Evolutionarily Conserved Role of Thioredoxin Systems in Determining Longevity
Abstract
1. Introduction
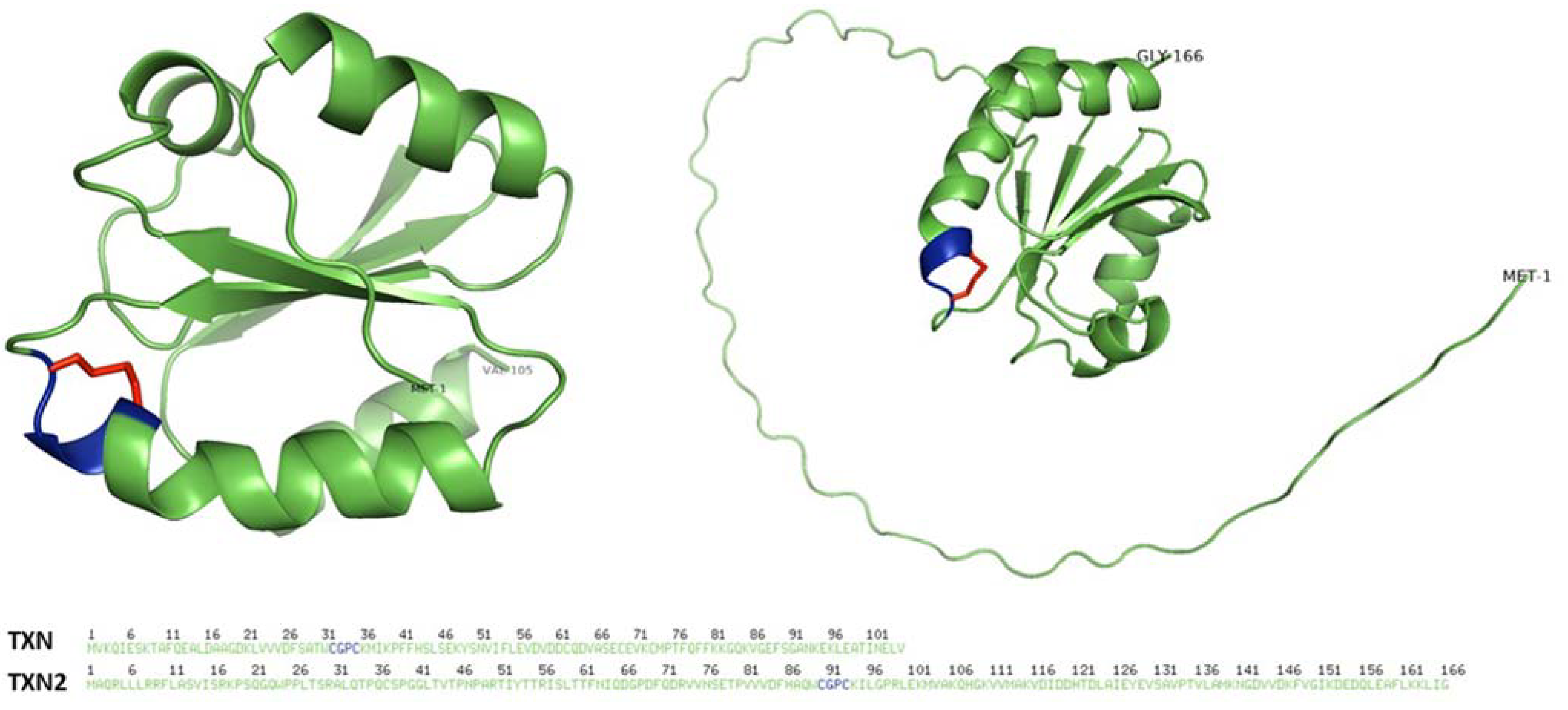
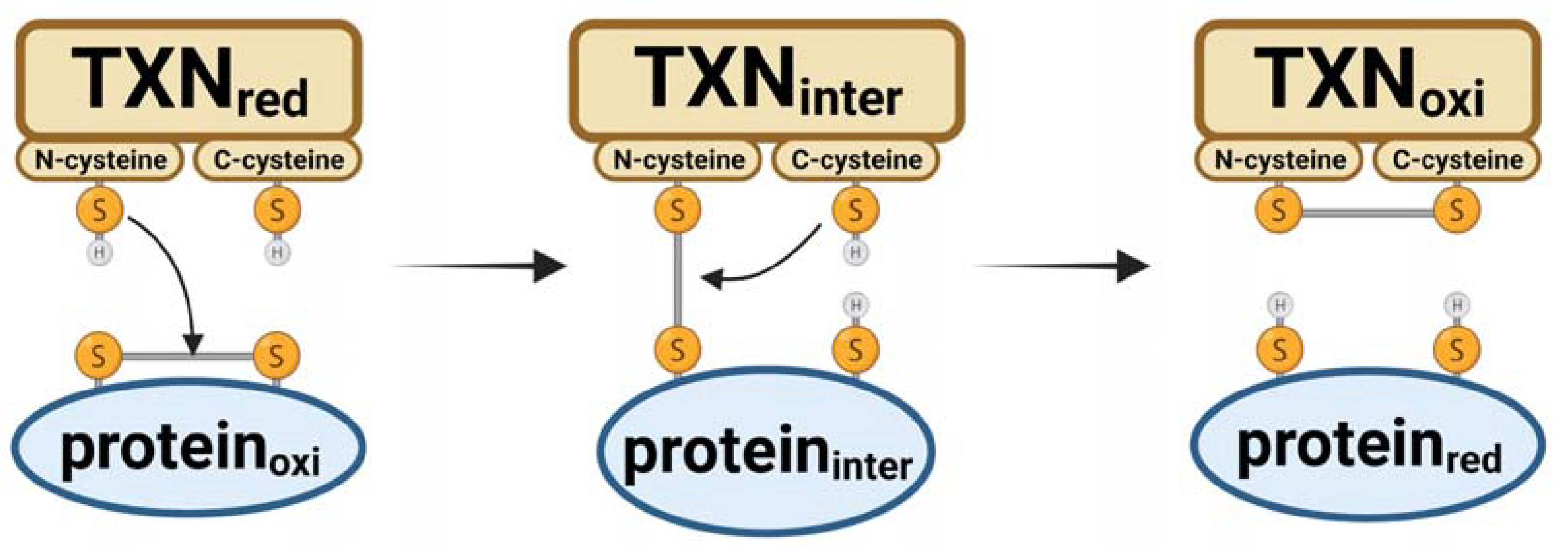
2. Antioxidant Roles of Thioredoxin and Thioredoxin Reductase
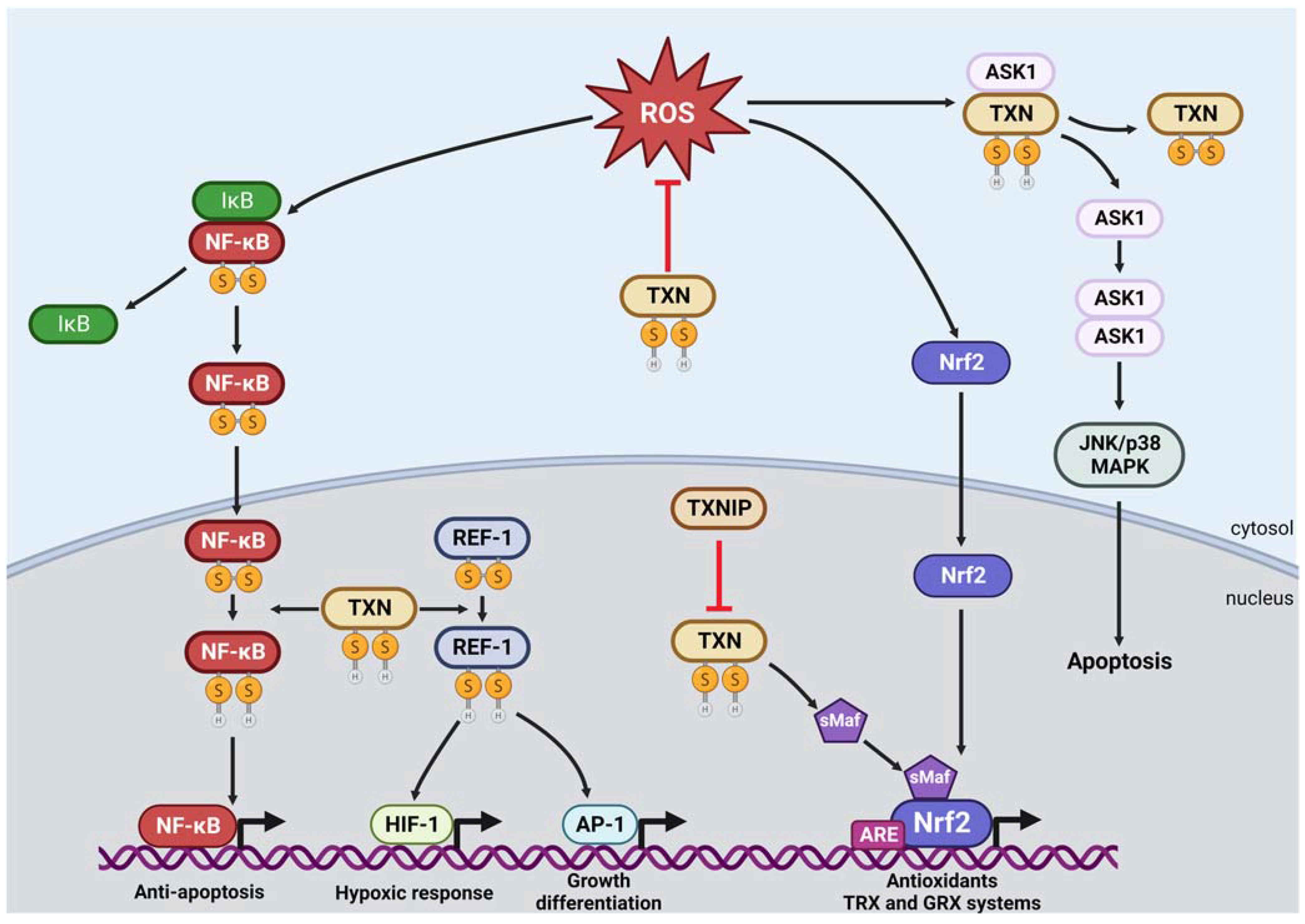
3. Additional Roles of Thioredoxin and Thioredoxin Reductase: Redox Signaling
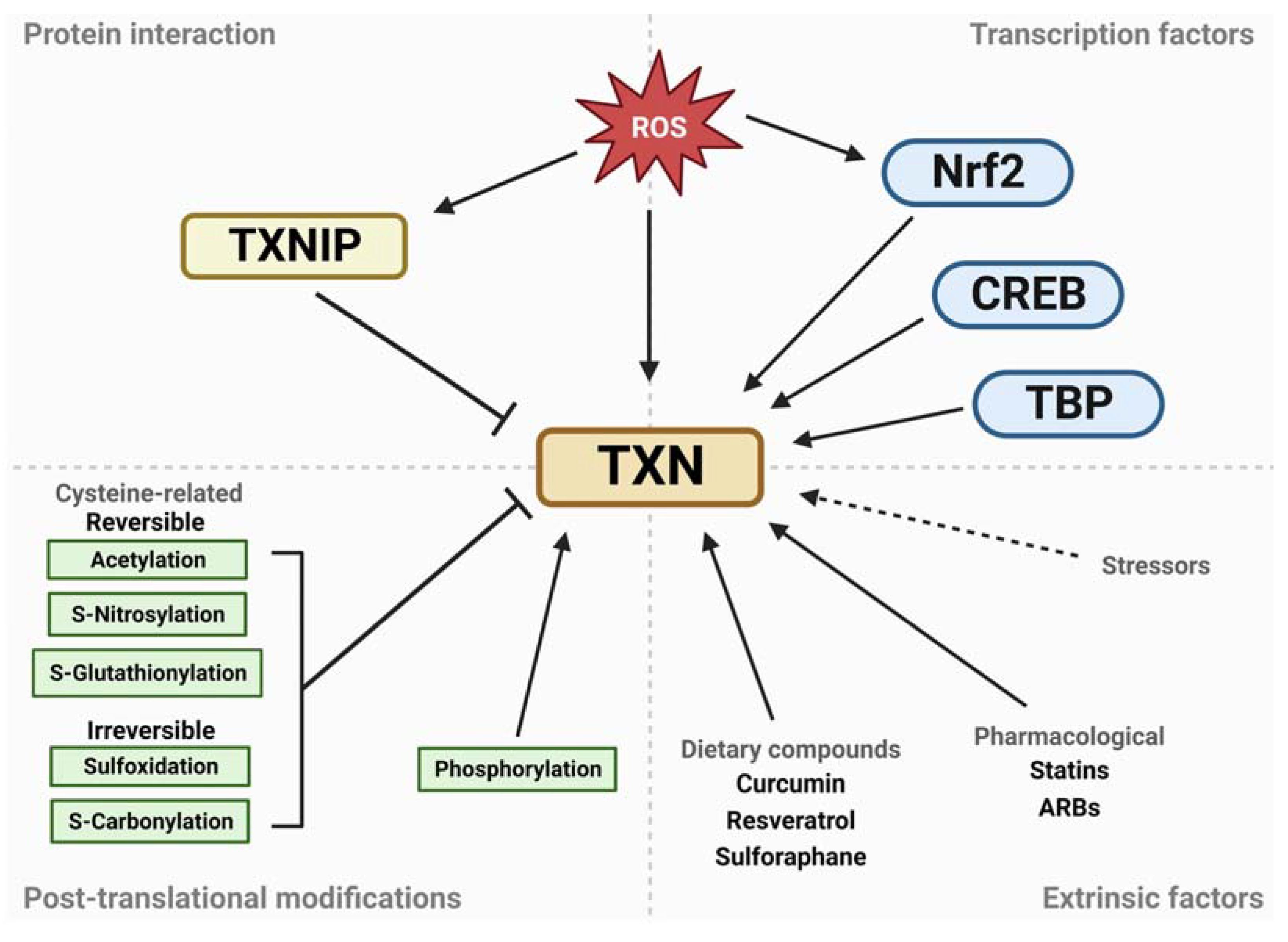
4. Regulation of the Thioredoxin System
5. Cytoplasmic Thioredoxin System Contributes to Lifespan in Yeast
6. Cytoplasmic Thioredoxin Is Important for Longevity in Caenorhabditis elegans
7. Contribution of Thioredoxin Systems to Lifespan in Drosophila
| Organism | Gene | Location | Modulation | Effect on Lifespan | References |
|---|---|---|---|---|---|
| Yeast | TRX1 | Cytoplasm | Disruption | ↓ | [69,70] |
| TRR1 | Cytoplasm | Disruption | ↓ | [73] | |
| TRX3 | Mitochondria | Disruption | = | [71,72] | |
| TRR2 | Mitochondria | Disruption | = | [71,72] | |
| C. elegans | trx-1 | Cytoplasm | Disruption | ↓ | [74,75,76,77] |
| trx-1::GFP | Cytoplasm | Overexpression | ↑ | [77] | |
| trxr-1 | Cytoplasm | Disruption | = | [74,78,79] | |
| trx-2 | Mitochondria | Disruption | = | [74,80] | |
| trxr-2 | Mitochondria | Disruption | =/↓ | [74,79,80] | |
| trx-3 | Cytoplasm/Nucleus Intestine | Disruption | = | [81] | |
| Drosophila | TrxT | Nucleus, male specific | Disruption | = | [82] |
| TrxT | Nucleus, male specific | Overexpression in all neurons | ↑ | [83] | |
| dhd | Nucleus, female specific | Disruption | = | [82] | |
| Trx-2 | Nucleus | Disruption | ↓ | [82,84] | |
| Trx-2 | Nucleus | Overexpression | ↑ | [82] | |
| TrxT-dhd-Trx-2 | Nucleus | Disruption | =/↓ | [82] | |
| Trxr-1 | Cytoplasm and Mitochondria | Disruption | Larval lethality | [85,86] | |
| Trxr-1 | Cytoplasm and Mitochondria | Overexpression | = | [87] | |
| Trxr-2 | Mitochondria | Overexpression | ↑ | [87] | |
| Vdup1 | Cytoplasm/Nucleus | Downregulation | ↑ | [88] | |
| Vdup1 | Cytoplasm/Nucleus | Overexpression | ↓ | [88] | |
| Mice | Txn1/Trx1 | Cytoplasmic | Disruption | Embryonic lethal | [89] |
| Txn1+/− | Cytoplasmic | Heterozygous disruption | = | [90,91] | |
| Human TXN | Cytoplasmic | Overexpression | ↑ | [92] | |
| Human TXN | Cytoplasmic | Overexpression | ↑ early lifespanin males | [93] | |
| Human TXN | Cytoplasmic | Overexpression | = | [94] | |
| Txnrd1 | Cytoplasmic | Disruption | Embryonic lethal | [95] | |
| Txn2/Trx2 | Mitochondria | Disruption | Embryonic lethal | [96] | |
| Txn2+/− | Mitochondria | Heterozygous disruption | = | [97] | |
| Human TXN2 | Mitochondria | Overexpression | ↑ early lifespan | [98] | |
| Human TXN1 + human TXN2 | Cytoplasmic and Mitochondria | Overexpression | ↓ | [99] | |
| Txn+/−; Txn2+/− | Cytoplasmic and Mitochondria | Heterozygous disruption | ↑ | [91] | |
| Txnrd2 | Mitochondria | Disruption | Embryonic lethal | [100] | |
| Rats | Human TXN | Cytoplasmic | Overexpression | = | [91] |
8. Cytoplasmic and Mitochondrial Thioredoxin Systems Are Required for Survival in Mice
9. Thioredoxin Reductase Variant Is Associated with Longevity in Humans
10. Discussion
10.1. Relative Importance of Cytoplasmic and Mitochondrial Thioredoxin Systems Differs between Species
10.2. Cytoplasmic and Mitochondrial Thioredoxin Systems Act Independently to Affect Longevity
10.3. Thioredoxin and Thioredoxin Reductase can Affect Lifespan Independently
10.4. The Effect of Thioredoxin and Thioredoxin Reductase on Lifespan Is Correlated with Effect on Resistance to Oxidative Stress
