A Rodent Model of Human-Dose-Equivalent 5-Fluorouracil: Toxicity in the Liver, Kidneys, and Lungs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
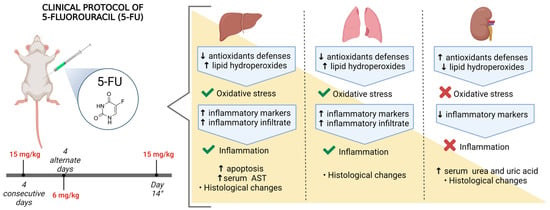
2.2. Experimental Protocol
2.3. Weights and Histological Analysis of Organs
2.3.1. Analysis of Liver Histology
2.3.2. Analysis of Kidney Histology
2.3.3. Analysis of Lung Histology
2.4. Evaluation of Apoptosis (TUNEL)
2.5. Oxidative Stress Markers
2.5.1. Reduced Glutathione (GSH) Measurements
2.5.2. Enzymatic Activity of Glutathione-S-Transferase (GST)
2.5.3. Catalase (CAT) Enzymatic Activity
2.5.4. Enzymatic Activity of Superoxide Dismutase (SOD)
2.5.5. Levels of Lipid Hydroperoxides (LOOH)
2.5.6. Total Protein Concentration
2.6. Analysis of Inflammatory Process Markers
2.6.1. Myeloperoxidase (MPO) Enzymatic Activity
2.6.2. Activity of the Enzyme N-Acetyl-Glucosaminidase (NAG)
2.6.3. Quantification of Nitric Oxide (NO)
2.6.4. Measurements of Interleukin Levels
2.7. Functional Analyses of Liver and Kidney
2.8. Statistical Analysis
3. Results
3.1. Treatment with 5-FU Promotes Morphological and Morphometric Alterations in the Liver, Kidney, and Lung
3.2. 5-FU Treatment Increases Cell Death in the Liver
3.3. 5-FU Treatment Modulates Oxidative Stress Markers
3.4. Inflammatory Response Was Modulated by 5-FU Treatment
3.5. 5-FU Treatment Impacts on the Function of the Liver and Kidneys
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- GLOBOCAN New Global Cancer Data. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 2 April 2023).
- National Cancer Institute Global Cancer Research. Available online: https://www.cancer.gov/research/areas/global-health#:~:text=Challenges%20in%20Global%20Cancer%20Research,-Addressing%20the%20rising&text=Drugs%20and%20vaccines%20are%20sometimes,less%20effective%20and%20more%20costly. (accessed on 31 March 2023).
- Xie, P.; Mo, J.L.; Liu, J.H.; Li, X.; Tan, L.M.; Zhang, W.; Zhou, H.H.; Liu, Z.Q. Pharmacogenomics of 5-Fluorouracil in Colorectal Cancer: Review and Update. Cell. Oncol. 2020, 43, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lucero-Prisno, D.E.; Zhang, L.; Xu, W.; Wong, S.H.; Ng, S.C.; Wong, M.C.S. Updated Epidemiology of Gastrointestinal Cancers in East Asia. Nat. Rev. Gastroenterol. Hepatol. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Model List of Essential Medicines. Available online: http://www.who.int/medicines/organization/par/edl/expcom13/eml13_en.pdf (accessed on 18 June 2020).
- de Miranda, J.A.L.; Barreto, J.E.F.; Martins, D.S.; de Souza Pimentel, P.V.; Da Silva Costa, D.V.; E Silva, R.R.; de Souza, L.K.M.; de Carvalho Lima, C.N.; Rocha, J.A.; de Freitas, A.P.F.; et al. Protective Effect of Cashew Gum (Anacardium occidentale L.) on 5-Fluorouracil-Induced Intestinal Mucositis. Pharmaceuticals 2019, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Genc, S.; Taghizadehghalehjoughi, A.; Yeni, Y.; Jafarizad, A.; Hacimuftuoglu, A.; Nikitovic, D.; Docea, A.O.; Mezhuev, Y.; Tsatsakis, A. Fe3O4 Nanoparticles in Combination with 5-FU Exert Antitumor Effects Superior to Those of the Active Drug in a Colon Cancer Cell Model. Pharmaceutics 2023, 15, 245. [Google Scholar] [CrossRef]
- Dongsar, T.T.; Dongsar, T.S.; Gupta, N.; Almalki, W.H.; Sahebkar, A.; Kesharwani, P. Emerging Potential of 5-Fluorouracil-Loaded Chitosan Nanoparticles in Cancer Therapy. J. Drug Deliv. Sci. Technol. 2023, 82, 104371. [Google Scholar] [CrossRef]
- Khafagy, N.; Hassen, S.; Ezat, F.; Elhawatky, A. Comparing the Efficacy of Micro-Needling Alone versus Micro-Needling with Topical 5-Fluorouracil in Treating Stable Non-Segmental Vitiligo. Egypt J. Hosp. Med. 2023, 90, 1960–1967. [Google Scholar] [CrossRef]
- Tong, Z.; Cheng, M.; Yu, Y.; Yu, J.; Yin, Y.; Liu, J.; Zhang, S.; Jiang, S.; Dong, M. Correlation between Pharmacokinetic Parameters of 5-Fluorouracil and Related Metabolites and Adverse Reactions in East-Asian Patients with Advanced Colorectal Cancer. Cancer Chemother. Pharmacol. 2022, 89, 323–330. [Google Scholar] [CrossRef]
