Impaired Melatonin Secretion, Oxidative Stress and Metabolic Syndrome in Night Shift Work
Abstract
:1. Epidemiological Data
2. Oxidative Stress and Working in Shifts
3. Melatonin Dysregulation—A Link between Oxidative Stress, Metabolic Syndrome and Working in Shifts
3.1. Melatonin—An Antioxidant Molecule
3.2. Antioxidative Effects Mediated by MT in Obesity
3.3. Antioxidative Effects Mediated by MT in the Vascular Wall
3.4. Antioxidative Effects Mediated by MT in Insulin Resistance and Diabetes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aleksynska, M.; Berg, J.; Foden, D.; Johnston, H.; Parent-Thirion, A.; Vanderleyden, J.; Vermeylen, G. Working Conditions in a Global Perspective; Research report/Eurofound; Publications Office of the European Union: Luxembourg, 2019. [CrossRef]
- Morikawa, Y.; Nakagawa, H.; Miura, K.; Soyama, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; Suwazono, Y.; Nogawa, K. Shift Work and the Risk of Diabetes Mellitus among Japanese Male Factory Workers. Scand. J. Work Environ. Health 2005, 31, 179–183. [Google Scholar] [CrossRef]
- Dochi, M.; Suwazono, Y.; Sakata, K.; Okubo, Y.; Oishi, M.; Tanaka, K.; Kobayashi, E.; Nogawa, K. Shift Work Is a Risk Factor for Increased Total Cholesterol Level: A 14-Year Prospective Cohort Study in 6886 Male Workers. Occup. Environ. Med. 2009, 66, 592–597. [Google Scholar] [CrossRef]
- de Assis, M.A.A.; Nahas, M.V.; Bellisle, F.; Kupek, E. Meals, Snacks and Food Choices in Brazilian Shift Workers with High Energy Expenditure. J. Hum. Nutr. Diet. 2003, 16, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Khosravipour, M.; Khanlari, P.; Khazaie, S.; Khosravipour, H.; Khazaie, H. A Systematic Review and Meta-Analysis of the Association between Shift Work and Metabolic Syndrome: The Roles of Sleep, Gender, and Type of Shift Work. Sleep Med. Rev. 2021, 57, 101427. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, B.; Knutsson, A.; Lindahl, B. Is There an Association between Shift Work and Having a Metabolic Syndrome? Results from a Population Based Study of 27,485 People. Occup. Environ. Med. 2001, 58, 747–752. [Google Scholar] [CrossRef] [PubMed]
- De Bacquer, D.; Van Risseghem, M.; Clays, E.; Kittel, F.; De Backer, G.; Braeckman, L. Rotating Shift Work and the Metabolic Syndrome: A Prospective Study. Int. J. Epidemiol. 2009, 38, 848–854. [Google Scholar] [CrossRef]
- Tucker, P.; Marquié, J.-C.; Folkard, S.; Ansiau, D.; Esquirol, Y. Shiftwork and Metabolic Dysfunction. Chronobiol. Int. 2012, 29, 549–555. [Google Scholar] [CrossRef]
- Ahn, C.W.; Shin, S.; Lee, S.; Park, H.-S.; Hong, N.; Rhee, Y. Association of Shift Work with Normal-Weight Obesity in Community-Dwelling Adults. Endocrinol. Metab. 2022, 37, 781–790. [Google Scholar] [CrossRef]
- Scuteri, A.; Laurent, S.; Cucca, F.; Cockcroft, J.; Cunha, P.G.; Mañas, L.R.; Mattace Raso, F.U.; Muiesan, M.L.; Ryliškytė, L.; Rietzschel, E.; et al. Metabolic Syndrome and Arteries Research (MARE) Consortium. Metabolic Syndrome across Europe: Different Clusters of Risk Factors. Eur. J. Prev. Cardiol. 2015, 22, 486–491. [Google Scholar] [CrossRef]
- Hu, G.; Qiao, Q.; Tuomilehto, J.; Balkau, B.; Borch-Johnsen, K.; Pyorala, K.; DECODE Study Group. Prevalence of the Metabolic Syndrome and Its Relation to All-Cause and Cardiovascular Mortality in Nondiabetic European Men and Women. Arch. Intern. Med. 2004, 164, 1066–1076. [Google Scholar] [CrossRef]
- Alegría, E.; Cordero, A.; Laclaustra, M.; Grima, A.; León, M.; Casasnovas, J.A.; Luengo, E.; del Río, A.; Ferreira, I. Prevalence of Metabolic Syndrome in the Spanish Working Population: MESYAS Registry. Rev. Esp. Cardiol. 2005, 58, 797–806. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, J.; Wang, C.; Wang, X.; Zhao, P.; Yuan, Y.; Ai, H.; Zhou, Q. The Racial Disparities in the Epidemic of Metabolic Syndrome with Increased Age: A Study from 28,049 Chinese and American Adults. Front. Public Health 2021, 9, 797183. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Duan, P.; Liu, B.; Li, T.; Wang, C.; Li, H.; Yang, T.; Gan, Y.; Wang, X.; et al. Is Shift Work Associated with a Higher Risk of Overweight or Obesity? A Systematic Review of Observational Studies with Meta-Analysis. Int. J. Epidemiol. 2018, 47, 1956–1971. [Google Scholar] [CrossRef]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating Night Shift Work and Risk of Type 2 Diabetes: Two Prospective Cohort Studies in Women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Zong, G.; Guo, Y.; Li, J.; Manson, J.E.; Hu, F.B.; Willett, W.C.; Schernhammer, E.S.; Bhupathiraju, S.N. Rotating Night Shift Work and Adherence to Unhealthy Lifestyle in Predicting Risk of Type 2 Diabetes: Results from Two Large US Cohorts of Female Nurses. BMJ 2018, 363, k4641. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Ishihara, J.; Kotemori, A.; Kokubo, Y.; Saito, I.; Yatsuya, H.; Yamagishi, K.; Sawada, N.; Iwasaki, M.; Iso, H.; et al. Association between Irregular Daily Routine and Risk of Incident Stroke and Coronary Heart Disease in a Large Japanese Population. Sci. Rep. 2022, 12, 15750. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Jong, C.J.; Mozaffari, M. Role of Oxidative Stress in Diabetes-Mediated Vascular Dysfunction: Unifying Hypothesis of Diabetes Revisited. Vascul. Pharmacol. 2012, 57, 139–149. [Google Scholar] [CrossRef]
- Brown, S.A. Circadian Metabolism: From Mechanisms to Metabolomics and Medicine. Trends Endocrinol. Metab. 2016, 27, 415–426. [Google Scholar] [CrossRef]
- Kiehn, J.-T.; Tsang, A.H.; Heyde, I.; Leinweber, B.; Kolbe, I.; Leliavski, A.; Oster, H. Circadian Rhythms in Adipose Tissue Physiology. Compr. Physiol. 2017, 7, 383–427. [Google Scholar] [CrossRef]
- Ashton, A.; Foster, R.G.; Jagannath, A. Photic Entrainment of the Circadian System. Int. J. Mol. Sci. 2022, 23, 729. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J. Partial Sleep Deprivation Reduces Phase Advances to Light in Humans. J. Biol. Rhythms 2010, 25, 460–468. [Google Scholar] [CrossRef]