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A. Thioredoxin. 6. The amino acid sequence of the protein from Escherichia coli B. Eur. J. Biochem. 1968, 6, 475–484. [Google Scholar] [CrossRef]
- Martin, J.L. Thioredoxin—A fold for all reasons. Structure 1995, 3, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Horibe, T.; Gomi, M.; Iguchi, D.; Ito, H.; Kitamura, Y.; Masuoka, T.; Tsujimoto, I.; Kimura, T.; Kikuchi, M. Different contributions of the three CXXC motifs of human protein-disulfide isomerase-related protein to isomerase activity and oxidative refolding. J. Biol. Chem. 2004, 279, 4604–4611. [Google Scholar] [CrossRef]
- Collet, J.F.; Messens, J. Structure, function, and mechanism of thioredoxin proteins. Antioxid. Redox Signal. 2010, 13, 1205–1216. [Google Scholar] [CrossRef]
- Lillig, C.H.; Holmgren, A. Thioredoxin and related molecules—From biology to health and disease. Antioxid. Redox Signal. 2007, 9, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, H.; Lu, J.; Holmgren, A. Glutathione and glutaredoxin act as a backup of human thioredoxin reductase 1 to reduce thioredoxin 1 preventing cell death by aurothioglucose. J. Biol. Chem. 2012, 287, 38210–38219. [Google Scholar] [CrossRef]
- Zhang, H.; Du, Y.; Zhang, X.; Lu, J.; Holmgren, A. Glutaredoxin 2 reduces both thioredoxin 2 and thioredoxin 1 and protects cells from apoptosis induced by auranofin and 4-hydroxynonenal. Antioxid. Redox Signal. 2014, 21, 669–681. [Google Scholar] [CrossRef]
- Das, K.C.; Das, C.K. Thioredoxin, a singlet oxygen quencher and hydroxyl radical scavenger: Redox independent functions. Biochem. Biophys. Res. Commun. 2000, 277, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Woo, H.A.; Kil, I.S.; Bae, S.H. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J. Biol. Chem. 2012, 287, 4403–4410. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Ikeda, Y. Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep. 2002, 7, 123–130. [Google Scholar] [CrossRef]
- Mustacich, D.; Powis, G. Thioredoxin reductase. Biochem. J. 2000, 346 Pt 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Arner, E.S.; Holmgren, A. Structure and mechanism of mammalian thioredoxin reductase: The active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proc. Natl. Acad. Sci. USA 2000, 97, 5854–5859. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Sun, Q.A.; Wu, Y.; Zappacosta, F.; Jeang, K.T.; Lee, B.J.; Hatfield, D.L.; Gladyshev, V.N. Redox regulation of cell signaling by selenocysteine in mammalian thioredoxin reductases. J. Biol. Chem. 1999, 274, 24522–24530. [Google Scholar] [CrossRef]
- Gladyshev, V.N.; Jeang, K.T.; Stadtman, T.C. Selenocysteine, identified as the penultimate C-terminal residue in human T-cell thioredoxin reductase, corresponds to TGA in the human placental gene. Proc. Natl. Acad. Sci. USA 1996, 93, 6146–6151. [Google Scholar] [CrossRef]
- Arner, E.S. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim. Biophys. Acta 2009, 1790, 495–526. [Google Scholar] [CrossRef]
- Zhao, R.; Masayasu, H.; Holmgren, A. Ebselen: A substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc. Natl. Acad. Sci. USA 2002, 99, 8579–8584. [Google Scholar] [CrossRef]
