Sulfated Undaria pinnatifida Polysaccharide Promotes Endocytosis of Nano-Calcium Oxalate Dihydrate by Repairing Subcellular Organelles in HK-2 Cells
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials and Apparatus
2.2. Experimental Methods
2.2.1. Cell Culture and Establishment of an Injury Model
- (1).
- Cell culture
- (2).
- Construction of a damage model
- (A).
- Normal control group: cultured in a serum-free medium;
- (B).
- Damage control group: the cells were injured with 2.8 mmol/L of oxalate solution for 3 h, removed by suction, and incubated with a serum-free medium for 12 h;
- (C).
- Polysaccharide repair group: after the cells were injured by 2.8 mmol/L of oxalate solution for 3 h, they were removed by suction and added to 60 μg/mL of UPP serum-free medium for repair and culture for 12 h.
2.2.2. Cytotoxicity of UPPs and Changes in Cell Viability, SOD, and MDA before and after UPP Repair
- (1).
- Cytotoxicity assay
- (2).
- Cell viability assay
- (3).
- SOD and MDA level detection
2.2.3. Cell Morphology and Cytoskeleton Observation
- (1).
- Observation of cell morphology
- (2).
- Cytoskeleton observation
2.2.4. Observation of the Cell Healing Rate
2.2.5. Detection of the ROS Expression and Mitochondrial Membrane Potential
- (1)
- ROS detection: 500 μL of DCFH-DA diluted at a ratio of 1:1000 was added, incubated at 37 °C for 30 min, and observed under a fluorescence microscope. Fluorescence images were analyzed using ImageJ for the semi-quantitative fluorescence analysis;
- (2)
- Mitochondrial membrane potential detection: 5 μg/mL of JC-1 working solution was added, stained at 37 °C for 1 h, washed with PBS three times, and observed using an inverted fluorescence microscope. Fluorescence images were analyzed using ImageJ for the semi-quantitative fluorescence analysis [29].
2.2.6. Detection of Intracellular Ca2+, Lysosomal Integrity and Autophagy
- (1).
- Detection of intracellular Ca2+: Cells were incubated with 2.5 μM Fluo-4 AM for 45 min in the dark, washed with PBS, fixed with 3.7% paraformaldehyde, and then stained with DAPI, and this was followed by direct observation using a confocal fluorescence microscope;
- (2).
- Detection of lysosomal integrity: Cells were stained with 5 μg/mL of AO working solution for 15 min, and then observed using an inverted fluorescence microscope after washing with PBS. Fluorescence images were analyzed semi-quantitatively by ImageJ;
- (3).
- Autophagy detection: Cells were washed two times with 1 × wash buffer, and 1 mL of 10% monodansylcadaverine (MDC) staining solution was added into each well and incubated in an incubator for 45 min in the dark. Once washed two times with a wash buffer, observation was made under a fluorescence microscope. Finally, ImageJ was used for the semi-quantitative fluorescence analysis of MDC.
2.2.7. Qualitative Observation and Quantitative Detection of Nano−COD Endocytosis
- (1).
- FITC fluorescent labeling and characterization of nano−COD: Based on the experimental method of our previous study [29], nano−COD was prepared using a two-step reaction.
- (2).
- Qualitative observation of endocytosis: The experimental treatment was the same as that described in Section 2.2.1. Once the repair was completed, the medium was aspirated and washed two times with PBS. Then, 200 μg/mL of the freshly prepared FITC-labeled COD was added to the medium and incubated for 6 h. Then, Lyso-Tracker Red (800 μL) was added to label the lysosomes for 2 h. The cells were fixed with paraformaldehyde, and the cell nuclei were stained with DAPI. The crystal distribution was observed under a confocal microscope;
- (3).
- Quantitative detection of endocytosis: The experimental treatment was the same as that described in Section 2.2.1. Once the repair was completed, the medium was aspirated and washed two times with PBS. Then, 200 μg/mL of the freshly prepared FITC-labeled COD was added and incubated for 6 h. The cells were treated with 5 mM EDTA for 5 min to remove the adhered crystals and collected, and the proportion of fluorescent cells was detected by flow cytometry.