- Saif, M.W.; Choma, A.; Salamone, S.J.; Chu, E. Pharmacokinetically Guided Dose Adjustment of 5-Fluorouracil: A Rational Approach to Improving Therapeutic Outcomes. J. Natl. Cancer Inst. 2009, 101, 1543–1552. [Google Scholar] [CrossRef]
- Wigle, T.J.; Tsvetkova, E.V.; Welch, S.A.; Kim, R.B. DPYD and Fluorouracil-Based Chemotherapy: Mini Review and Case Report. Pharmaceutics 2019, 11, 199. [Google Scholar] [CrossRef]
- Yu, Q.Q.; Zhang, H.; Guo, Y.; Han, B.; Jiang, P. The Intestinal Redox System and Its Significance in Chemotherapy-Induced Intestinal Mucositis. Oxidative Med. Cell. Longev. 2022, 2022, 7255497. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L. Novel Strategies to Improve the Anticancer Action of 5-Fluorouracil by Using Drug Delivery Systems. Molecules 2008, 13, 2340–2369. [Google Scholar] [CrossRef] [PubMed]
- AlDosari, S.M.; Banawas, S.; Ghafour, H.S.; Tlili, I.; Le, Q.H. Drug Release Using Nanoparticles in the Cancer Cells on 2-D Materials in Order to Target Drug Delivery: A Numerical Simulation via Molecular Dynamics Method. Eng. Anal. Bound. Elements 2023, 148, 34–40. [Google Scholar] [CrossRef]
- de Andrade, G.L.; da Silva Souza, B.; Araújo, D.D.; de Freitas, C.D.T.; de Oliveira, J.S. Protective Effect of Plumeria Pudica Latex Proteins on Intestinal Mucositis Induced by 5-Fluorouracil. Mini-Rev. Med. Chem. 2023, 23, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Fumoto, S.; Nishi, J.; Nakashima, M.; Sasaki, H.; Nakamura, J.; Nishida, K. Absorption and Distribution Characteristics of 5-Fluorouracil (5-FU) after an Application to the Liver Surface in Rats in Order to Reduce Systemic Side Effects. Biol. Pharm. Bull. 2008, 31, 1049–1052. [Google Scholar] [CrossRef]
- Coronado-Cerda, E.E.; Franco-Molina, M.A.; Mendoza-Gamboa, E.; Prado-García, H.; Rivera-Morales, L.G.; Zapata-Benavides, P.; Rodríguez-Salazar, M.D.C.; Caballero-Hernandez, D.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C. In Vivo Chemoprotective Activity of Bovine Dialyzable Leukocyte Extract in Mouse Bone Marrow Cells against Damage Induced by 5-Fluorouracil. J. Immunol. Res. 2016, 2016, 6942321. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, D.; Roberts, L.A.; Marchesi, J.R.; Kinross, J.M. Gut Microbiota Modulation of Efficacy and Toxicity of Cancer Chemotherapy and Immunotherapy. Gastroenterology 2022, 164, 198–213. [Google Scholar] [CrossRef]
- Fabiano, L.C.; Da Silva, M.C.; Da Costa, K.C.; De Freitas, P.L.Z.; Neves, C.Q.; Borges, S.C.; Breithaupt-Faloppa, A.C.; Buttow, N.C. 5-Fluorouracil Administration Using Clinical Treatment Protocol Causes Mucositis in the Ileum in Wistar Rats. Res. Soc. Dev. 2020, 9, e1529119661. [Google Scholar] [CrossRef]
- Kuan, H.Y.; Smith, D.E.; Ensminger, W.D.; Knol, J.A.; DeRemer, S.J.; Yang, Z.; Stetson, P.L. Regional Pharmacokinetics of 5-Fluorouracil in Dogs: Role of the Liver, Gastrointestinal Tract, and Lungs. Cancer Res. 1998, 58, 1688–1694. [Google Scholar] [PubMed]
- Corbacioglu, S.; Jabbour, E.J.; Mohty, M. Risk Factors for Development of and Progression of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol. Blood Marrow Transplant. 2019, 25, 1271–1280. [Google Scholar] [CrossRef]
- Alessandrino, F.; Qin, L.; Cruz, G.; Sahu, S.; Rosenthal, M.H.; Meyerhardt, J.A.; Shinagare, A.B. 5-Fluorouracil Induced Liver Toxicity in Patients with Colorectal Cancer: Role of Computed Tomography Texture Analysis as a Potential Biomarker. Abdom. Radiol. 2019, 44, 3099–3106. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Maghrabi, I.A. Camel Milk Ameliorates 5-Fluorouracil-Induced Renal Injury in Rats: Targeting MAPKs, NF-ΚB and PI3K/Akt/ENOS Pathways. Cell. Physiol. Biochem. 2018, 46, 1628–1642. [Google Scholar] [CrossRef] [PubMed]
