila melanogaster. PLoS ONE 2009, 4, e7580. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; DesMarais, T.L.; Tong, Z.; Yao, Y.; Costa, M. Oxidative stress alters global histone modification and DNA methylation. Free. Radic. Biol. Med. 2015, 82, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Nayak, M.; Das, D.; Pradhan, J.; Ahmed, R.; Laureano-Melo, R.; Dandapat, J. Epigenetic signature in neural plasticity: The journey so far and journey ahead. Heliyon 2022, 8, e12292. [Google Scholar] [CrossRef]
- Chiu, J.C.; Low, K.H.; Pike, D.H.; Yildirim, E.; Edery, I. Assaying Locomotor Activity to Study Circadian Rhythms and Sleep Parameters in Drosophila. J. Vis. Exp. 2010, 43, e2157. [Google Scholar] [CrossRef] [Green Version]
- Bogomolova, E.V.; Rauschenbach, I.; Adonyeva, N.V.; Alekseev, A.A.; Faddeeva, N.V.; Gruntenko, N.E. Dopamine down-regulates activity of alkaline phosphatase in Drosophila: The role of D2-like receptors. J. Insect Physiol. 2010, 56, 1155–1159. [Google Scholar] [CrossRef]
- Faust, K.; Gehrke, S.; Yang, Y.; Yang, L.; Beal, M.F.; Lu, B. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci. 2009, 10, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filošević Vujnović, A.; Rubinić, M.; Starčević, I.; Andretić Waldowski, R. Influence of Redox and Dopamine Regulation in Cocaine-Induced Phenotypes Using Drosophila. Antioxidants 2023, 12, 933. https://doi.org/10.3390/antiox12040933
Filošević Vujnović A, Rubinić M, Starčević I, Andretić Waldowski R. Influence of Redox and Dopamine Regulation in Cocaine-Induced Phenotypes Using Drosophila. Antioxidants. 2023; 12(4):933. https://doi.org/10.3390/antiox12040933
Chicago/Turabian StyleFilošević Vujnović, Ana, Marko Rubinić, Ivona Starčević, and Rozi Andretić Waldowski. 2023. "Influence of Redox and Dopamine Regulation in Cocaine-Induced Phenotypes Using Drosophila" Antioxidants 12, no. 4: 933. https://doi.org/10.3390/antiox12040933
APA StyleFilošević Vujnović, A., Rubinić, M., Starčević, I., & Andretić Waldowski, R. (2023). Influence of Redox and Dopamine Regulation in Cocaine-Induced Phenotypes Using Drosophila. Antioxidants, 12(4), 933. https://doi.org/10.3390/antiox12040933