2.2.8. Statistical Analysis
3. Results
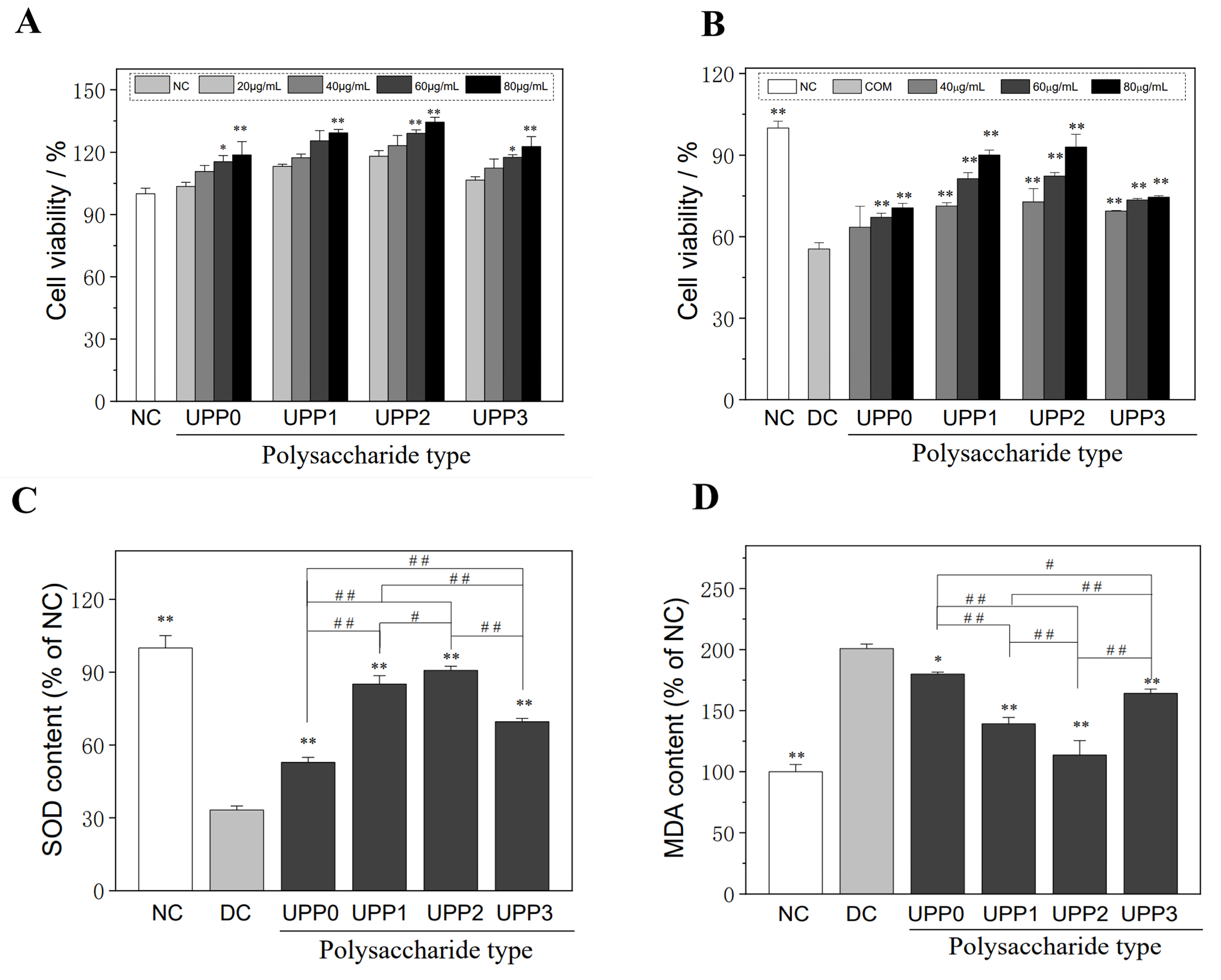
3.1. UPPs Are Non-Cytotoxic, Which Can Improve the Viability of Injured Cells
- (1).
- UPPs are not cytotoxic
- (2).
- UPPs can improve the viability of damaged cells
3.2. UPPs Improve the Cellular Antioxidant Capacity and Reduce Lipid Peroxidative Damage
- (1).
- UPPs increase SOD expression
- (2).