- Südhoff, T.; Enderle, M.D.; Pahlke, M.; Petz, C.; Teschendorf, C.; Graeven, U.; Schmiegel, W. 5-Fluorouracil Induces Arterial Vasocontractions. Ann. Oncol. 2004, 15, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Akindele, A.J.; Oludadepo, G.O.; Amagon, K.I.; Singh, D.; Osiagwu, D.D. Protective Effect of Carvedilol Alone and Coadministered with Diltiazem and Prednisolone on Doxorubicin and 5-Fluorouracil-Induced Hepatotoxicity and Nephrotoxicity in Rats. Pharmacol. Res. Perspect. 2018, 6, e00381. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Nicco, C.; Chéreau, C.; Laurent, A.; Weill, B.; Goldwasser, F.; Batteux, F. Improvement of the Therapeutic Index of Anticancer Drugs by the Superoxide Dismutase Mimic Mangafodipir. J. Natl. Cancer Inst. 2006, 98, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Riul, S.; Aguillar, O.M. Quimioterapia Antineoplásica: Revisão Da Literatura. Rev. Min. Enferm. 1999, 3, 60–67. [Google Scholar]
- Sommer, J.; Mahli, A.; Freese, K.; Schiergens, T.S.; Kuecuekoktay, F.S.; Teufel, A.; Thasler, W.E.; Müller, M.; Bosserhoff, A.K.; Hellerbrand, C. Analysis of Molecular Mechanisms of 5-Fluorouracil-Induced Steatosis and Inflammation in Vitro and in Mice. Oncotarget 2017, 8, 13059–13072. [Google Scholar] [CrossRef]
- Kim, J.J.; Tannock, I.F. Repopulation of Cancer Cells during Therapy: An Important Cause of Treatment Failure. Nat. Rev. Cancer 2005, 5, 516–525. [Google Scholar] [CrossRef]
- Eurofarma Flusan (Fluorouracila)—Bula Para o Profissional Da Saúde 2015, 1–14. Available online: https://eurofarma.com.br/produtos/flusan (accessed on 25 April 2023).
- Marcelino, M.C.D.S.; Magalhães, W.V.; Fonseca, F.L.A.; Nucci, R.A.B.; Maifrino, L.B.M. Effects of Resistance Training on Kidney Morphology of Aged Ovariectomized Rats. Acta Histochem. 2020, 122, 151613. [Google Scholar] [CrossRef]
- Borges, S.C.; Ferreira, P.E.B.; da Silva, L.M.; de Paula Werner, M.F.; Irache, J.M.; Cavalcanti, O.A.; Buttow, N.C. Evaluation of the Treatment with Resveratrol-Loaded Nanoparticles in Intestinal Injury Model Caused by Ischemia and Reperfusion. Toxicology 2018, 396–397, 13–22. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Tiwari, V.; Kuhad, A.; Chopra, K. Emblica Officinalis Corrects Functional, Biochemical and Molecular Deficits in Experimental Diabetic Neuropathy by Targeting the Oxido-Nitrosative Stress Mediated Inflammatory Cascade. Phytother. Res. 2011, 25, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.V.S.; Bon-Frauches, A.C.; Silva, A.M.H.P.; Lima-Júnior, R.C.P.; Martins, C.S.; Leitão, R.F.C.; Freitas, G.B.; Castelucci, P.; Bolick, D.T.; Guerrant, R.L.; et al. 5-Fluorouracil Induces Enteric Neuron Death and Glial Activation during Intestinal Mucositis via a S100B-RAGE-NFκB-Dependent Pathway. Sci. Rep. 2019, 9, 665. [Google Scholar] [CrossRef]
- Leocádio, P.C.L.; Antunes, M.M.; Teixeira, L.G.; Leonel, A.J.; Alvarez-Leite, J.I.; Machado, D.C.C.; Generoso, S.V.; Cardoso, V.N.; Correia, M.I.T.D. L-Arginine Pretreatment Reduces Intestinal Mucositis as Induced by 5-FU in Mice. Nutr. Cancer 2015, 67, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Gelen, V.; Şengül, E.; Yıldırım, S.; Senturk, E.; Tekin, S.; Kükürt, A. The Protective Effects of Hesperidin and Curcumin on 5-Fluorouracil–Induced Nephrotoxicity in Mice. Environ. Sci. Pollut. Res. 2021, 28, 47046–47055. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; An, L.; Yan, D.; Hiroshi, M.; Ding, W.; Zhang, M.; Xu, G.; Sun, Y.; Yuan, G.; Wang, M.; et al. Combined Antitumor Effects of P-5m Octapeptide and 5-fluorouracil on a Murine Model of H22 Hepatoma Ascites. Exp. Ther. Med. 2018, 16, 1586–1592. [Google Scholar] [CrossRef]