- Tamura, T.; Sato, K.; Komori, K.; Imai, T.; Kuwahara, M.; Okugochi, T.; Mihara, H.; Esaki, N.; Inagaki, K. Selenite reduction by the thioredoxin system: Kinetics and identification of protein-bound selenide. Biosci. Biotechnol. Biochem. 2011, 75, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Mendiratta, S.; Hill, K.E.; Burk, R.F. Reduction of dehydroascorbate to ascorbate by the selenoenzyme thioredoxin reductase. J. Biol. Chem. 1997, 272, 22607–22610. [Google Scholar] [CrossRef]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Nordman, T.; Olsson, J.M.; Damdimopoulos, A.; Bjorkhem-Bergman, L.; Nalvarte, I.; Eriksson, L.C.; Arner, E.S.; Spyrou, G.; Bjornstedt, M. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J. Biol. Chem. 2003, 278, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Nalvarte, I.; Damdimopoulos, A.E.; Spyrou, G. Human mitochondrial thioredoxin reductase reduces cytochrome c and confers resistance to complex III inhibition. Free Radic. Biol. Med. 2004, 36, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Hashemy, S.I.; Ungerstedt, J.S.; Zahedi Avval, F.; Holmgren, A. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. J. Biol. Chem. 2006, 281, 10691–10697. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A.; Lyckeborg, C. Enzymatic reduction of alloxan by thioredoxin and NADPH-thioredoxin reductase. Proc. Natl. Acad. Sci. USA 1980, 77, 5149–5152. [Google Scholar] [CrossRef]
- Holmgren, A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox Signal. 2000, 2, 811–820. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.M.; Lee, R.T. Thioredoxin and thioredoxin target proteins: From molecular mechanisms to functional significance. Antioxid. Redox Signal. 2013, 18, 1165–1207. [Google Scholar] [CrossRef]
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998, 17, 2596–2606. [Google Scholar] [CrossRef]
- Wei, B.; Li, F. Mechanisms of Trx2/ASK1-Mediated Mitochondrial Injury in Pemphigus Vulgaris. Biomed. Res. Int. 2021, 2021, 2471518. [Google Scholar] [CrossRef]
- Saxena, G.; Chen, J.; Shalev, A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010, 285, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, D.; Hu, D.; Zhou, X.; Zhou, Y. The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 2018, 103, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Oslowski, C.M.; Hara, T.; O’Sullivan-Murphy, B.; Kanekura, K.; Lu, S.; Hara, M.; Ishigaki, S.; Zhu, L.J.; Hayashi, E.; Hui, S.T.; et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 2012, 16, 265–273. [Google Scholar] [CrossRef]
- Qayyum, N.; Haseeb, M.; Kim, M.S.; Choi, S. Role of thioredoxin-interacting protein in diseases and its therapeutic outlook. Int. J. Mol. Sci. 2021, 22, 2754. [Google Scholar] [CrossRef]
- Wang, C.Y.; Mayo, M.W.; Korneluk, R.G.; Goeddel, D.V.; Baldwin, A.S., Jr. NF-kappaB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998, 281, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Matthews, J.R.; Wakasugi, N.; Virelizier, J.L.; Yodoi, J.; Hay, R.T. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992, 20, 3821–3830. [Google Scholar] [CrossRef]
- Ando, K.; Hirao, S.; Kabe, Y.; Ogura, Y.; Sato, I.; Yamaguchi, Y.; Wada, T.; Handa, H. A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 2008, 36, 4327–4336. [Google Scholar] [CrossRef]
- Ramana, C.V.; Boldogh, I.; Izumi, T.; Mitra, S. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc. Natl. Acad. Sci. USA 1998, 95, 5061–5066. [Google Scholar] [CrossRef]
- Morel, Y.; Barouki, R. Repression of gene expression by oxidative stress. Biochem. J. 1999, 342 Pt 3, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Matsui, M.; Iwata, S.; Nishiyama, A.; Mori, K.; Yodoi, J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. USA 1997, 94, 3633–3638. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.E.; Arany, Z.; Livingston, D.M.; Bunn, H.F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J. Biol. Chem. 1996, 271, 32253–32259. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Hirota, K.; Mimura, J.; Abe, H.; Yodoi, J.; Sogawa, K.; Poellinger, L.; Fujii-Kuriyama, Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: Their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999, 18, 1905–1914. [Google Scholar] [CrossRef]