- UPPs reduce the malondialdehyde (MDA) levels
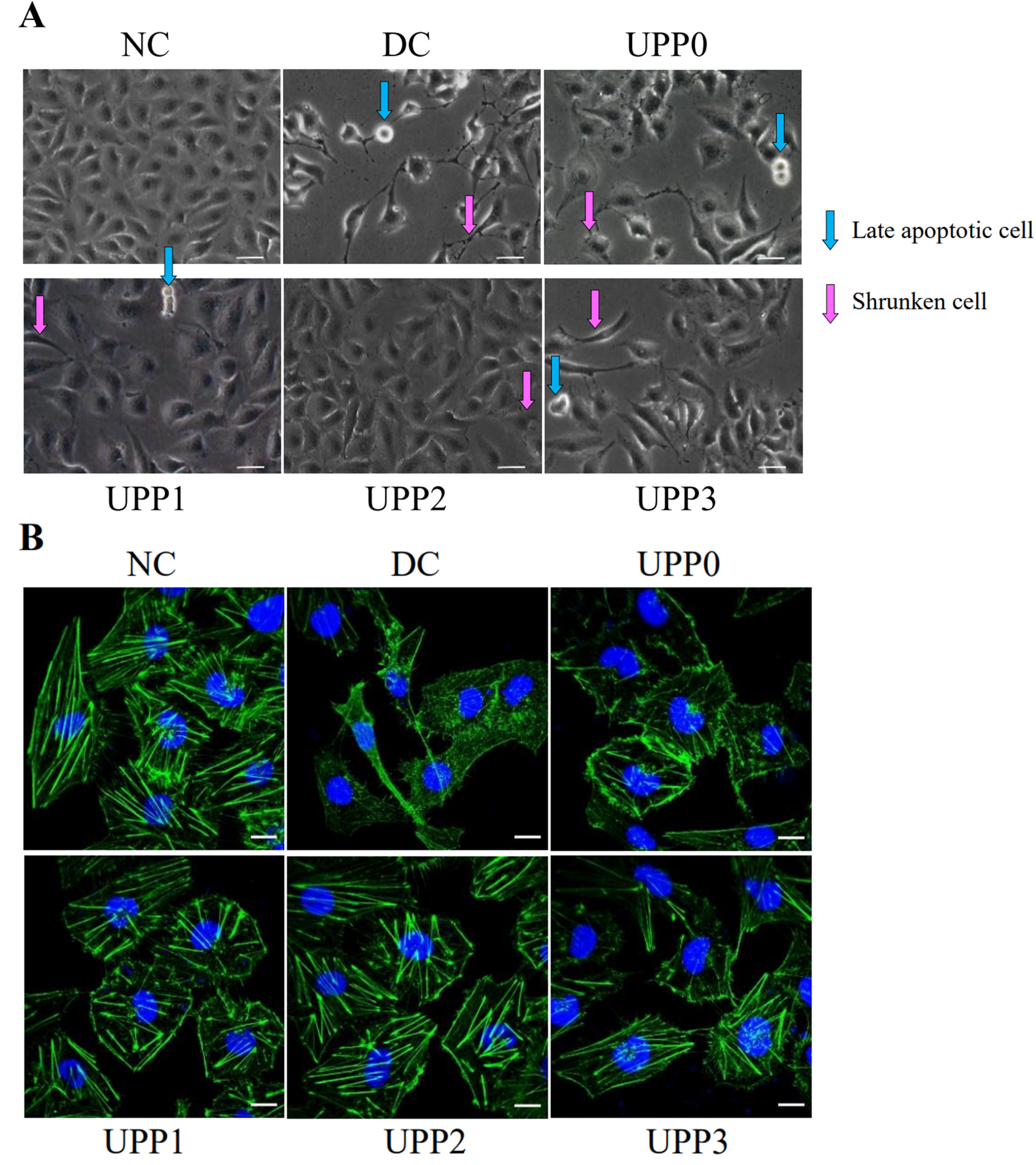
3.3. UPPs Improve Cell Morphology and Cytoskeleton
- (1).
- UPPs improve cell morphology
- (2).
- UPPs improve the cytoskeleton
3.4. UPPs Promote the Healing of Injured Cells
3.5. UPPs Reduce ROS Production and Increase Intracellular Mitochondrial Membrane Potential (Δψm)
- (1).
- Reduced intracellular ROS production
- (2).