- Cottone, L.; Capobianco, A.; Gualteroni, C.; Perrotta, C.; Bianchi, M.E.; Rovere-Querini, P.; Manfredi, A.A. 5-Fluorouracil Causes Leukocytes Attraction in the Peritoneal Cavity by Activating Autophagy and HMGB1 Release in Colon Carcinoma Cells. Int. J. Cancer 2015, 136, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Gupta, S.K.; Verma, M. Dihydropyrimidine Dehydrogenase in the Metabolism of the Anticancer Drugs. Cancer Chemother. Pharmacol. 2019, 84, 1157–1166. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Funakoshi, T.; Horimatsu, T.; Miyamoto, S.; Matsubara, T.; Yanagita, M.; Nakagawa, S.; Yonezawa, A.; Matsubara, K.; Muto, M. Accumulation of Alpha-Fluoro-Beta-Alanine and Fluoro Mono Acetate in a Patient with 5-Fluorouracil-Associated Hyperammonemia. Cancer Chemother. Pharmacol. 2017, 79, 629–633. [Google Scholar] [CrossRef]
- Poole, C.; Gardiner, J.; Twelves, C.; Johnston, P.; Harper, P.; Cassidy, J.; Monkhouse, J.; Banken, L.; Weidekamm, E.; Reigner, B. Effect of Renal Impairment on the Pharmacokinetics and Tolerability of Capecitabine (Xeloda) in Cancer Patients. Cancer Chemother. Pharmacol. 2002, 49, 225–234. [Google Scholar] [CrossRef]
- Pinedo, H.M.; Peters, G.F. Fluorouracil: Biochemistry and Pharmacology. Am. Soc. Clin. Oncol. 1988, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Soong, R.; Diasio, R.B. Advances and Challenges in Fluoropyrimidine Pharmacogenomics and Pharmacogenetics. Pharmacogenomics 2005, 6, 835–847. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The Current State of Serum Biomarkers of Hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative Stress Is Progressively Enhanced with Advancing Stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Conklin, K.A. Chemotherapy-Associated Oxidative Stress: Impact on Chemotherapeutic Effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef]
- Akpinar, B.; Bracht, E.V.; Reijnders, D.; Safarikova, B.; Jelinkova, I.; Grandien, A.; Hyrslova Vaculova, A.; Zhivotovsky, B.; Olsson, M. 5-Fluorouracil-Induced RNA Stress Engages a TRAIL-DISC-Dependent Apoptosis Axis Facilitated by P53. Oncotarget 2015, 6, 43679–43697. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.D. Reactive Oxygen Species and Programmed Cell Death. Trends Biochem. Sci. 1996, 21, 83–86. [Google Scholar] [CrossRef]
- Nigam, S.; Schewe, T. Phospholipase A2s and Lipid Peroxidation. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2000, 1488, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Olino, K.; Gleisner, A.L.; Torbenson, M.; Schulick, R.; Choti, M.A. Preoperative Chemotherapy for Colorectal Liver Metastases: Impact on Hepatic Histology and Postoperative Outcome. J. Gastrointest. Surg. 2007, 11, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, R.; Hijona, E.; Emparanza, J.; Alústiza, J.M.; Hijona, L.; Macarulla, M.T.; Portillo, M.P.; Herreros-Villanueva, M.; Beguiristain, A.; Arenas, J.; et al. Effect of Neoadjuvant Chemotherapy in Hepatic Steatosis. Chemotherapy 2012, 58, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.; Edal, A.L.; Madsen, E.L.; Fenger, C.; Poulsen, M.R.; Petersen, O.F. Reversible Hepatic Steatosis in Patients Treated with Interferon Alfa-2A and 5-fluorouracil. Cancer 1995, 75, 2592–2596. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Hellerbrand, C. Non-Alcoholic Fatty Liver Disease, Obesity and the Metabolic Syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 451–496. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.E. The Biological Effects of Interleukin 6. Med. Res. Rev. 1996, 16, 87–109. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, Y.; Che, X.; Hou, K.; Zhang, C.; Li, C.; Wen, T.; Wang, S.; Cheng, Y.; Liu, Y.; et al. 5-FU-Induced Upregulation of Exosomal PD-L1 Causes Immunosuppression in Advanced Gastric Cancer Patients. Front. Oncol. 2020, 10, 492. [Google Scholar] [CrossRef]