- Cuesta, M.; Boudreau, P.; Cermakian, N.; Boivin, D.B. Rapid Resetting of Human Peripheral Clocks by Phototherapy during Simulated Night Shift Work. Sci. Rep. 2017, 7, 16310. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Mariani, S.; Weng, J.; Marinac, C.R.; Kaplan, E.R.; Rueschman, M.; Mitchell, J.A.; James, P.; Hipp, J.A.; Cespedes Feliciano, E.M.; et al. Zeitgebers and Their Association with Rest-Activity Patterns. Chronobiol. Int. 2019, 36, 203–213. [Google Scholar] [CrossRef]
- Weinert, D.; Gubin, D. The Impact of Physical Activity on the Circadian System: Benefits for Health, Performance and Wellbeing. Appl. Sci. 2022, 12, 9220. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Mendonça, F.M.; de Sousa, F.R.; Barbosa, A.L.; Martins, S.C.; Araújo, R.L.; Soares, R.; Abreu, C. Metabolic Syndrome and Risk of Cancer: Which Link? Metabolism 2015, 64, 182–189. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.C.; Hong, H.; Weidemann, B.J.; Ramsey, K.M.; Affinati, A.H.; Schmidt, M.S.; Cedernaes, J.; Omura, C.; Braun, R.; Lee, C.; et al. NAD+ Controls Circadian Reprogramming through PER2 Nuclear Translocation to Counter Aging. Mol. Cell 2020, 78, 835–849.e7. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 Mediates Central Circadian Control in the SCN by a Mechanism That Decays with Aging. Cell 2013, 153, 1448–1460. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK Signalling Pathway Coordinates Cell Growth, Autophagy and Metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Brenna, A.; Albrecht, U. Phosphorylation and Circadian Molecular Timing. Front. Physiol. 2020, 11, 612510. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.J.; Andrews, J.L.; McDearmon, E.L.; Campbell, K.S.; Barber, B.K.; Miller, B.H.; Walker, J.R.; Hogenesch, J.B.; Takahashi, J.S.; Esser, K.A. Identification of the Circadian Transcriptome in Adult Mouse Skeletal Muscle. Physiol. Genomics 2007, 31, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, M.-S.; Li, R.; Liu, V.Y.; Fu, L.; Moore, D.D.; Ma, K.; Yechoor, V.K. Loss of Bmal1 Leads to Uncoupling and Impaired Glucose-Stimulated Insulin Secretion in β-Cells. Islets 2011, 3, 381–388. [Google Scholar] [CrossRef]
- Jacobi, D.; Liu, S.; Burkewitz, K.; Kory, N.; Knudsen, N.H.; Alexander, R.K.; Unluturk, U.; Li, X.; Kong, X.; Hyde, A.L.; et al. Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness. Cell Metab. 2015, 22, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.; Kvandová, M.; Daiber, A.; Stamm, P.; Frenis, K.; Schulz, E.; Münzel, T.; Kröller-Schön, S. The AMP-Activated Protein Kinase Plays a Role in Antioxidant Defense and Regulation of Vascular Inflammation. Antioxidants 2020, 9, 525. [Google Scholar] [CrossRef]
- Kröller-Schön, S.; Jansen, T.; Hauptmann, F.; Schüler, A.; Heeren, T.; Hausding, M.; Oelze, M.; Viollet, B.; Keaney, J.F.; Wenzel, P.; et al. A1AMP-Activated Protein Kinase Mediates Vascular Protective Effects of Exercise. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1632–1641. [Google Scholar] [CrossRef]
- Zippel, N.; Malik, R.A.; Frömel, T.; Popp, R.; Bess, E.; Strilic, B.; Wettschureck, N.; Fleming, I.; Fisslthaler, B. Transforming Growth Factor-β-Activated Kinase 1 Regulates Angiogenesis via AMP-Activated Protein Kinase-A1 and Redox Balance in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2792–2799. [Google Scholar] [CrossRef]
- Schulz, E.; Dopheide, J.; Schuhmacher, S.; Thomas, S.R.; Chen, K.; Daiber, A.; Wenzel, P.; Münzel, T.; Keaney, J.F. Suppression of the JNK Pathway by Induction of a Metabolic Stress Response Prevents Vascular Injury and Dysfunction. Circulation 2008, 118, 1347–1357. [Google Scholar] [CrossRef]
- Zou, M.-H.; Hou, X.-Y.; Shi, C.-M.; Nagata, D.; Walsh, K.; Cohen, R.A. Modulation by Peroxynitrite of Akt- and AMP-Activated Kinase-Dependent Ser1179 Phosphorylation of Endothelial Nitric Oxide Synthase. J. Biol. Chem. 2002, 277, 32552–32557. [Google Scholar] [CrossRef]
- Sardon Puig, L.; Valera-Alberni, M.; Cantó, C.; Pillon, N.J. Circadian Rhythms and Mitochondria: Connecting the Dots. Front. Genet. 2018, 9, 452. [Google Scholar] [CrossRef]
- Schmitt, K.; Grimm, A.; Dallmann, R.; Oettinghaus, B.; Restelli, L.M.; Witzig, M.; Ishihara, N.; Mihara, K.; Ripperger, J.A.; Albrecht, U.; et al. Circadian Control of DRP1 Activity Regulates Mitochondrial Dynamics and Bioenergetics. Cell Metab. 2018, 27, 657–666.e5. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, S.; Gómez-Valadés, A.G.; Schneeberger, M.; Varela, L.; Haddad-Tóvolli, R.; Altirriba, J.; Noguera, E.; Drougard, A.; Flores-Martínez, Á.; Imbernón, M.; et al. Mitochondrial Dynamics Mediated by Mitofusin 1 Is Required for POMC Neuron Glucose-Sensing and Insulin Release Control. Cell Metab. 2017, 25, 1390–1399.e6. [Google Scholar] [CrossRef]
- Ulas, T.; Buyukhatipoglu, H.; Kirhan, I.; Dal, M.S.; Ulas, S.; Demir, M.E.; Eren, M.A.; Ucar, M.; Hazar, A.; Kurkcuoglu, I.C.; et al. Evaluation of Oxidative Stress Parameters and Metabolic Activities of Nurses Working Day and Night Shifts. Rev. Esc. Enferm. USP 2013, 47, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, I.; Nakano, M.; Ikushima, M.; Hara, Y.; Yoshimine, T.; Haraga, M.; Nakatani, J.; Kawamoto, R.; Kasai, H. Effect of Work Conditions and Work Environments on the Formation of 8-OH-DG in Nurses and Non-Nurse Female Workers. J. UOEH 2008, 30, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, P.; Mirick, D.K.; Randolph, T.W.; Gong, J.; Buchanan, D.T.; Zhang, J.J.; Davis, S. Oxidative DNA Damage during Night Shift Work. Occup. Environ. Med. 2017, 74, 680–683. [Google Scholar] [CrossRef]
- Demir, I.; Toker, A.; Zengin, S.; Laloglu, E.; Aksoy, H. Oxidative Stress and Insulin Resistance in Policemen Working Shifts. Int. Arch. Occup. Environ. Health 2016, 89, 407–412. [Google Scholar] [CrossRef]