- Increased intracellular Δψm
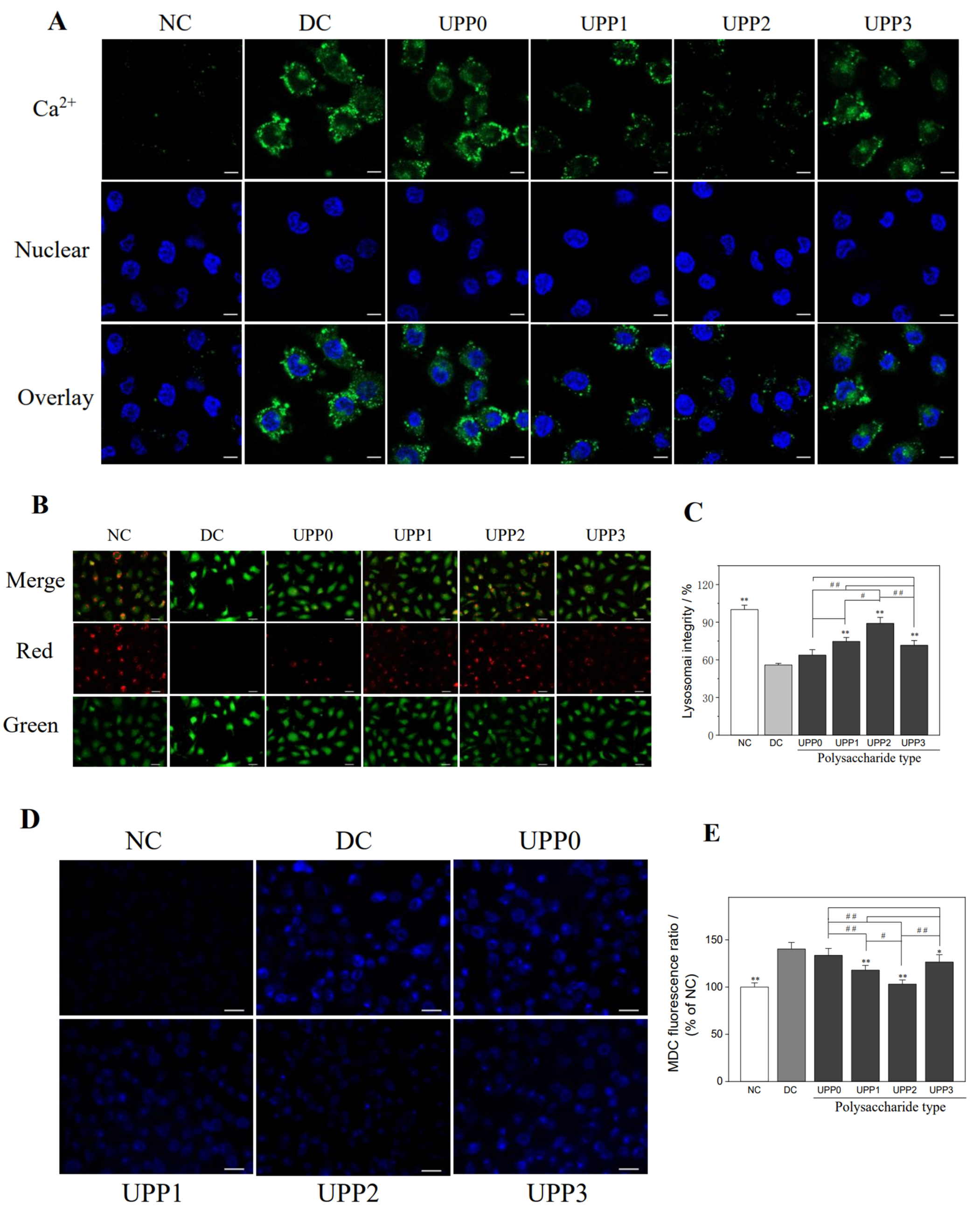
3.6. UPPs Reduce the Intracellular Ca2+ Concentration
3.7. UPPs Improve the Lysosomal Integrity
3.8. UPPs Reduce Autophagic Activity
3.9. UPP Repair Promotes Cellular Endocytosis of Nano−COD
- (1).
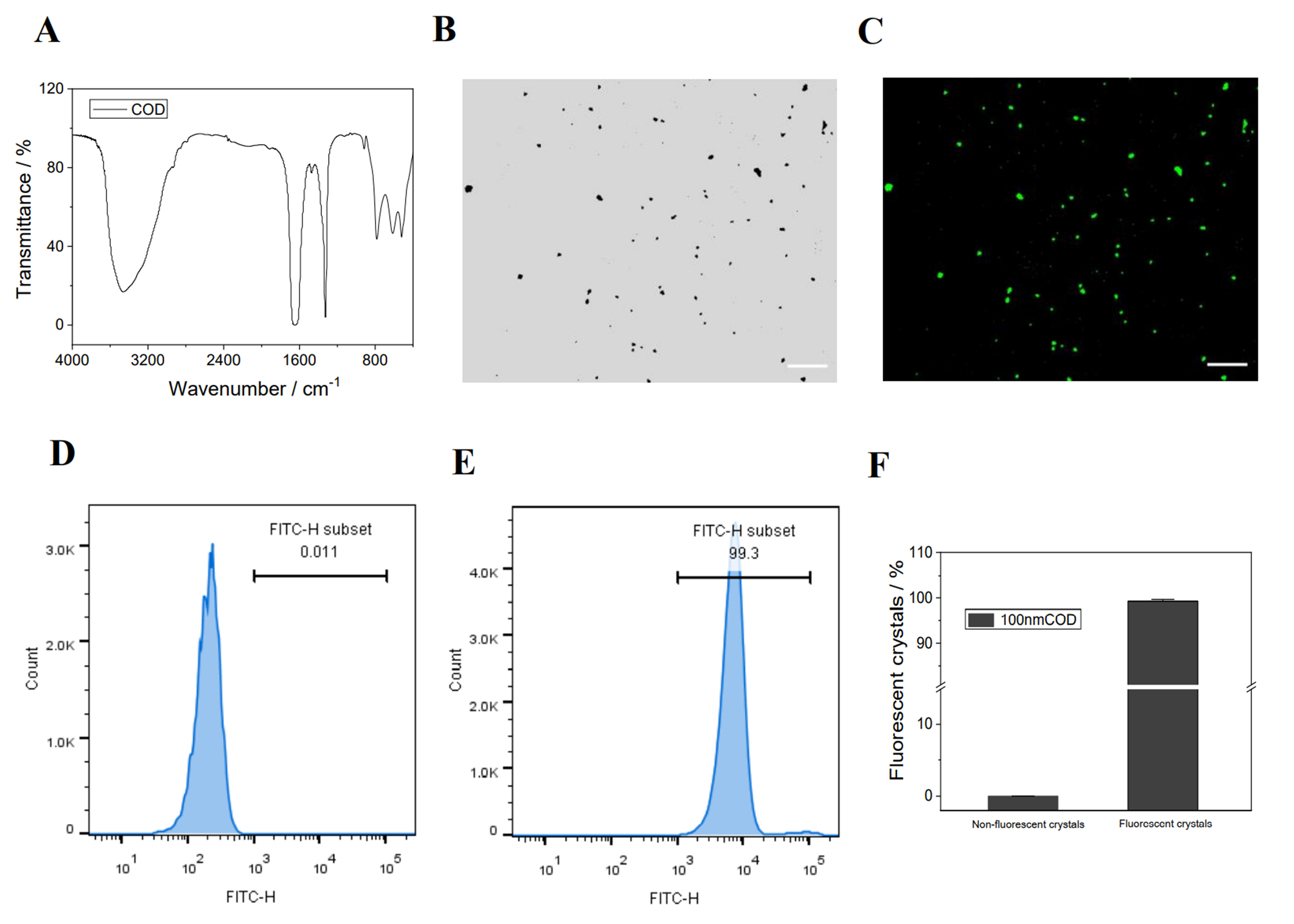
- Synthesis, fluorescent labeling, and characterization of nano−COD crystals
- (2).
- Qualitative and quantitative detection of nano−COD endocytosis by cells
4. Discussion
4.1. Sulfated Polysaccharides Can Repair Damaged Cells
- (1).
- The introduction of –OSO3− groups changes the configuration and orientation of polysaccharides, making it easier to expose hydroxyl groups in aqueous solution [46];
- (2).
- The strong acidic environment generated by the introduction of –OSO3− substituents into sulfated polysaccharide molecules leads to weaker dissociation energy of hydrogen bonds, thereby increasing the hydrogen-donating ability of polysaccharide derivatives [47];
- (3).
- The introduction of –OSO3− activates the hydrogen atoms of the anomeric carbon and increases the hydrogen-donating capacity [43].
4.2. Reasons for the High Activity of UPPs with Moderate –OSO3− Content
4.3. UPPs Promote Crystal Endocytosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornell, L.D.; Amer, H.; Viehman, J.K.; Mehta, R.A.; Lieske, J.C.; Lorenz, E.C.; Milliner, D.S. Posttransplant recurrence of calcium oxalate crystals in patients with primary hyperoxaluria: Incidence, risk factors, and effect on renal allograft function. Am. J. Transplant. 2022, 22, 85–95. [Google Scholar] [CrossRef]
- Li, C.Y.; Liu, L.; Zhao, Y.W.; Chen, J.Y.; Sun, X.Y.; Ouyang, J.M. Inhibition of calcium oxalate formation and antioxidant activity of carboxymethylated Poria cocos polysaccharides. Oxid. Med. Cell. Longev. 2021, 2021, 6653593. [Google Scholar] [CrossRef]
- Schubert, G. Stone analysis. Urol. Res. 2006, 34, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Machida, Y. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials 1999, 20, 175–182. [Google Scholar] [CrossRef]
- Daudon, M.; Jungers, P.; Bazin, D.; Williams, J.C. Recurrence rates of urinary calculi according to stone composition and morphology. Urolithiasis 2018, 46, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Hackett, R.L. Retention of calcium oxalate crystals in renal tubules. Scanning Microsc. 1991, 5, 707–711; Discussion 711–702. [Google Scholar] [PubMed]
- Khan, S.R.; Hackett, R.L. Calcium oxalate urolithiasis in the rat: Is it a model for human stone disease? A review of recent literature. Scanning Electron Microsc. 1985, 1985, 759–774. [Google Scholar]
- Oliver, J.; Macdowell, M.; Whang, R.; Welt, L.G. The renal lesions of electrolyte imbalance. IV. The intranephronic calculosis of experimental magnesium depletion. J. Exp. Med. 1966, 124, 263–278. [Google Scholar] [CrossRef]
- Delatte, L.C.; Miñón-Cifuentes, J.; Medina, J.A. New studies on papillary calculi. J. Urol. 1987, 137, 1024–1029. [Google Scholar] [CrossRef]