- Matthews, V.B.; Allen, T.L.; Risis, S.; Chan, M.H.S.; Henstridge, D.C.; Watson, N.; Zaffino, L.A.; Babb, J.R.; Boon, J.; Meikle, P.J.; et al. Interleukin-6-Deficient Mice Develop Hepatic Inflammation and Systemic Insulin Resistance. Diabetologia 2010, 53, 2431–2441. [Google Scholar] [CrossRef]
- Chun, Y.S.; Laurent, A.; Maru, D.; Vauthey, J.N. Management of Chemotherapy-Associated Hepatotoxicity in Colorectal Liver Metastases. Lancet Oncol. 2009, 10, 278–286. [Google Scholar] [CrossRef]
- Brunt, E.M. Nonalcoholic Steatohepatitis. Liver Dis. 2004, 24, 20. [Google Scholar] [CrossRef][Green Version]
- Brunt, E.M. Grading and Staging the Histopathological Lesions of Chronic Hepatitis: The Knodell Histology Activity Index and Beyond. Hepatology 2000, 31, 241–246. [Google Scholar] [CrossRef]
- Brancatelli, G.; Furlan, A.; Calandra, A.; Dioguardi Burgio, M. Hepatic Sinusoidal Dilatation. Abdom. Radiol. 2018, 43, 2011–2022. [Google Scholar] [CrossRef]
- Neufeld, T.P.; Edgar, B.A. Connections between Growth and the Cell Cycle. Curr. Opin. Cell Biol. 1998, 10, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Arafah, A.; Rehman, M.U.; Ahmad, A.; Alkharfy, K.M.; Alqahtani, S.; Jan, B.L.; Almatroudi, N.M. Myricetin (3,3′,4′,5,5′,7-Hexahydroxyflavone) Prevents 5-Fluorouracil-Induced Cardiotoxicity. ACS Omega 2022, 7, 4514–4524. [Google Scholar] [CrossRef] [PubMed]
- Backus, H.H.J.; Dukers, D.F.; van Groeningen, C.J.; Vos, W.; Bloemena, E.; Wouters, D.; van Riel, J.M.G.H.; Smid, K.; Giaccone, G.; Pinedo, H.M.; et al. 5-Fluorouracil Induced Fas Upregulation Associated with Apoptosis in Liver Metastases of Colorectal Cancer Patients. Ann. Oncol. 2001, 12, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Kaplowitz, N.; Fernández-Checa, J.C. Mitochondrial Glutathione: Features, Regulation and Role in Disease. Biochim. Biophys. Acta. 2014, 1830, 3317–3328. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, F.R.; Shang, J.Y.; Liu, Y.Y.; Lv, X.F.; Yuan, J.N.; Zhang, T.T.; Li, K.; Lin, X.C.; Liu, X.; et al. Renal Inhibition of MiR-181a Ameliorates 5-Fluorouracil-Induced Mesangial Cell Apoptosis and Nephrotoxicity. Cell Death Dis. 2018, 9, 610. [Google Scholar] [CrossRef]
- Allen, M.J.; Vaughan, M.; Webb, A.; Johnston, S.; Savage, P.; Eisen, T.; Bate, S.; Moore, J.; Ahern, R.; Gore, M.E. Protracted Venous Infusion 5-Fluorouracil in Combination with Subcutaneous Interleukin-2 and Alpha-Interferon in Patients with Metastatic Renal Cell Cancer: A Phase II Study. Br. J. Cancer 2000, 83, 980–985. [Google Scholar] [CrossRef]
- Matsushita, K.; Toyoda, T.; Yamada, T.; Morikawa, T.; Ogawa, K. Comprehensive Expression Analysis of MRNA and MicroRNA for the Investigation of Compensatory Mechanisms in the Rat Kidney after Unilateral Nephrectomy. J. Appl. Toxicol. 2020, 40, 1373–1383. [Google Scholar] [CrossRef]
- Thomson, S.C.; Deng, A.; Bao, D.; Satriano, J.; Blantz, R.C.; Vallon, V. Ornithine Decarboxylase, Kidney Size, and the Tubular Hypothesis of Glomerular Hyperfiltration in Experimental Diabetes. J. Clin. Investig. 2001, 107, 217–224. [Google Scholar] [CrossRef][Green Version]
- Koseki, J.; Matsui, H.; Konno, M.; Nishida, N.; Kawamoto, K.; Kano, Y.; Mori, M.; Doki, Y.; Ishii, H. A Trans-Omics Mathematical Analysis Reveals Novel Functions of the Ornithine Metabolic Pathway in Cancer Stem Cells. Sci. Rep. 2016, 6, 20726. [Google Scholar] [CrossRef]
- Tobar, A.; Ori, Y.; Benchetrit, S.; Milo, G.; Herman-Edelstein, M.; Zingerman, B.; Lev, N.; Gafter, U.; Chagnac, A. Proximal Tubular Hypertrophy and Enlarged Glomerular and Proximal Tubular Urinary Space in Obese Subjects with Proteinuria. PLoS ONE 2013, 8, e75547. [Google Scholar] [CrossRef]
- Baldelomar, E.J.; Charlton, J.R.; Beeman, S.C.; Bennett, K.M. Measuring Rat Kidney Glomerular Number and Size in Vivo with MRI. Am. J. Physiol. Ren. Physiol. 2018, 314, F399–F406. [Google Scholar] [CrossRef]