- Teixeira, K.R.C.; Dos Santos, C.P.; de Medeiros, L.A.; Mendes, J.A.; Cunha, T.M.; De Angelis, K.; Penha-Silva, N.; de Oliveira, E.P.; Crispim, C.A. Night Workers Have Lower Levels of Antioxidant Defenses and Higher Levels of Oxidative Stress Damage When Compared to Day Workers. Sci. Rep. 2019, 9, 4455. [Google Scholar] [CrossRef]
- Thosar, S.S.; Berman, A.M.; Herzig, M.X.; McHill, A.W.; Bowles, N.P.; Swanson, C.M.; Clemons, N.A.; Butler, M.P.; Clemons, A.A.; Emens, J.S.; et al. Circadian Rhythm of Vascular Function in Midlife Adults. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1203–1211. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Wei, T.; Li, C.; Heng, Y.; Gao, X.; Zhang, G.; Wang, H.; Zhao, X.; Meng, Z.; Zhang, Y.; Hou, H. Association between Night-Shift Work and Level of Melatonin: Systematic Review and Meta-Analysis. Sleep Med. 2020, 75, 502–509. [Google Scholar] [CrossRef]
- Corbalán-Tutau, D.; Madrid, J.A.; Nicolás, F.; Garaulet, M. Daily Profile in Two Circadian Markers “Melatonin and Cortisol” and Associations with Metabolic Syndrome Components. Physiol. Behav. 2014, 123, 231–235. [Google Scholar] [CrossRef]
- Robeva, R.; Kirilov, G.; Tomova, A.; Kumanov, P. Melatonin-Insulin Interactions in Patients with Metabolic Syndrome. J. Pineal Res. 2008, 44, 52–56. [Google Scholar] [CrossRef]
- Zeman, M.; Dulková, K.; Bada, V.; Herichová, I. Plasma Melatonin Concentrations in Hypertensive Patients with the Dipping and Non-Dipping Blood Pressure Profile. Life Sci. 2005, 76, 1795–1803. [Google Scholar] [CrossRef]
- Forman, J.P.; Curhan, G.C.; Schernhammer, E.S. Urinary Melatonin and Risk of Incident Hypertension among Young Women. J. Hypertens. 2010, 28, 446–451. [Google Scholar] [CrossRef]
- Rubio-Sastre, P.; Scheer, F.A.J.L.; Gómez-Abellán, P.; Madrid, J.A.; Garaulet, M. Acute Melatonin Administration in Humans Impairs Glucose Tolerance in Both the Morning and Evening. Sleep 2014, 37, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Ahmed, S.; Jackson, J.M.L.; Sack, R.L. Melatonin Shifts Human Orcadian Rhythms According to a Phase-Response Curve. Chronobiol. Int. 1992, 9, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.M.; Figueiro, M.G. Measuring Light at Night and Melatonin Levels in Shift Workers: A Review of the Literature. Biol. Res. Nurs. 2017, 19, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Lee, E.; Moon, J.-H.; Kim, Y.; Lee, H.-J. Circadian Disruption and Increase of Oxidative Stress in Male and Female Volunteers after Bright Light Exposure before Bed Time. Mol. Cell. Toxicol. 2019, 15, 221–229. [Google Scholar] [CrossRef]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal Melatonin: Analysis of Its Subcellular Distribution and Daily Fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef]
- Skinner, D.C.; Malpaux, B. High Melatonin Concentrations in Third Ventricular Cerebrospinal Fluid Are Not Due to Galen Vein Blood Recirculating through the Choroid Plexus. Endocrinology 1999, 140, 4399–4405. [Google Scholar] [CrossRef] [PubMed]
- Raikhlin, N.T.; Kvetnoy, I.M.; Tolkachev, V.N. Melatonin May Be Synthesised in Enterochromaffin Cells. Nature 1975, 255, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Summer, J.; Anis, R. How Long Does Melatonin Last? Sleep Foundation. Available online: https://www.sleepfoundation.org/melatonin/how-long-does-melatonin-last (accessed on 8 March 2023).
- Kovacs, J.; Langer, M.; Brodner, W.; Waldhauser, F. Both Urinary Excreted Melatonin and 6-Hydroxy-Melatonin Sulfate Are Good Representatives of Endogenous Serum Melatonin Concentration in Human. Pediatr. Res. 1999, 45, 92. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an Antioxidant: Under Promises but over Delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Kohlmeier, M. Chapter 8-Amino Acids and Nitrogen Compounds. In Nutrient Metabolism, 2nd ed.; Kohlmeier, M., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 265–477. [Google Scholar] [CrossRef]
- Blanchard-Fillion, B.; Servy, C.; Ducrocq, C. 1-Nitrosomelatonin Is a Spontaneous NO-Releasing Compound. Free Radic. Res. 2001, 35, 857–866. [Google Scholar] [CrossRef]
- Hardeland, R.; Tan, D.-X.; Reiter, R.J. Kynuramines, Metabolites of Melatonin and Other Indoles: The Resurrection of an Almost Forgotten Class of Biogenic Amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef]
- Ressmeyer, A.-R.; Mayo, J.C.; Zelosko, V.; Sáinz, R.M.; Tan, D.-X.; Poeggeler, B.; Antolín, I.; Zsizsik, B.K.; Reiter, R.J.; Hardeland, R. Antioxidant Properties of the Melatonin Metabolite N1-Acetyl-5-Methoxykynuramine (AMK): Scavenging of Free Radicals and Prevention of Protein Destruction. Redox. Rep. 2003, 8, 205–213. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual Role of Mitochondria in Producing Melatonin and Driving GPCR Signaling to Block Cytochrome c Release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef]
- Markus, R.P.; Sousa, K.S.; da Silveira Cruz-Machado, S.; Fernandes, P.A.; Ferreira, Z.S. Possible Role of Pineal and Extra-Pineal Melatonin in Surveillance, Immunity, and First-Line Defense. Int. J. Mol. Sci. 2021, 22, 12143. [Google Scholar] [CrossRef]
- Martín, M.; Macías, M.; Escames, G.; Reiter, R.J.; Agapito, M.T.; Ortiz, G.G.; Acuña-Castroviejo, D. Melatonin-Induced Increased Activity of the Respiratory Chain Complexes I and IV Can Prevent Mitochondrial Damage Induced by Ruthenium Red in Vivo. J. Pineal Res. 2000, 28, 242–248. [Google Scholar] [CrossRef]
- Hardeland, R. Antioxidative Protection by Melatonin: Multiplicity of Mechanisms from Radical Detoxification to Radical Avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Ma, X.; Wang, S.; Cheng, H.; Ouyang, H.; Ma, X. Melatonin Attenuates Ischemia/Reperfusion-Induced Oxidative Stress by Activating Mitochondrial Fusion in Cardiomyocytes. Oxid. Med. Cell. Longev. 2022, 2022, 7105181. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Qi, W. Biochemical Reactivity of Melatonin with Reactive Oxygen and Nitrogen Species: A Review of the Evidence. Cell. Biochem. Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; He, Y.; Jia, L.; Yang, C.S.; Reiter, R.J.; Zhang, J. Antioxidant and Pro-Oxidant Activities of Melatonin in the Presence of Copper and Polyphenols In Vitro and In Vivo. Cells 2019, 8, 903. [Google Scholar] [CrossRef]