- Thamilselvan, S.; Byer, K.J.; Hackett, R.L.; Khan, S.R. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J. Urol. 2000, 164, 224–229. [Google Scholar] [CrossRef]
- Chanthick, C.; Thongboonkerd, V. Hyaluronic acid promotes calcium oxalate crystal growth, crystal-cell adhesion, and crystal invasion through extracellular matrix. Toxicol. Vitr. 2022, 80, 105320. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Sintiprungrat, K.; Chaiyarit, S.; Thongboonkerd, V. Macropinocytosis is the major mechanism for endocytosis of calcium oxalate crystals into renal tubular cells. Cell Biochem. Biophys. 2013, 67, 1171–1179. [Google Scholar] [CrossRef]
- Chaiyarit, S.; Singhto, N.; Thongboonkerd, V. Calcium oxalate monohydrate crystals internalized into renal tubular cells are degraded and dissolved by endolysosomes. Chem. Biol. Interact. 2016, 246, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Cheng, X.Y.; Sun, X.Y.; Chen XW Ouyang, J.M. Interaction between nanometer calcium oxalate and renal epithelial cells repaired with carboxymethylated polysaccharides. Biomater. Adv. 2022, 137, 212854. [Google Scholar] [CrossRef]
- Huang, F.; Sun, X.Y.; Chen, X.W.; Ouyang, J.M. Effects of selenized astragalus polysaccharide on the adhesion and endocytosis of nanocalcium oxalate dihydrate after the repair of damaged HK-2 cells. ACS Biomater. Sci. Eng. 2021, 7, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Hu, C.; Zou, X.; Lin, Y.; Xia, Y.; You, L. Chemistry and immunostimulatory activity of a polysaccharide from Undaria pinnatifida. Food Chem. Toxicol. 2019, 128, 119–128. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structural dependence of sulfated polysaccharide for diabetes management: Fucoidan from Undaria pinnatifida inhibiting α-glucosidase more strongly than α-amylase and amyloglucosidase. Front. Pharmacol. 2020, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, X.; Lu, W.; He, Y.; Wang, Y.; Liu, F. Effect of sulfated polysaccharide from Undaria pinnatifida (SPUP) on proliferation, migration, and apoptosis of human prostatic cancer. Int. J. Polym. Sci. 2019, 2019, 7690764. [Google Scholar] [CrossRef]
- Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Gong, B.; Li, H.S.; Xie, M.Y. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocolloid. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Sulfated modification, characterization and bioactivities of an acidic polysaccharide fraction from an edible mushroom Pleurotus eous (Berk.) Sacc. Heliyon 2021, 7, e05964. [Google Scholar] [CrossRef]
- Ahmad, M.M. Recent trends in chemical modification and antioxidant activities of plants-based polysaccharides: A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100045. [Google Scholar] [CrossRef]
- Rodrigues, J.A.G.; Quinderé, A.L.G.; Benevides, N.M.B. In vitro effects of an Acanthophora muscoides (Ceramiales, Rhodophyta) native and modified sulfated polysaccharide fraction on thrombin generation. Acta Sci. Technol. 2021, 43, e49082. [Google Scholar] [CrossRef]