- Brenner, B.M.; Garcia, D.L.; Anderson, S. Glomeruli and Blood Pressure: Less of One, More the Other? Am. J. Hypertens. 1988, 1, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Assayag, M.; Rouvier, P.; Gauthier, M.; Costel, G.; Cluzel, P.; Mercadal, L.; Deray, G.; Isnard Bagnis, C. Renal Failure during Chemotherapy: Renal Biopsy for Assessing Subacute Nephrotoxicity of Pemetrexed. BMC Cancer 2017, 17, 770. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Harshil, B.; Ishwarlal, J. Renal Function Tests; StatPearls Publishing LLC: Tampa, FL, USA, 2020; p. 5. [Google Scholar] [CrossRef]
- Slack, A.; Yeoman, A.; Wendon, J. Renal Dysfunction in Chronic Liver Disease. Crit. Care 2010, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Takabatake, T.; Ohta, H.; Ishida, Y.; Hara, H.; Ushiogi, Y.; Hattori, N. Low Serum Creatinine Levels in Severe Hepatic Disease. Arch. Intern. Med. 1988, 148, 1313–1315. [Google Scholar] [CrossRef]
- Moore, C.L.; Savenka, A.V.; Basnakian, A.G. Tunel Assay: A Powerful Tool for Kidney Injury Evaluation. Int. J. Mol. Sci. 2021, 22, 412. [Google Scholar] [CrossRef]
- Lopes, A.J.; Noronha, A.J.; Mafort, T.T. Mecanismos de Defesa Do Sistema Respiratório. Rev. Hosp. UniveRsitáRio Pedro Ernesto UeRJ 2010, 9, 10–16. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kode, A. Oxidant and Antioxidant Balance in the Airways and Airway Diseases. Eur. J. Pharmacol. 2006, 533, 222–239. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and Mitophagy in Acute Kidney Injury. Autophagy 2022, 19, 401–414. [Google Scholar] [CrossRef]
- Larson-Casey, J.L.; Deshane, J.S.; Ryan, A.J.; Thannickal, V.J.; Carter, A.B. Macrophage Akt1 Kinase-Mediated Mitophagy Modulates Apoptosis Resistance and Pulmonary Fibrosis. Immunity 2016, 44, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Wildner, D.; Boxberger, F.; Wein, A.; Wolff, K.; Albrecht, H.; Männlein, G.; Janka, R.; Amann, K.; Siebler, J.; Hohenberger, W.; et al. Granulomatous Lung Disease Requiring Mechanical Ventilation Induced by a Single Application of Oxaliplatin-Based Chemotherapy for Colorectal Cancer: A Case Report. Case Rep. Oncol. Med. 2013, 2013, 683948. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; MacNee, W. Oxidative Stress and Regulation of GSH in Lung Inflammation. Eur. Respir. J. 2000, 16, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Dominguez, A.; Martinez, W.; Sanabria, F. Pulmonary Toxicity Due to 5-Fluorouracil (5-FU) Manifested as Diffuse Alveolar Hemorrhage: Case Report. Am. J. Respir. Crit. Care Med. 2018, 197, 2–4. [Google Scholar] [CrossRef]
- Bailey, S.A.; Zidell, R.H.; Perry, R.W. Relationships Between Organ Weight and Body/Brain Weight in the Rat: WhaT Is the Best Analytical Endpoint? Toxicol. Pathol. 2004, 32, 448–466. [Google Scholar] [CrossRef]
- Abuohashish, H.M.; Zaghloul, E.H.; El Sharkawy, A.S.; Abbas, E.M.; Ahmed, M.M.; Al-Rejaie, S.S. Pharmacological Effects of Marine-Derived Enterococcus Faecium EA9 against Acute Lung Injury and Inflammation in Cecal Ligated and Punctured Septic Rats. Biomed. Res. Int. 2021, 2021, 5801700. [Google Scholar] [CrossRef]
- Rojas, M.; Woods, C.R.; Mora, A.L.; Xu, J.; Brigham, K.L. Endotoxin-Induced Lung Injury in Mice: Structural, Functional, and Biochemical Responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, 333–341. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Liao, Z.; Gao, S.; Hua, L.; Ye, X.; Wang, Y.; Jiang, S.; Wang, N.; Zhou, D.; et al. PM 2.5 Induced Pulmonary Fibrosis in Vivo and in Vitro. Ecotoxicol. Environ. Saf. 2019, 171, 112–121. [Google Scholar] [CrossRef]
- Bulstrode, N.W.; Mudera, V.; McGrouther, D.A.; Grobbelaar, A.O.; Cambrey, A.D. 5-Fluorouracil Selectively Inhibits Collagen Synthesis. Plast. Reconstr. Surg. 2005, 116, 209–221. [Google Scholar] [CrossRef]
- Khaw, P.T.; Sherwood, M.B.; MacKay, S.L.D.; Rossi, M.J.; Schultz, G. Five-Minute Treatments with Fluorouracil, Floxuridine, and Mitomycin Have Long-Term Effects on Human Tenon’s Capsule Fibroblasts. Arch. Ophthalmol. 1992, 110, 1150–1154. [Google Scholar] [CrossRef]