- Theurey, P.; Rieusset, J. Mitochondria-Associated Membranes Response to Nutrient Availability and Role in Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 32–45. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.-R.; Liu, B. Mitochondrial Sirtuin 3: New Emerging Biological Function and Therapeutic Target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef]
- Cecon, E.; Oishi, A.; Jockers, R. Melatonin Receptors: Molecular Pharmacology and Signalling in the Context of System Bias. Br. J. Pharmacol. 2018, 175, 3263–3280. [Google Scholar] [CrossRef] [PubMed]
- Antolín, I.; Rodríguez, C.; Saínz, R.M.; Mayo, J.C.; Uría, H.; Kotler, M.L.; Rodríguez-Colunga, M.J.; Tolivia, D.; Menéndez-Peláez, A. Neurohormone Melatonin Prevents Cell Damage: Effect on Gene Expression for Antioxidant Enzymes. FASEB J. 1996, 10, 882–890. [Google Scholar] [CrossRef]
- Kotler, M.; Rodríguez, C.; Sáinz, R.M.; Antolín, I.; Menéndez-Peláez, A. Melatonin Increases Gene Expression for Antioxidant Enzymes in Rat Brain Cortex. J. Pineal Res. 1998, 24, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Acuña-Castroviejo, D.; Tan, D.X.; Burkhardt, S. Free Radical-Mediated Molecular Damage. Mechanisms for the Protective Actions of Melatonin in the Central Nervous System. Ann. N. Y. Acad. Sci. 2001, 939, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Abdulwahab, D.A.; El-Missiry, M.A.; Shabana, S.; Othman, A.I.; Amer, M.E. Melatonin Protects the Heart and Pancreas by Improving Glucose Homeostasis, Oxidative Stress, Inflammation and Apoptosis in T2DM-Induced Rats. Heliyon 2021, 7, e06474. [Google Scholar] [CrossRef]
- Amer, M.E.; Othamn, A.I.; El-Missiry, M.A. Melatonin Ameliorates Diabetes-Induced Brain Injury in Rats. Acta Histochem. 2021, 123, 151677. [Google Scholar] [CrossRef]
- Urata, Y.; Honma, S.; Goto, S.; Todoroki, S.; Iida, T.; Cho, S.; Honma, K.; Kondo, T. Melatonin Induces Gamma-Glutamylcysteine Synthetase Mediated by Activator Protein-1 in Human Vascular Endothelial Cells. Free Radic. Biol. Med. 1999, 27, 838–847. [Google Scholar] [CrossRef]
- Sewerynek, E.; Abe, M.; Reiter, R.J.; Barlow-Walden, L.R.; Chen, L.; McCabe, T.J.; Roman, L.J.; Diaz-Lopez, B. Melatonin Administration Prevents Lipopolysaccharide-Induced Oxidative Damage in Phenobarbital-Treated Animals. J. Cell. Biochem. 1995, 58, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and Beige Adipose Tissue: A Novel Therapeutic Strategy for Obesity and Type 2 Diabetes Mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef]
- Jiménez-Aranda, A.; Fernández-Vázquez, G.; Campos, D.; Tassi, M.; Velasco-Perez, L.; Tan, D.-X.; Reiter, R.J.; Agil, A. Melatonin Induces Browning of Inguinal White Adipose Tissue in Zucker Diabetic Fatty Rats. J. Pineal Res. 2013, 55, 416–423. [Google Scholar] [CrossRef]
- Salagre, D.; Chayah, M.; Molina-Carballo, A.; Oliveras-López, M.-J.; Munoz-Hoyos, A.; Navarro-Alarcón, M.; Fernández-Vázquez, G.; Agil, A. Melatonin Induces Fat Browning by Transdifferentiation of White Adipocytes and de Novo Differentiation of Mesenchymal Stem Cells. Food Funct. 2022, 13, 3760–3775. [Google Scholar] [CrossRef]
- Xu, L.; Li, D.; Li, H.; Zhang, O.; Huang, Y.; Shao, H.; Wang, Y.; Cai, S.; Zhu, Y.; Jin, S.; et al. Suppression of Obesity by Melatonin through Increasing Energy Expenditure and Accelerating Lipolysis in Mice Fed a High-Fat Diet. Nutr. Diabetes 2022, 12, 42. [Google Scholar] [CrossRef]
- Pan, R.; Chen, Y. Management of Oxidative Stress: Crosstalk Between Brown/Beige Adipose Tissues and Skeletal Muscles. Front. Physiol. 2021, 12, 712372. [Google Scholar] [CrossRef]
- Dlasková, A.; Clarke, K.J.; Porter, R.K. The Role of UCP 1 in Production of Reactive Oxygen Species by Mitochondria Isolated from Brown Adipose Tissue. Biochim. Biophys. Acta 2010, 1797, 1470–1476. [Google Scholar] [CrossRef]
- Chusyd, D.E.; Wang, D.; Huffman, D.M.; Nagy, T.R. Relationships between Rodent White Adipose Fat Pads and Human White Adipose Fat Depots. Front. Nutr. 2016, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Obayemi, M.J.; Akintayo, C.O.; Oniyide, A.A.; Aturamu, A.; Badejogbin, O.C.; Atuma, C.L.; Saidi, A.O.; Mahmud, H.; Olaniyi, K.S. Protective Role of Melatonin against Adipose-Hepatic Metabolic Comorbidities in Experimentally Induced Obese Rat Model. PLoS ONE 2021, 16, e0260546. [Google Scholar] [CrossRef]
- Agil, A.; Navarro-Alarcon, M.; Ali, F.A.Z.; Albrakati, A.; Salagre, D.; Campoy, C.; Elmahallawy, E.K. Melatonin Enhances the Mitochondrial Functionality of Brown Adipose Tissue in Obese-Diabetic Rats. Antioxidants 2021, 10, 1482. [Google Scholar] [CrossRef]
- de Farias, T.d.S.M.; Cruz, M.M.; de Sa, R.C.d.C.; Severi, I.; Perugini, J.; Senzacqua, M.; Cerutti, S.M.; Giordano, A.; Cinti, S.; Alonso-Vale, M.I.C. Melatonin Supplementation Decreases Hypertrophic Obesity and Inflammation Induced by High-Fat Diet in Mice. Front. Endocrinol. 2019, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, I.G.B.; Junior, M.D.F.; Lopes, P.R.; Campos, D.B.T.; Ferreira-Neto, M.L.; Santos, E.H.R.; Mathias, P.C.d.F.; Francisco, F.A.; Koike, B.D.V.; de Castro, C.H.; et al. Forced Internal Desynchrony Induces Cardiometabolic Alterations in Adult Rats. J. Endocrinol. 2019, 242, 25–36. [Google Scholar] [CrossRef]
- Chivchibashi-Pavlova, D.; Stoyanov, G.S.; Bratoeva, K. Effects of Melatonin Supplementation on the Aortic Wall in a Diet-Induced Obesity Rat Model. Cureus 2023, 15, e33333. [Google Scholar] [CrossRef]