- Yu, Y.; Song, Q.; Huang, L.; Shen, M.; Yu, Q.; Chen, Y.; Xie, J. Immunomodulatory activities of sulfated Cyclocarya paliurus polysaccharides with different degrees of substitution on mouse spleen lymphocytes. J. Funct. Foods 2020, 64, 103706. [Google Scholar] [CrossRef]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Hwang, Y.; Lee, Y.S.; Bae, S.H.; Lee, M.S. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. 2021, 2021, 1–29. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends Food Sci. Tech. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Mukherjee, S.; Jana, S.; Khawas, S.; Kicuntod, J.; Marschall, M.; Ray, B.; Ray, S. Synthesis, molecular features and biological activities of modified plant polysaccharides. Carbohyd. Polym. 2022, 289, 119299. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, H.; Shen, M.; Yu, Q.; Chen, Y.; Xie, J. Sulfation modification enhances the intestinal regulation of Cyclocarya paliurus polysaccharides in cyclophosphamide-treated mice via restoring intestinal mucosal barrier function and modulating gut microbiota. Food Funct. 2021, 12, 12278–12290. [Google Scholar] [CrossRef]
- Sun, X.Y.; Zhang, H.; Deng, J.W.; Yu, B.X.; Zhang, Y.H.; Ouyang, J.M. Regulatory effects of damaged renal epithelial cells after repair by Porphyra yezoensis polysaccharides with different Sulfation degree on the calcium oxalate crystal–cell interaction. Int. J. Nanomed. 2021, 16, 8087. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Sun, X.Y.; Tang, G.H.; Ouyang, J.M. Sulfated Undaria pinnatifida polysaccharide inhibits the formation of kidney stones by inhibiting HK-2 cell damage and reducing the adhesion of nano-calcium oxalate crystals. Biomater. Adv. 2022, 134, 112564. [Google Scholar] [CrossRef]
- Sun, X.Y.; Ouyang, J.M.; Liu, A.J.; Ding, Y.M.; Li, Y.B.; Gan, Q.Z. Preparation, characterization, and in vitro cytotoxicity of COM and COD crystals with various sizes. Mater. Sci. Eng. C 2015, 57, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ladrech, S.; Eybalin, M.; Puel, J.L.; Lenoir, M. Epithelial-mesenchymal transition, and collective and individual cell migration regulate epithelial changes in the amikacin-damaged organ of Corti. Histochem. Cell. Bio. 2017, 148, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wang, J.; Ren, G.; Qin, F.; Zhao, Z.; Li, K.; Lin, Y. Superoxide-like Cu/GO single-atom catalysts nanozyme with high specificity and activity for removing superoxide free radicals. Nano Res. 2022, 15, 8804–8809. [Google Scholar] [CrossRef]
- Buwono, N.R.; Risjani, Y.; Soegianto, A. Oxidative stress responses of microplastic-contaminated gambusia affinis obtained from the Brantas River in East Java, Indonesia. Chemosphere 2022, 293, 133543. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.Y.; Sun, X.Y.; Ouyang, J.M. Effects of physical properties of nano-sized hydroxyapatite crystals on cellular toxicity in renal epithelial cells. Mater. Sci. Eng. C 2019, 103, 109807. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Jiang, Y.T.; Lin, D.L.; Lin, W.H.; Xue, H.W. Phosphatidic acid suppresses autophagy through petitive inhibition by binding GAPC (glyceraldehyde-3-phosphate dehydrogenase) and PGK (phosphoglycerate kinase) proteins. Autophagy 2022, 18, 2656–2670. [Google Scholar] [CrossRef]