- Jastrzab, A.; Skrzydlewska, E. Thioredoxin-dependent system. Application of inhibitors. J. Enzym. Inhib. Med. Chem. 2021, 36, 362–371. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, M.; Gao, J.; Dai, G.; Guan, Q.; Du, C. Upregulation of Thioredoxin Reductase 1 Expression by Flavan-3-Ols Protects Human Kidney Proximal Tubular Cells from Hypoxia-Induced Cell Death. Antioxidants 2022, 11, 1399. [Google Scholar] [CrossRef]
- Karlenius, T.C.; Shah, F.; Di Trapani, G.; Clarke, F.M.; Tonissen, K.F. Cycling hypoxia up-regulates thioredoxin levels in human MDA-MB-231 breast cancer cells. Biochem. Biophys. Res. Commun. 2012, 419, 350–355. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Hawkes, H.J.; Karlenius, T.C.; Tonissen, K.F. Regulation of the human thioredoxin gene promoter and its key substrates: A study of functional and putative regulatory elements. Biochim. Biophys. Acta 2014, 1840, 303–314. [Google Scholar] [CrossRef]
- Kim, Y.C.; Yamaguchi, Y.; Kondo, N.; Masutani, H.; Yodoi, J. Thioredoxin-dependent redox regulation of the antioxidant responsive element (ARE) in electrophile response. Oncogene 2003, 22, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Rundlof, A.K.; Janard, M.; Miranda-Vizuete, A.; Arner, E.S. Evidence for intriguingly complex transcription of human thioredoxin reductase 1. Free Radic. Biol. Med. 2004, 36, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, W.; Liu, S.; Yu, L.; Chen, Z. Thioredoxin-1 phosphorylated at T100 is needed for its anti-apoptotic activity in HepG2 cancer cells. Life Sci. 2010, 87, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, R.; Holmgren, A. Thioredoxin and thioredoxin reductase in relation to reversible S-nitrosylation. Antioxid. Redox Signal. 2013, 18, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, S.; Bonetto, V.; Fratelli, M.; Gianazza, E.; Eberini, I.; Massignan, T.; Salmona, M.; Chang, G.; Holmgren, A.; Ghezzi, P. Glutathionylation of human thioredoxin: A possible crosstalk between the glutathione and thioredoxin systems. Proc. Natl. Acad. Sci. USA 2002, 99, 9745–9749. [Google Scholar] [CrossRef]
- Spindel, O.N.; World, C.; Berk, B.C. Thioredoxin interacting protein: Redox dependent and independent regulatory mechanisms. Antioxid. Redox Signal. 2012, 16, 587–596. [Google Scholar] [CrossRef]
- Patwari, P.; Higgins, L.J.; Chutkow, W.A.; Yoshioka, J.; Lee, R.T. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006, 281, 21884–21891. [Google Scholar] [CrossRef]
- Zitman-Gal, T.; Green, J.; Pasmanik-Chor, M.; Oron-Karni, V.; Bernheim, J. Endothelial pro-atherosclerotic response to extracellular diabetic-like environment: Possible role of thioredoxin-interacting protein. Nephrol. Dial. Transplant. 2010, 25, 2141–2149. [Google Scholar] [CrossRef]
- Parikh, H.; Carlsson, E.; Chutkow, W.A.; Johansson, L.E.; Storgaard, H.; Poulsen, P.; Saxena, R.; Ladd, C.; Schulze, P.C.; Mazzini, M.J.; et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007, 4, e158. [Google Scholar] [CrossRef]