- Occleston, N.L.; Daniels, J.T.; Tarnuzzer, R.W.; Sethi, K.K.; Alexander, R.A.; Bhattacharya, S.S.; Schultz, G.S.; Khaw, P.T. Single Exposures to Antiproliferatives: Long-Term Effects on Ocular Fibroblast Wound-Healing Behavior. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1998–2007. [Google Scholar]
- Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.W.; Seol, Y.J.; Zhang, Y.S.; Shin, S.R.; Zhao, L.; Aleman, J.; et al. Multi-Tissue Interactions in an Integrated Three-Tissue Organ-on-a-Chip Platform. Sci. Rep. 2017, 7, 8837. [Google Scholar] [CrossRef] [PubMed]
- Petak, I.; Tillman, D.M.; Houghton, J.A. P53 Dependence of Fas Induction and Acute Apoptosis in Response to 5-Fluorouracil-Leucovorin in Human Colon Carcinoma Cell Lines. Clin. Cancer Res. 2000, 6, 4432–4441. [Google Scholar] [PubMed]








| Analysis | C | 5-FU | p Value | |
|---|---|---|---|---|
| Liver | Liver relative weight (%) | 3.803 ± 0.1355 | 4.241 ± 0.1904 | 0.0851 |
| Sinusoidal diameter (μm2) | 5.562 ± 0.0734 | 6.022 ± 0.0975 | 0.0027 * | |
| Core area (μm2) | 34.74 ± 0.8656 | 38.21 ± 0.7748 | 0.0113 * | |
| Hepatocyte area (μm2) | 224.2 ± 4.641 | 261.4 ± 3.727 | <0.0001 * | |
| Kidney | Relative kidney weight (%) | 0.8786 ± 0.0331 | 0.9214 ± 0.0191 | 0.2834 |
| Area of the corpuscle (μm2) | 5674 ± 179.2 | 6959 ± 232.8 | 0.0009 * | |
| Area of the visceral layer (μm2) | 4302 ± 173.7 | 5052 ± 179.7 | 0.0111 * | |
| Bowman space (μm2) | 1372 ± 54.9 | 1907 ± 98.12 | 0.0005 * | |
| Number of corpuscles (mm2) | 126 ± 1.976 | 107.7 ± 2.032 | <0.0001 * | |
| External area PT (μm2) | 13.15 ± 0.3372 | 14.3 ± 0.5622 | 0.1036 | |
| Internal area PT (μm2) | 3.229 ± 0.121 | 3.428 ± 0.2261 | 0.4524 | |
| PT thickness (μm2) | 9.92 ± 0.2286 | 10.88 ± 0.3859 | 0.0545 | |
| External area DT (μm2) | 9.40 ± 8.405–9.524 | 10.31 ± 9.783–10.84 | 0.0006 * | |
| Internal area DT (μm2) | 3.102 ± 0.1058 | 3.456 ± 0.1027 | 0.0334 * | |
| DT thickness (μm2) | 5.862 ± 0.1464 | 6.857 ± 0.1495 | 0.0005 * | |
| Lung | Relative lung weight (%) | 0.57 ± 0.0377 | 0.8043 ± 0.0277 | 0.0003 * |
| Collagen type I (%/area) | 28,616 ± 2325 | 13,718 ± 813.2 | <0.0001 * | |
| Collagen type III (%/area) | 9677 ± 1115 | 3872 ± 300.4 | 0.0003 * | |
| Total collagen (%/area) | 37,813 ± 2566 | 18,175 ± 906.1 | <0.0001 * | |
| Hemorrhagic focus (mm2) | 0 ± 0 | 39.99 ± 13.55 | 0.0121 * | |
| Perivascular focal infiltrate (mm2) | 202.1 ± 77.22 | 1342 ± 383.1 | 0.0129 * | |
| Diffuse infiltrate in the parenchyma (mm2) | 13.57 ± −6.18–33.33 | 82.07± 26.25–137.9 | 0.0105 * | |
| Edema (μm2) | 305.4 ± 35.38 | 706 ± 154.2 | 0.0263 * | |
| Alveolar area (μm2) | 719.2 ± 50.19 | 531.4 ± 36.32 | 0.0104 * | |
| Thickness of the alveolar septum (μm2) | 10 ± 0.4817 | 12.54 ± 0.402 | 0.0016 * |
| Description | Score | Results (n = 7) | ||
|---|---|---|---|---|
| C | 5-FU | |||
| Steatosis | 0–33% of the lobes | 1 (mild) | 100% | 0% |
| 34–66% of the lobes | 2 (moderate) | 0% | 28.6% | |
| >66% of the lobes | 3 (severe) | 0% | 71.4% | |
| Inflammatory portal infiltrate | Absent | 0 | 28.6% | 0% |
| Focus on 1/3 of the portal tracts | 1 (mild) | 42.9% | 14.2% | |
| Focus on > 1/3 and < 2/3 of portal tracts | 2 (moderate) | 28.5% | 71.4% | |
| Focus on ≥ 2/3 of portal tracts | 3 (severe) | 0% | 14.3% | |
| Inflammatory lobular infiltrate | Absent | 0 | 14.3% | 0% |
| <2 foci per field | 1 (mild) | 85.7% | 57.1% | |
| 2–4 foci per field | 2 (moderate) | 0% | 42.9% | |
| >4 foci per field | 3 (severe) | 0% | 0% | |
| Analysis | C | 5-FU | p Value | |
|---|---|---|---|---|
| Liver | CAT (μmol/min/mg protein) | 0.054 ± 0.0059 | 0.0301 ± 0.0022 | 0.0025 * |
| SOD (U SOD/mg protein) | 1.032 ± 0.0724 | 0.8616 ± 0.0452 | 0.0696 | |
| GST (μmol/min/mg protein) | 0.0584 ± 0.0026 | 0.0501 ± 0.002 | 0.0267 * | |