- Sudhakar, J.N.; Lu, H.H.; Chiang, H.Y.; Suen, C.S.; Hwang, M.J.; Wu, S.Y.; Shui, J.W. Lumenal Galectin-9-Lamp2 interaction regulates lysosome and autophagy to prevent pathogenesis in the intestine and pancreas. Nat. Commun. 2020, 11, 4286. [Google Scholar] [CrossRef]
- Chen, X.W.; Huang, W.B.; Sun, X.Y.; Xiong, P.; Ouyang, J.M. Antioxidant activity of sulfated Porphyra yezoensis polysaccharides and their regulating effect on calcium oxalate crystal growth. Mater. Sci. Eng. C 2021, 128, 112338. [Google Scholar] [CrossRef]
- Sun, X.Y.; Gan, Q.Z.; Ouyang, J.M. Size-dependent cellular uptake mechanism and cytotoxicity toward calcium oxalate on Vero cells. Sci. Rep. 2017, 7, 41949. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; García-Domínguez, E.; Borrás, C. Recent approaches to determine static and dynamic redox state-related parameters. Antioxidants 2022, 11, 864. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Liu, J. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845. [Google Scholar] [CrossRef]
- Joshi, S.; Khan, S.R. Opportunities for future therapeutic interventions for hyperoxaluria: Targeting oxidative stress. Expert Opin. Ther. Targets. 2019, 23, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: Evidence from clinical and experimental investigations. J. Urol. 2013, 189, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhao, M.; Ouyang, K.; Chen, S.; Zhang, Y.; Liu, X.; Wang, W. Sulfated modification, structures, antioxidant activities and mechanism of Cyclocarya paliurus polysaccharides protecting dendritic cells against oxidant stress. Ind. Crop. Prod. 2021, 164, 113353. [Google Scholar] [CrossRef]
- Zhao, B.; Tao, F.; Wang, J.; Zhang, J. The sulfated modification and antioxidative activity of polysaccharides from Potentilla anserine L. New J. Chem. 2020, 44, 4726–4735. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Wang, Y.; Zhang, R.; Zhang, C.; Wang, C.; Yan, X. Preparation of highly substituted sulfated alfalfa polysaccharides and evaluation of their biological activity. Foods 2022, 11, 737. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Sulfated modification of the polysaccharides from ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2015, 186, 231–238. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Marrache, S.; Dhar, S. The energy blocker inside the power house: Mitochondria targeted delivery of 3-bromopyruvate. Chem. Sci. 2015, 6, 1832–1845. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Javadov, S.; Margreiter, R.; Grimm, M.; Hagenbuchner, J.; Ausserlechner, M.J. The role of mitochondria in the mechanisms of cardiac ischemia-reperfusion injury. Antioxidants 2019, 8, 454. [Google Scholar] [CrossRef]
- Nakamura, S.; Shigeyama, S.; Minami, S.; Shima, T.; Akayama, S.; Matsuda, T.; Yoshimori, T. LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nat. Cell Biol. 2020, 22, 1252–1263. [Google Scholar] [CrossRef]