- Bedarida, T.; Baron, S.; Vibert, F.; Ayer, A.; Henrion, D.; Thioulouse, E.; Marchiol, C.; Beaudeux, J.L.; Cottart, C.H.; Nivet-Antoine, V. resveratrol decreases TXNIP mRNA and protein nuclear expressions with an arterial function improvement in old mice. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 720–729. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, B.; Duan, D.; Wu, J.; Fang, J. Curcumin targeting the thioredoxin system elevates oxidative stress in HeLa cells. Toxicol. Appl. Pharmacol. 2012, 262, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Lu, J.; Holmgren, A. Thioredoxin reductase is irreversibly modified by curcumin: A novel molecular mechanism for its anticancer activity. J. Biol. Chem. 2005, 280, 25284–25290. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.; Howie, A.F.; Beckett, G.J.; Mithen, R.; Bao, Y. Sulforaphane, erucin, and iberin up-regulate thioredoxin reductase 1 expression in human MCF-7 cells. J. Agric. Food Chem. 2005, 53, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Skogastierna, C.; Johansson, M.; Parini, P.; Eriksson, M.; Eriksson, L.C.; Ekstrom, L.; Bjorkhem-Bergman, L. Statins inhibit expression of thioredoxin reductase 1 in rat and human liver and reduce tumour development. Biochem. Biophys. Res. Commun. 2012, 417, 1046–1051. [Google Scholar] [CrossRef]
- Mansouri, A.; Reiner, Z.; Ruscica, M.; Tedeschi-Reiner, E.; Radbakhsh, S.; Bagheri Ekta, M.; Sahebkar, A. Antioxidant effects of statins by modulating Nrf2 and Nrf2/HO-1 signaling in different diseases. J. Clin. Med. 2022, 11, 1313. [Google Scholar] [CrossRef]
- Ebrahimian, T.; Sairam, M.R.; Schiffrin, E.L.; Touyz, R.M. Cardiac hypertrophy is associated with altered thioredoxin and ASK-1 signaling in a mouse model of menopause. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1481–H1488. [Google Scholar] [CrossRef]
- Kim, H.; Baek, C.H.; Lee, R.B.; Chang, J.W.; Yang, W.S.; Lee, S.K. Anti-Fibrotic Effect of Losartan, an Angiotensin II Receptor Blocker, Is Mediated through Inhibition of ER Stress via Up-Regulation of SIRT1, Followed by Induction of HO-1 and Thioredoxin. Int. J. Mol. Sci. 2017, 18, 305. [Google Scholar] [CrossRef]
- Gellert, M.; Hossain, M.F.; Berens, F.J.F.; Bruhn, L.W.; Urbainsky, C.; Liebscher, V.; Lillig, C.H. Substrate specificity of thioredoxins and glutaredoxins—Towards a functional classification. Heliyon 2019, 5, e02943. [Google Scholar] [CrossRef]
- Matecic, M.; Smith, D.L.; Pan, X.; Maqani, N.; Bekiranov, S.; Boeke, J.D.; Smith, J.S. A microarray-based genetic screen for yeast chronological aging factors. PLoS Genet. 2010, 6, e1000921. [Google Scholar] [CrossRef]
- Laschober, G.T.; Ruli, D.; Hofer, E.; Muck, C.; Carmona-Gutierrez, D.; Ring, J.; Hutter, E.; Ruckenstuhl, C.; Micutkova, L.; Brunauer, R.; et al. Identification of evolutionarily conserved genetic regulators of cellular aging. Aging Cell 2010, 9, 1084–1097. [Google Scholar] [CrossRef]
- Unlu, E.S.; Koc, A. Effects of deleting mitochondrial antioxidant genes on life span. Ann. N. Y. Acad. Sci. 2007, 1100, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.B.; Koc, A. Assessment of chronological lifespan dependent molecular damages in yeast lacking mitochondrial antioxidant genes. Biochem. Biophys. Res. Commun. 2010, 400, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Picazo, C.; Matallana, E.; Aranda, A. Yeast thioredoxin reductase Trr1p controls TORC1-regulated processes. Sci. Rep. 2018, 8, 16500. [Google Scholar] [CrossRef]
- Harris-Gauthier, N.; Traa, A.; AlOkda, A.; Moldakozhayev, A.; Anglas, U.; Soo, S.K.; Van Raamsdonk, J.M. Mitochondrial thioredoxin system is required for enhanced stress resistance and extended longevity in long-lived mitochondrial mutants. Redox Biol. 2022, 53, 102335. [Google Scholar] [CrossRef] [PubMed]