- Anwar, M.M.; Meki, A.R.; Rahma, H.H. Inhibitory Effects of Melatonin on Vascular Reactivity: Possible Role of Vasoactive Mediators. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 357–367. [Google Scholar] [CrossRef]
- Girouard, H.; de Champlain, J. Inhibitory Effect of Melatonin on Alpha1-Adrenergic-Induced Vasoconstriction in Mesenteric Beds of Spontaneously Hypertensive Rats. Am. J. Hypertens. 2004, 17, 339–346. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Zingarelli, B.; Gilad, E.; Hake, P.; Salzman, A.L.; Szabó, C. Protective Effect of Melatonin in Carrageenan-Induced Models of Local Inflammation: Relationship to Its Inhibitory Effect on Nitric Oxide Production and Its Peroxynitrite Scavenging Activity. J. Pineal Res. 1997, 23, 106–116. [Google Scholar] [CrossRef]
- Tamura, E.K.; Silva, C.L.M.; Markus, R.P. Melatonin Inhibits Endothelial Nitric Oxide Production in Vitro. J. Pineal Res. 2006, 41, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Pechánová, O.; Zicha, J.; Paulis, L.; Zenebe, W.; Dobesová, Z.; Kojsová, S.; Jendeková, L.; Sládková, M.; Dovinová, I.; Simko, F.; et al. The Effect of N-Acetylcysteine and Melatonin in Adult Spontaneously Hypertensive Rats with Established Hypertension. Eur. J. Pharmacol. 2007, 561, 129–136. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, H.; Jin, C.; Qiu, F.; Wu, Y.; Shi, L. Melatonin Mediates Vasodilation through Both Direct and Indirect Activation of BKCa Channels. J. Mol. Endocrinol. 2017, 59, 219–233. [Google Scholar] [CrossRef]
- Rezzani, R.; Porteri, E.; De Ciuceis, C.; Bonomini, F.; Rodella, L.F.; Paiardi, S.; Boari, G.E.M.; Platto, C.; Pilu, A.; Avanzi, D.; et al. Effects of Melatonin and Pycnogenol on Small Artery Structure and Function in Spontaneously Hypertensive Rats. Hypertension 2010, 55, 1373–1380. [Google Scholar] [CrossRef]
- Benova, M.; Herichova, I.; Stebelova, K.; Paulis, L.; Krajcirovicova, K.; Simko, F.; Zeman, M. Effect of L-NAME-Induced Hypertension on Melatonin Receptors and Melatonin Levels in the Pineal Gland and the Peripheral Organs of Rats. Hypertens. Res. 2009, 32, 242–247. [Google Scholar] [CrossRef]
- Li, H.-Y.; Leu, Y.-L.; Wu, Y.-C.; Wang, S.-H. Melatonin Inhibits in Vitro Smooth Muscle Cell Inflammation and Proliferation and Atherosclerosis in Apolipoprotein E-Deficient Mice. J. Agric. Food Chem. 2019, 67, 1889–1901. [Google Scholar] [CrossRef]
- Li, P.; Xie, C.; Zhong, J.; Guo, Z.; Guo, K.; Tu, Q. Melatonin Attenuates Ox-LDL-Induced Endothelial Dysfunction by Reducing ER Stress and Inhibiting JNK/Mff Signaling. Oxid. Med. Cell. Longev. 2021, 2021, 5589612. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Fang, X.-L.; Fang, N.; Wang, X.-B.; Qian, H.-Y.; Cao, Z.; Cheng, Y.; Wang, B.-N.; Wang, Y. Melatonin Ameliorates Vascular Endothelial Dysfunction, Inflammation, and Atherosclerosis by Suppressing the TLR4/NF-ΚB System in High-Fat-Fed Rabbits. J. Pineal Res. 2013, 55, 388–398. [Google Scholar] [CrossRef]
- Raaz, U.; Schellinger, I.N.; Chernogubova, E.; Warnecke, C.; Kayama, Y.; Penov, K.; Hennigs, J.K.; Salomons, F.; Eken, S.; Emrich, F.C.; et al. Transcription Factor Runx2 Promotes Aortic Fibrosis and Stiffness in Type 2 Diabetes Mellitus. Circ. Res. 2015, 117, 513–524. [Google Scholar] [CrossRef]
- Chen, F.; Liu, H.; Wang, X.; Li, Z.; Zhang, J.; Pei, Y.; Zheng, Z.; Wang, J. Melatonin Activates Autophagy via the NF-ΚB Signaling Pathway to Prevent Extracellular Matrix Degeneration in Intervertebral Disc. Osteoarthritis. Cartilage 2020, 28, 1121–1132. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a Master Regulator of Cell Death and Inflammation: Molecular Mechanisms and Clinical Implications for Newborn Care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Ding, K.; Xu, J.; Wang, H.; Zhang, L.; Wu, Y.; Li, T. Melatonin Protects the Brain from Apoptosis by Enhancement of Autophagy after Traumatic Brain Injury in Mice. Neurochem. Int. 2015, 91, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, L.; Wu, C.; Hu, Q.; Gu, C.; Yan, F.; Li, J.; Yan, W.; Chen, G. Melatonin-Enhanced Autophagy Protects against Neural Apoptosis via a Mitochondrial Pathway in Early Brain Injury Following a Subarachnoid Hemorrhage. J. Pineal Res. 2014, 56, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, D.; Zhu, P.; Ma, Q.; Toan, S.; Wang, J.; Hu, S.; Chen, Y.; Zhang, Y. Inhibitory Effect of Melatonin on Necroptosis via Repressing the Ripk3-PGAM5-CypD-MPTP Pathway Attenuates Cardiac Microvascular Ischemia-Reperfusion Injury. J. Pineal Res. 2018, 65, e12503. [Google Scholar] [CrossRef]
- Ma, S.; Chen, J.; Feng, J.; Zhang, R.; Fan, M.; Han, D.; Li, X.; Li, C.; Ren, J.; Wang, Y.; et al. Melatonin Ameliorates the Progression of Atherosclerosis via Mitophagy Activation and NLRP3 Inflammasome Inhibition. Oxid. Med. Cell. Longev. 2018, 2018, 9286458. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xu, J.; Tian, F.; Hu, S.; Chen, Y.; Fu, Z. Melatonin Attenuates Myocardial Ischemia-Reperfusion Injury via Improving Mitochondrial Fusion/Mitophagy and Activating the AMPK-OPA1 Signaling Pathways. J. Pineal Res. 2019, 66, e12542. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Byun, J.-K.; Park, M.; Woo Kim, S.; Lee, S.; Kim, J.-G.; Lee, I.-K.; Choi, Y.-K.; Park, K.-G. Melatonin Inhibits Vascular Smooth Muscle Cell Proliferation and Apoptosis through Upregulation of Sestrin2. Exp. Ther. Med. 2020, 19, 3454–3460. [Google Scholar] [CrossRef]
- Fan, Y.-J.; Zong, W.-X. The Cellular Decision between Apoptosis and Autophagy. Chin. J. Cancer. 2013, 32, 121–129. [Google Scholar] [CrossRef]
- Campos, L.A.; Cipolla-Neto, J.; Amaral, F.G.; Michelini, L.C.; Bader, M.; Baltatu, O.C. The Angiotensin-Melatonin Axis. Int. J. Hypertens. 2013, 2013, 521783. [Google Scholar] [CrossRef]
- Herichová, I.; Mravec, B.; Stebelová, K.; Krizanová, O.; Jurkovicová, D.; Kvetnanský, R.; Zeman, M. Rhythmic Clock Gene Expression in Heart, Kidney and Some Brain Nuclei Involved in Blood Pressure Control in Hypertensive TGR(MREN-2)27 Rats. Mol. Cell. Biochem. 2007, 296, 25–34. [Google Scholar] [CrossRef]