| GSH (μg GSH/g tissue) | 738.8 ± 646.5–956.4 | 428.3 ± 291–479.6 | 0.0006 * | |
| LOOH (mmol/mg tissue) | 29.77 ± 27.23–31.7 | 32.98 ± 29.43–42.65 | 0.0262 * | |
| Kidney | CAT (μmol/min/mg protein) | 0.0543 ± 0.0136 | 0.1413 ± 0.0142 | 0.0008 * |
| SOD (U SOD/mg protein) | 0.7785 ± 0.0629 | 1.138 ± 0.0468 | 0.0006 * | |
| GST (μmol/min/mg protein) | 0.0315 ± 0.0034 | 0.0406 ± 0.0027 | 0.0558 | |
| GSH (μg GSH/g tissue) | 550.5 ± 32.4 | 388.1 ± 20.39 | 0.0011 * | |
| LOOH (mmol/mg tissue) | 66.5 ± 1.646 | 58.59 ± 1.779 | 0.0068 * | |
| Lung | CAT (μmol/min/mg protein) | 0.024 ± 0.0043 | 0.012 ± 0.0009 | 0.0180 * |
| SOD (U SOD/mg protein) | 1.773 ± 0.1024 | 1.388 ± 0.0781 | 0.0112 * | |
| GST (μmol/min/mg protein) | 0.0098 ± 0.0008 | 0.0066 ± 0.0004 | 0.0045 * | |
| GSH (μg GSH/g tissue) | 453.6 ± 413.2–555.1 | 287.9 ± 246.4–405.3 | 0.0175 * | |
| LOOH (mmol/mg tissue) | 11.25 ± 2.918 | 26.6 ± 1.134 | 0.0004 * | |
| Total protein (µg/mL) | 10.15 ± 0.3855 (n = 6) | 8.19 ± 0.4223 (n = 6) | 0.0064 * |
| Analysis | C | 5-FU | p Value | |
|---|---|---|---|---|
| Liver | MPO (mD.O./mg protein) | 0.1415 ± 0.0096 | 0.1818 ± 0.0139 | 0.0347 * |
| NAG (mD.O./mg protein) | 1.651 ± 0.1065 | 2.093 ± 0.1348 | 0.0245 * | |
| NO (μM/µL) | 95.92 ± 10.3 | 199.6 ± 22.44 | 0.0012 * | |
| IL-1β (pg/mL) | 4686 ± 585.2 | 6773 ± 568.9 | 0.0252 * | |
| IL-6 (pg/mL) | 13,126 ± 1599 | 7902 ± 1311 | 0.0266 * | |
| IL-10 (pg/mL) | 118.7 ± 44.02–334.2 | 174.4 ± 97.41–259.4 | 0.5350 | |
| Kidney | MPO (mD.O./mg protein) | 0.1437 ± 0.0098 | 0,1143 ± 0.0054 | 0.0215 * |
| NAG (mD.O./mg protein) | 3.53 ± 0.225 | 2.847 ± 0.1235 | 0.0208 * | |
| NO (μM/µL) | 101.2 ± 29.16 (n = 6) | 127.4 ± 42.229 (n = 6) | 0.6209 | |
| IL-1β (pg/mL) | 10,217 ± 863.9 | 10,511 ± 789.9 (n = 6) | 0.8088 | |
| IL-6 (pg/mL) | 112,944 ± 10,176–408,206 | 54,769 ± −12,492–27,669 | 0.5350 | |
| IL-10 (pg/mL) | 1795 ± 504 | 3112 ± 949.6 | 0.2441 | |
| Lung | MPO (mD.O./mg protein) | 0.2656 ± 0.0401 | 0.4043 ± 0.0223 | 0.0105 * |
| NAG (mD.O./mg protein) | 2.618 ± 0.3137 | 4.999 ± 0.605 | 0.0044 * | |
| NO (μM/µL) | 17.96 ± 3.816–158.1 | 235.3 ± 209.8–297.2 | 0.0006 * | |
| IL-6 (pg/mL) | 1682 ± 380.3 | 834.9 ± 75.82 | 0.0495 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.C.; Fabiano, L.C.; da Costa Salomão, K.C.; de Freitas, P.L.Z.; Neves, C.Q.; Borges, S.C.; de Souza Carvalho, M.d.G.; Breithaupt-Faloppa, A.C.; de Thomaz, A.A.; dos Santos, A.M.; et al. A Rodent Model of Human-Dose-Equivalent 5-Fluorouracil: Toxicity in the Liver, Kidneys, and Lungs. Antioxidants 2023, 12, 1005. https://doi.org/10.3390/antiox12051005
da Silva MC, Fabiano LC, da Costa Salomão KC, de Freitas PLZ, Neves CQ, Borges SC, de Souza Carvalho MdG, Breithaupt-Faloppa AC, de Thomaz AA, dos Santos AM, et al. A Rodent Model of Human-Dose-Equivalent 5-Fluorouracil: Toxicity in the Liver, Kidneys, and Lungs. Antioxidants. 2023; 12(5):1005. https://doi.org/10.3390/antiox12051005
Chicago/Turabian Styleda Silva, Mariana Conceição, Lilian Catarim Fabiano, Karile Cristina da Costa Salomão, Pedro Luiz Zonta de Freitas, Camila Quaglio Neves, Stephanie Carvalho Borges, Maria das Graças de Souza Carvalho, Ana Cristina Breithaupt-Faloppa, André Alexandre de Thomaz, Aline Mara dos Santos, and et al. 2023. "A Rodent Model of Human-Dose-Equivalent 5-Fluorouracil: Toxicity in the Liver, Kidneys, and Lungs" Antioxidants 12, no. 5: 1005. https://doi.org/10.3390/antiox12051005
APA Styleda Silva, M. C., Fabiano, L. C., da Costa Salomão, K. C., de Freitas, P. L. Z., Neves, C. Q., Borges, S. C., de Souza Carvalho, M. d. G., Breithaupt-Faloppa, A. C., de Thomaz, A. A., dos Santos, A. M., & Buttow, N. C. (2023). A Rodent Model of Human-Dose-Equivalent 5-Fluorouracil: Toxicity in the Liver, Kidneys, and Lungs. Antioxidants, 12(5), 1005. https://doi.org/10.3390/antiox12051005