- Jee, C.; Vanoaica, L.; Lee, J.; Park, B.J.; Ahnn, J. Thioredoxin is related to life span regulation and oxidative stress response in Caenorhabditis elegans. Genes Cells 2005, 10, 1203–1210. [Google Scholar] [CrossRef]
- Miranda-Vizuete, A.; Fierro Gonzalez, J.C.; Gahmon, G.; Burghoorn, J.; Navas, P.; Swoboda, P. Lifespan decrease in a Caenorhabditis elegans mutant lacking TRX-1, a thioredoxin expressed in ASJ sensory neurons. FEBS Lett. 2006, 580, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Fierro-Gonzalez, J.C.; Gonzalez-Barrios, M.; Miranda-Vizuete, A.; Swoboda, P. The thioredoxin TRX-1 regulates adult lifespan extension induced by dietary restriction in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2011, 406, 478–482. [Google Scholar] [CrossRef]
- Stenvall, J.; Fierro-Gonzalez, J.C.; Swoboda, P.; Saamarthy, K.; Cheng, Q.; Cacho-Valadez, B.; Arner, E.S.; Persson, O.P.; Miranda-Vizuete, A.; Tuck, S. Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2011, 108, 1064–1069. [Google Scholar] [CrossRef]
- Yu, D.; Pendergraff, H.; Liu, J.; Kordasiewicz, H.B.; Cleveland, D.W.; Swayze, E.E.; Lima, W.F.; Crooke, S.T.; Prakash, T.P.; Corey, D.R. Single-Stranded RNAs Use RNAi to Potently and Allele-Selectively Inhibit Mutant Huntingtin Expression. Cell 2012, 150, 895–908. [Google Scholar] [CrossRef]
- Cacho-Valadez, B.; Munoz-Lobato, F.; Pedrajas, J.R.; Cabello, J.; Fierro-Gonzalez, J.C.; Navas, P.; Swoboda, P.; Link, C.D.; Miranda-Vizuete, A. The characterization of the Caenorhabditis elegans mitochondrial thioredoxin system uncovers an unexpected protective role of thioredoxin reductase 2 in beta-amyloid peptide toxicity. Antioxid. Redox Signal. 2012, 16, 1384–1400. [Google Scholar] [CrossRef]
- Jimenez-Hidalgo, M.; Kurz, C.L.; Pedrajas, J.R.; Naranjo-Galindo, F.J.; Gonzalez-Barrios, M.; Cabello, J.; Saez, A.G.; Lozano, E.; Button, E.L.; Veal, E.A.; et al. Functional characterization of thioredoxin 3 (TRX-3), a Caenorhabditis elegans intestine-specific thioredoxin. Free Radic. Biol. Med. 2014, 68, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.J.; Larsson, J. Thioredoxin-2 affects lifespan and oxidative stress in Drosophila. Hereditas 2007, 144, 25–32. [Google Scholar] [CrossRef]
- Umeda-Kameyama, Y.; Tsuda, M.; Ohkura, C.; Matsuo, T.; Namba, Y.; Ohuchi, Y.; Aigaki, T. Thioredoxin suppresses Parkin-associated endothelin receptor-like receptor-induced neurotoxicity and extends longevity in Drosophila. J. Biol. Chem. 2007, 282, 11180–11187. [Google Scholar] [CrossRef]
- Tsuda, M.; Ootaka, R.; Ohkura, C.; Kishita, Y.; Seong, K.H.; Matsuo, T.; Aigaki, T. Loss of Trx-2 enhances oxidative stress-dependent phenotypes in Drosophila. FEBS Lett. 2010, 584, 3398–3401. [Google Scholar] [CrossRef] [PubMed]
- Missirlis, F.; Phillips, J.P.; Jackle, H. Cooperative action of antioxidant defense systems in Drosophila. Curr. Biol. 2001, 11, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Missirlis, F.; Ulschmid, J.K.; Hirosawa-Takamori, M.; Gronke, S.; Schafer, U.; Becker, K.; Phillips, J.P.; Jackle, H. Mitochondrial and cytoplasmic thioredoxin reductase variants encoded by a single Drosophila gene are both essential for viability. J. Biol. Chem. 2002, 277, 11521–11526. [Google Scholar] [CrossRef]
- Pickering, A.M.; Lehr, M.; Gendron, C.M.; Pletcher, S.D.; Miller, R.A. Mitochondrial thioredoxin reductase 2 is elevated in long-lived primate as well as rodent species and extends fly mean lifespan. Aging Cell 2017, 16, 683–692. [Google Scholar] [CrossRef]