- Ishigaki, S.; Ohashi, N.; Matsuyama, T.; Isobe, S.; Tsuji, N.; Iwakura, T.; Fujikura, T.; Tsuji, T.; Kato, A.; Miyajima, H.; et al. Melatonin Ameliorates Intrarenal Renin-Angiotensin System in a 5/6 Nephrectomy Rat Model. Clin. Exp. Nephrol. 2018, 22, 539–549. [Google Scholar] [CrossRef]
- Ishigaki, S.; Ohashi, N.; Isobe, S.; Tsuji, N.; Iwakura, T.; Ono, M.; Sakao, Y.; Tsuji, T.; Kato, A.; Miyajima, H.; et al. Impaired Endogenous Nighttime Melatonin Secretion Relates to Intrarenal Renin-Angiotensin System Activation and Renal Damage in Patients with Chronic Kidney Disease. Clin. Exp. Nephrol. 2016, 20, 878–884, Correction in Clin. Exp. Nephrol. 2019, 23, 289–290. [Google Scholar] [CrossRef]
- Cook, J.S.; Sauder, C.L.; Ray, C.A. Melatonin Differentially Affects Vascular Blood Flow in Humans. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H670–H674. [Google Scholar] [CrossRef]
- Tamura, E.K.; Cecon, E.; Monteiro, A.W.A.; Silva, C.L.M.; Markus, R.P. Melatonin Inhibits LPS-Induced NO Production in Rat Endothelial Cells. J. Pineal Res. 2009, 46, 268–274. [Google Scholar] [CrossRef]
- Yildiz, M.; Akdemir, O. Assessment of the Effects of Physiological Release of Melatonin on Arterial Distensibility and Blood Pressure. Cardiol. Young 2009, 19, 198–203. [Google Scholar] [CrossRef]
- Grossman, E.; Laudon, M.; Zisapel, N. Effect of Melatonin on Nocturnal Blood Pressure: Meta-Analysis of Randomized Controlled Trials. Vasc. Health Risk Manag. 2011, 7, 577–584. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse Metabolic and Cardiovascular Consequences of Circadian Misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef]
- Banks, S.; Dorrian, J.; Grant, C.; Coates, A. Chapter 17-Circadian Misalignment and Metabolic Consequences: Shiftwork and Altered Meal Times. In Modulation of Sleep by Obesity, Diabetes, Age, and Diet; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 155–164. [Google Scholar] [CrossRef]
- Kervezee, L.; Kosmadopoulos, A.; Boivin, D.B. Metabolic and Cardiovascular Consequences of Shift Work: The Role of Circadian Disruption and Sleep Disturbances. Eur. J. Neurosci. 2020, 51, 396–412. [Google Scholar] [CrossRef]
- Grant, L.K.; Ftouni, S.; Nijagal, B.; De Souza, D.P.; Tull, D.; McConville, M.J.; Rajaratnam, S.M.W.; Lockley, S.W.; Anderson, C. Circadian and Wake-Dependent Changes in Human Plasma Polar Metabolites during Prolonged Wakefulness: A Preliminary Analysis. Sci. Rep. 2019, 9, 4428. [Google Scholar] [CrossRef]
- Song, J.; Jiang, X.; Juan, J.; Cao, Y.; Chibnik, L.B.; Hofman, A.; Wu, T.; Hu, Y. Role of Metabolic Syndrome and Its Components as Mediators of the Genetic Effect on Type 2 Diabetes: A Family-Based Study in China. J. Diabetes 2019, 11, 552–562. [Google Scholar] [CrossRef]
- de Luis Román, D.A.; Primo, D.; Aller, R.; Izaola, O. Association of the Rs10830963 Polymorphism in MTNR1B with Fasting Glucose, Serum Adipokine Levels and Components of Metabolic Syndrome in Adult Obese Subjects. Nutr. Hosp. 2019, 36, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Caro-Gomez, M.A.; Naranjo-González, C.A.; Gallego-Lopera, N.; Parra-Marín, M.V.; Valencia, D.M.; Arcos, E.G.; Villegas-Perrasse, A.; Bedoya-Berrío, G. Association of Native American Ancestry and Common Variants in ACE, ADIPOR2, MTNR1B, GCK, TCF7L2 and FTO Genes with Glycemic Traits in Colombian Population. Gene 2018, 677, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Vejrazkova, D.; Vankova, M.; Vcelak, J.; Krejci, H.; Anderlova, K.; Tura, A.; Pacini, G.; Sumova, A.; Sladek, M.; Bendlova, B. The Rs10830963 Polymorphism of the MTNR1B Gene: Association With Abnormal Glucose, Insulin and C-Peptide Kinetics. Front. Endocrinol. 2022, 13, 868364. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Jin, Y. Effects of MTNR1B Genetic Variants on the Risk of Type 2 Diabetes Mellitus: A Meta-Analysis. Mol. Genet. Genomic Med. 2019, 7, e611. [Google Scholar] [CrossRef]
- Mousavi, R.; Alizadeh, M.; Asghari Jafarabadi, M.; Heidari, L.; Nikbakht, R.; Babaahmadi Rezaei, H.; Karandish, M. Effects of Melatonin and/or Magnesium Supplementation on Biomarkers of Inflammation and Oxidative Stress in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2022, 200, 1010–1019. [Google Scholar] [CrossRef]
- Romo-Nava, F.; Alvarez-Icaza González, D.; Fresán-Orellana, A.; Saracco Alvarez, R.; Becerra-Palars, C.; Moreno, J.; Ontiveros Uribe, M.P.; Berlanga, C.; Heinze, G.; Buijs, R.M. Melatonin Attenuates Antipsychotic Metabolic Effects: An Eight-Week Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Clinical Trial. Bipolar Disord. 2014, 16, 410–421. [Google Scholar] [CrossRef]
- Agahi, M.; Akasheh, N.; Ahmadvand, A.; Akbari, H.; Izadpanah, F. Effect of Melatonin in Reducing Second-Generation Antipsychotic Metabolic Effects: A Double Blind Controlled Clinical Trial. Diabetes Metab. Syndr. 2018, 12, 9–15. [Google Scholar] [CrossRef]
- Modabbernia, A.; Heidari, P.; Soleimani, R.; Sobhani, A.; Roshan, Z.A.; Taslimi, S.; Ashrafi, M.; Modabbernia, M.J. Melatonin for Prevention of Metabolic Side-Effects of Olanzapine in Patients with First-Episode Schizophrenia: Randomized Double-Blind Placebo-Controlled Study. J. Psychiatr. Res. 2014, 53, 133–140. [Google Scholar] [CrossRef]
- Awni, N.; Ahmed, T.S.; Sarhat, E.R.; Ali, N.H.; Abass, K.S. Altered Serum Levels of Melatonin, Antioxidant Enzymes and Oxidative Stress in Individuals with Diabetes Mellitus Type 2. Rev. Latinoam. Hipertens. 2022, V17, 138–141. [Google Scholar] [CrossRef]
- Bach, A.G.; Mühlbauer, E.; Peschke, E. Adrenoceptor Expression and Diurnal Rhythms of Melatonin and Its Precursors in the Pineal Gland of Type 2 Diabetic Goto-Kakizaki Rats. Endocrinology 2010, 151, 2483–2493. [Google Scholar] [CrossRef]