- Oberacker, T.; Bajorat, J.; Ziola, S.; Schroeder, A.; Roth, D.; Kastl, L.; Edgar, B.A.; Wagner, W.; Gulow, K.; Krammer, P.H. Enhanced expression of thioredoxin-interacting-protein regulates oxidative DNA damage and aging. FEBS Lett. 2018, 592, 2297–2307. [Google Scholar] [CrossRef]
- Matsui, M.; Oshima, M.; Oshima, H.; Takaku, K.; Maruyama, T.; Yodoi, J.; Taketo, M.M. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev. Biol. 1996, 178, 179–185. [Google Scholar] [CrossRef]
- Cunningham, G.M.; Roman, M.G.; Flores, L.C.; Hubbard, G.B.; Salmon, A.B.; Zhang, Y.; Gelfond, J.; Ikeno, Y. The paradoxical role of thioredoxin on oxidative stress and aging. Arch. Biochem. Biophys. 2015, 576, 32–38. [Google Scholar] [CrossRef]
- Roman, M.G.; Flores, L.C.; Cunningham, G.M.; Cheng, C.; Allen, C.; Hubbard, G.B.; Bai, Y.; Saunders, T.L.; Ikeno, Y. Thioredoxin and aging: What have we learned from the survival studies? Aging Pathobiol. Ther. 2020, 2, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, A.; Hamuro, J.; Nakamura, H.; Kondo, N.; Hirabayashi, Y.; Ishizaki-Koizumi, S.; Hirakawa, T.; Inoue, T.; Yodoi, J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid. Redox Signal. 2002, 4, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.I.; Cortez, L.A.; Lew, C.M.; Rodriguez, M.; Webb, C.R.; Van Remmen, H.; Chaudhuri, A.; Qi, W.; Lee, S.; Bokov, A.; et al. Thioredoxin 1 overexpression extends mainly the earlier part of life span in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1286–1299. [Google Scholar] [CrossRef]
- Flores, L.C.; Roman, M.G.; Cunningham, G.M.; Cheng, C.; Dube, S.; Allen, C.; Van Remmen, H.; Hubbard, G.B.; Saunders, T.L.; Ikeno, Y. Continuous overexpression of thioredoxin 1 enhances cancer development and does not extend maximum lifespan in male C57BL/6 mice. Pathobiol. Aging Age Relat. Dis. 2018, 8, 1533754. [Google Scholar] [CrossRef] [PubMed]
- Jakupoglu, C.; Przemeck, G.K.; Schneider, M.; Moreno, S.G.; Mayr, N.; Hatzopoulos, A.K.; de Angelis, M.H.; Wurst, W.; Bornkamm, G.W.; Brielmeier, M.; et al. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol. Cell. Biol. 2005, 25, 1980–1988. [Google Scholar] [CrossRef]
- Nonn, L.; Williams, R.R.; Erickson, R.P.; Powis, G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol. Cell. Biol. 2003, 23, 916–922. [Google Scholar] [CrossRef]
- Perez, V.I.; Lew, C.M.; Cortez, L.A.; Webb, C.R.; Rodriguez, M.; Liu, Y.; Qi, W.; Li, Y.; Chaudhuri, A.; Van Remmen, H.; et al. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic. Biol. Med. 2008, 44, 882–892. [Google Scholar] [CrossRef]
- Roman, M.G.; Flores, L.C.; Cunningham, G.M.; Cheng, C.; Dube, S.; Allen, C.; Van Remmen, H.; Bai, Y.; Hubbard, G.B.; Saunders, T.L.; et al. Thioredoxin overexpression in mitochondria showed minimum effects on aging and age-related diseases in male C57BL/6 mice. Aging Pathobiol. Ther. 2020, 2, 20–31. [Google Scholar] [CrossRef]
- Cunningham, G.M.; Flores, L.C.; Roman, M.G.; Cheng, C.; Dube, S.; Allen, C.; Valentine, J.M.; Hubbard, G.B.; Bai, Y.; Saunders, T.L.; et al. Thioredoxin overexpression in both the cytosol and mitochondria accelerates age-related disease and shortens lifespan in male C57BL/6 mice. Geroscience 2018, 40, 453–468. [Google Scholar] [CrossRef]
- Conrad, M.; Jakupoglu, C.; Moreno, S.G.; Lippl, S.; Banjac, A.; Schneider, M.; Beck, H.; Hatzopoulos, A.K.; Just, U.; Sinowatz, F.; et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol. Cell. Biol. 2004, 24, 9414–9423. [Google Scholar] [CrossRef]
- Takagi, Y.; Mitsui, A.; Nishiyama, A.; Nozaki, K.; Sono, H.; Gon, Y.; Hashimoto, N.; Yodoi, J. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc. Natl. Acad. Sci. USA 1999, 96, 4131–4136. [Google Scholar] [CrossRef]