- Mihailović, M.; Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Grdović, N.; Vidaković, M. The Influence of Plant Extracts and Phytoconstituents on Antioxidant Enzymes Activity and Gene Expression in the Prevention and Treatment of Impaired Glucose Homeostasis and Diabetes Complications. Antioxidants 2021, 10, 480. [Google Scholar] [CrossRef]
- Hurt, E.M.; Thomas, S.B.; Peng, B.; Farrar, W.L. Molecular Consequences of SOD2 Expression in Epigenetically Silenced Pancreatic Carcinoma Cell Lines. Br. J. Cancer 2007, 97, 1116–1123. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mishra, M. Epigenetic Regulation of Redox Signaling in Diabetic Retinopathy: Role of Nrf2. Free Radic. Biol. Med. 2017, 103, 155–164. [Google Scholar] [CrossRef]
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low Antioxidant Enzyme Gene Expression in Pancreatic Islets Compared with Various Other Mouse Tissues. Free Radic. Biol. Med. 1996, 20, 463–466. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Reiter, R.J.; Asemi, Z. Melatonin Administration Lowers Biomarkers of Oxidative Stress and Cardio-Metabolic Risk in Type 2 Diabetic Patients with Coronary Heart Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 191–196. [Google Scholar] [CrossRef]
- She, M.; Deng, X.; Guo, Z.; Laudon, M.; Hu, Z.; Liao, D.; Hu, X.; Luo, Y.; Shen, Q.; Su, Z.; et al. NEU-P11, a Novel Melatonin Agonist, Inhibits Weight Gain and Improves Insulin Sensitivity in High-Fat/High-Sucrose-Fed Rats. Pharmacol. Res. 2009, 59, 248–253. [Google Scholar] [CrossRef]
- Ji, Z.-Z.; Xu, Y.-C. Melatonin Protects Podocytes from Angiotensin II-Induced Injury in an in Vitro Diabetic Nephropathy Model. Mol. Med. Rep. 2016, 14, 920–926. [Google Scholar] [CrossRef]
- Santos-Ledo, A.; de Luxán-Delgado, B.; Caballero, B.; Potes, Y.; Rodríguez-González, S.; Boga, J.A.; Coto-Montes, A.; García-Macia, M. Melatonin Ameliorates Autophagy Impairment in a Metabolic Syndrome Model. Antioxidants 2021, 10, 796. [Google Scholar] [CrossRef]
- Peschke, E.; Schucht, H.; Mühlbauer, E. Long-Term Enteral Administration of Melatonin Reduces Plasma Insulin and Increases Expression of Pineal Insulin Receptors in Both Wistar and Type 2-Diabetic Goto-Kakizaki Rats. J. Pineal Res. 2010, 49, 373–381. [Google Scholar] [CrossRef]
- Peschke, E.; Bähr, I.; Mühlbauer, E. Melatonin and Pancreatic Islets: Interrelationships between Melatonin, Insulin and Glucagon. Int. J. Mol. Sci. 2013, 14, 6981–7015. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jung, H.S.; Kwon, M.J.; Jang, J.E.; Kim, T.N.; Lee, S.H.; Kim, M.-K.; Park, J.H. Melatonin Protects INS-1 Pancreatic β-Cells from Apoptosis and Senescence Induced by Glucotoxicity and Glucolipotoxicity. Islets 2020, 12, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Costes, S.; Boss, M.; Thomas, A.P.; Matveyenko, A.V. Activation of Melatonin Signaling Promotes β-Cell Survival and Function. Mol. Endocrinol. 2015, 29, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Delpino, F.M.; Figueiredo, L.M.; Nunes, B.P. Effects of Melatonin Supplementation on Diabetes: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Clin. Nutr. 2021, 40, 4595–4605. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-J.; Kim, K.; Kim, S.-Y.; Kim, J.-H.; Suh, C.; Son, B.-C.; Lee, C.-K.; Choi, J. Effects of Shift Work on Abdominal Obesity among 20-39-Year-Old Female Nurses: A 5-Year Retrospective Longitudinal Study. Ann. Occup. Environ. Med. 2016, 28, 69. [Google Scholar] [CrossRef] [PubMed]
- Suwazono, Y.; Dochi, M.; Sakata, K.; Okubo, Y.; Oishi, M.; Tanaka, K.; Kobayashi, E.; Nogawa, K. Shift Work Is a Risk Factor for Increased Blood Pressure in Japanese Men: A 14-Year Historical Cohort Study. Hypertension 2008, 52, 581–586. [Google Scholar] [CrossRef]
- Kubo, T.; Fujino, Y.; Nakamura, T.; Kunimoto, M.; Tabata, H.; Tsuchiya, T.; Kadowaki, K.; Odoi, H.; Oyama, I.; Matsuda, S. An Industry-Based Cohort Study of the Association between Weight Gain and Hypertension Risk among Rotating Shift Workers. J. Occup. Environ. Med. 2013, 55, 1041–1045. [Google Scholar] [CrossRef]
- Ferguson, J.M.; Costello, S.; Neophytou, A.M.; Balmes, J.R.; Bradshaw, P.T.; Cullen, M.R.; Eisen, E.A. Night and Rotational Work Exposure within the Last 12 Months and Risk of Incident Hypertension. Scand. J. Work Environ. Health 2019, 45, 256–266. [Google Scholar] [CrossRef]
- Vimalananda, V.G.; Palmer, J.R.; Gerlovin, H.; Wise, L.A.; Rosenzweig, J.L.; Rosenberg, L.; Ruiz Narváez, E.A. Night-Shift Work and Incident Diabetes among African-American Women. Diabetologia 2015, 58, 699–706. [Google Scholar] [CrossRef]
- Hansen, A.B.; Stayner, L.; Hansen, J.; Andersen, Z.J. Night Shift Work and Incidence of Diabetes in the Danish Nurse Cohort. Occup. Environ. Med. 2016, 73, 262–268. [Google Scholar] [CrossRef]
- Chen, J.-D.; Lin, Y.-C.; Hsiao, S.-T. Obesity and High Blood Pressure of 12-Hour Night Shift Female Clean-Room Workers. Chronobiol. Int. 2010, 27, 334–344. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Liu, C.-S.; Hu, K.-C.; Cheng, Y.-F.; Karhula, K.; Härmä, M. Night Shift Work and the Risk of Metabolic Syndrome: Findings from an 8-Year Hospital Cohort. PLoS ONE 2021, 16, e0261349. [Google Scholar] [CrossRef]
- Karlsson, B.H.; Knutsson, A.K.; Lindahl, B.O.; Alfredsson, L.S. Metabolic Disturbances in Male Workers with Rotating Three-Shift Work. Results of the WOLF Study. Int. Arch. Occup. Environ. Health 2003, 76, 424–430. [Google Scholar] [CrossRef]
- Kawada, T.; Otsuka, T. Effect of Shift Work on the Development of Metabolic Syndrome after 3 Years in Japanese Male Workers. Arch. Environ. Occup. Health 2014, 69, 55–61. [Google Scholar] [CrossRef]
- Guo, Y.; Rong, Y.; Huang, X.; Lai, H.; Luo, X.; Zhang, Z.; Liu, Y.; He, M.; Wu, T.; Chen, W. Shift Work and the Relationship with Metabolic Syndrome in Chinese Aged Workers. PLoS ONE 2015, 10, e0120632. [Google Scholar] [CrossRef]
 Activation;
Activation;  Suppression.