- Perez, V.I.; Bokov, A.; Van Remmen, H.; Mele, J.; Ran, Q.; Ikeno, Y.; Richardson, A. Is the oxidative stress theory of aging dead? Biochim. Et Biophys. Acta 2009, 1790, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Chocron, E.S.; Mdaki, K.; Jiang, N.; Cropper, J.; Pickering, A.M. Mitochondrial TrxR2 regulates metabolism and protects from metabolic disease through enhanced TCA and ETC function. Commun. Biol. 2022, 5, 467. [Google Scholar] [CrossRef] [PubMed]
- Soerensen, M.; Dato, S.; Tan, Q.; Thinggaard, M.; Kleindorp, R.; Beekman, M.; Jacobsen, R.; Suchiman, H.E.; de Craen, A.J.; Westendorp, R.G.; et al. Human longevity and variation in GH/IGF-1/insulin signaling, DNA damage signaling and repair and pro/antioxidant pathway genes: Cross sectional and longitudinal studies. Exp. Gerontol. 2012, 47, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Dato, S.; Soerensen, M.; Lagani, V.; Montesanto, A.; Passarino, G.; Christensen, K.; Tan, Q.; Christiansen, L. Contribution of genetic polymorphisms on functional status at very old age: A gene-based analysis of 38 genes (311 SNPs) in the oxidative stress pathway. Exp. Gerontol. 2014, 52, 23–29. [Google Scholar] [CrossRef]
- Dato, S.; De Rango, F.; Crocco, P.; Passarino, G.; Rose, G. Antioxidants and quality of aging: Further evidences for a major role of TXNRD1 gene variability on physical performance at old age. Oxid. Med. Cell. Longev. 2015, 2015, 926067. [Google Scholar] [CrossRef]
- Jurado, P.; de Lorenzo, V.; Fernandez, L.A. Thioredoxin fusions increase folding of single chain Fv antibodies in the cytoplasm of Escherichia coli: Evidence that chaperone activity is the prime effect of thioredoxin. J. Mol. Biol. 2006, 357, 49–61. [Google Scholar] [CrossRef]
- Du, H.; Kim, S.; Hur, Y.S.; Lee, M.S.; Lee, S.H.; Cheon, C.I. A cytosolic thioredoxin acts as a molecular chaperone for peroxisome matrix proteins as well as antioxidant in peroxisome. Mol. Cells 2015, 38, 187–194. [Google Scholar] [CrossRef]
- Liu, Y.; Min, W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ. Res. 2002, 90, 1259–1266. [Google Scholar] [CrossRef]
- Sanzo-Machuca, Á.; Monje Moreno, J.M.; Casado-Navarro, R.; Karakuzu, O.; Guerrero-Gómez, D.; Fierro-González, J.C.; Swoboda, P.; Muñoz, M.J.; Garsin, D.A.; Pedrajas, J.R.; et al. Redox-dependent and redox-independent functions of Caenorhabditis elegans thioredoxin 1. Redox Biol. 2019, 24, 101178. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, W.; Ren, J.; Hall, Q.; Zhang, Y.; Kaplan, J.M. Thioredoxin shapes the C. elegans sensory response to Pseudomonas produced nitric oxide. eLife 2018, 7, e36833. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlOkda, A.; Van Raamsdonk, J.M. Evolutionarily Conserved Role of Thioredoxin Systems in Determining Longevity. Antioxidants 2023, 12, 944. https://doi.org/10.3390/antiox12040944
AlOkda A, Van Raamsdonk JM. Evolutionarily Conserved Role of Thioredoxin Systems in Determining Longevity. Antioxidants. 2023; 12(4):944. https://doi.org/10.3390/antiox12040944
Chicago/Turabian StyleAlOkda, Abdelrahman, and Jeremy M. Van Raamsdonk. 2023. "Evolutionarily Conserved Role of Thioredoxin Systems in Determining Longevity" Antioxidants 12, no. 4: 944. https://doi.org/10.3390/antiox12040944
APA StyleAlOkda, A., & Van Raamsdonk, J. M. (2023). Evolutionarily Conserved Role of Thioredoxin Systems in Determining Longevity. Antioxidants, 12(4), 944. https://doi.org/10.3390/antiox12040944