Suppression.
 Activation;
Activation;  Suppression.
Suppression.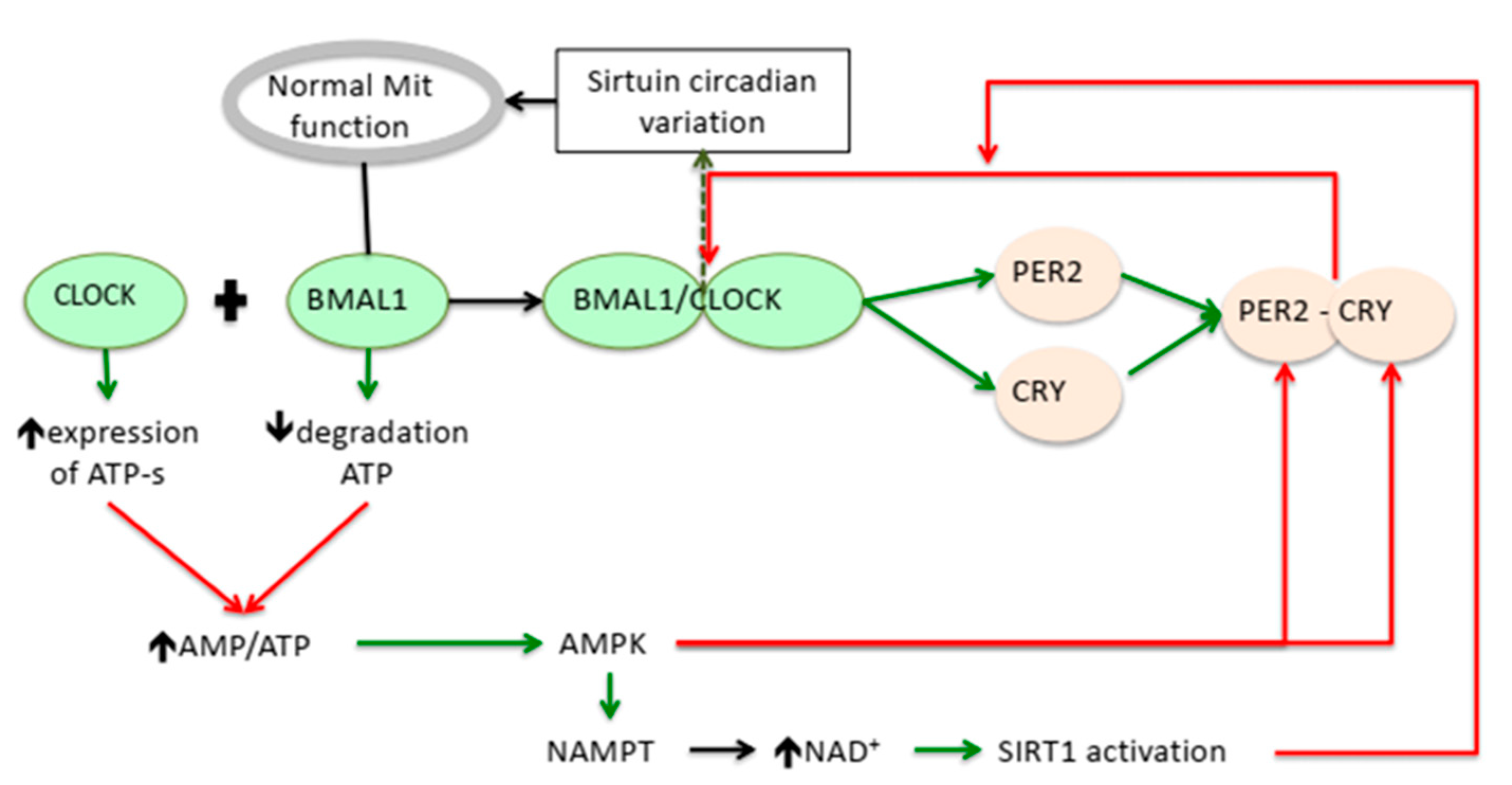


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohor, S.; Mandanach, C.; Maftei, A.; Zugravu, C.A.; Oțelea, M.R. Impaired Melatonin Secretion, Oxidative Stress and Metabolic Syndrome in Night Shift Work. Antioxidants 2023, 12, 959. https://doi.org/10.3390/antiox12040959
Hohor S, Mandanach C, Maftei A, Zugravu CA, Oțelea MR. Impaired Melatonin Secretion, Oxidative Stress and Metabolic Syndrome in Night Shift Work. Antioxidants. 2023; 12(4):959. https://doi.org/10.3390/antiox12040959
Chicago/Turabian StyleHohor, Sorina, Cristina Mandanach, Andreea Maftei, Corina Aurelia Zugravu, and Marina Ruxandra Oțelea. 2023. "Impaired Melatonin Secretion, Oxidative Stress and Metabolic Syndrome in Night Shift Work" Antioxidants 12, no. 4: 959. https://doi.org/10.3390/antiox12040959
APA StyleHohor, S., Mandanach, C., Maftei, A., Zugravu, C. A., & Oțelea, M. R. (2023). Impaired Melatonin Secretion, Oxidative Stress and Metabolic Syndrome in Night Shift Work. Antioxidants, 12(4), 959. https://doi.org/10.3390/antiox12040959