- Mulay, S.R.; Honarpisheh, M.M.; Foresto-Neto, O.; Shi, C.; Desai, J.; Zhao, Z.B.; Anders, H.J. Mitochondria permeability transition versus necroptosis in oxalate-induced AKI. J. Am. Soc. Nephrol. 2019, 30, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lim, H.; Kim, J.; Hwang, Y.; Lee, Y.S.; Bae, S.H.; Lee, M.S. Lysosomal Ca2+-mediated TFEB activation modulates mitophagy and functional adaptation of pancreatic β-cells to metabolic stress. Nat. Commun. 2022, 13, 1300. [Google Scholar] [CrossRef] [PubMed]
- Lumlertgul, N.; Siribamrungwong, M.; Jaber, B.L.; Jaber, B.L.; Susantitaphong, P. Secondary oxalate nephropathy: A systematic review. Kidney Int. Rep. 2018, 3, 1363–1372. [Google Scholar] [CrossRef]
- Sun, X.Y.; Zhang, H.; Liu, J.; Ouyang, J.M. Repair activity and crystal adhesion inhibition of polysaccharides with different molecular weights from red algae Porphyra yezoensis against oxalate-induced oxidative damage in renal epithelial cells. Food Funct. 2019, 10, 3851–3867. [Google Scholar] [CrossRef]
- Jang, S.; Javadov, S. Association between ROS production, swelling and the respirasome integrity in cardiac mitochondria. Arch. Biochem. Biophys. 2017, 630, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Tang, Z.; Bai, Y.; Wan, M.; Yu, M.; Chen, J.; Ge, M. Astragalus polysaccharide protects against cadmium-induced autophagy injury through reactive oxygen species (ROS) pathway in chicken embryo fibroblast. Biol. Trace Elem. Res. 2022, 200, 318–329. [Google Scholar] [CrossRef]
- Huang, F.; Chen, J.Y.; Ouyang, J.M. Comparison of the inhibition of high phosphate-induced smooth muscle cell calcification by porphyra yezoensis and astragalus polysaccharides. J. Funct. Foods 2020, 73, 104160. [Google Scholar] [CrossRef]
- Li, M.; Luo, T.; Huang, Y.; Su, J.; Li, D.; Chen, X.; Zhang, Y.; Huang, L.; Li, S.; Jiao, C.; et al. Polysaccharide from pycnoporus sanguineus ameliorates dextran sulfate sodium-induced colitis via helper T cells repertoire modulation and autophagy suppression. Phytother. Res. 2020, 34, 2649–2664. [Google Scholar] [CrossRef]
- Li, C.; Duan, S.; Li, Y.; Pan, X.; Han, L. Polysaccharides in natural products that repair the damage to intestinal mucosa caused by cyclophosphamide and their mechanisms: A review. Carbohyd. Polym. 2021, 261, 117876. [Google Scholar] [CrossRef]
- Ying, L.; Pan, Y.; Wang, Y.; Xu, P. Physicochemical properties, in vitro antioxidant activities and protective effects of Liubao tea polysaccharides on HUVEC. J. Tea Sci. 2017, 37, 25–37. [Google Scholar]
- Dinoro, J.; Maher, M.; Talebian, S.; Jafarkhani, M.; Mehrali, M.; Orive, G.; Dolatshahi-Pirouz, A. Sulfated polysaccharide-based scaffolds for orthopaedic tissue engineering. Biomaterials 2019, 214, 119214. [Google Scholar] [CrossRef]
- Wu, D.T.; He, Y.; Fu, M.X.; Gan, R.Y.; Hu, Y.C.; Peng, L.X.; Zou, L. Structural characteristics and biological activities of a pectic-polysaccharide from okra affected by ultrasound assisted metal-free fenton reaction. Food Hydrocolloid. 2022, 122, 107085. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, L.; Pan, Y.; Zheng, X.; Sun, Q.; Wang, Z.; Qiao, H. Effect of molecular weight on the antibacterial activity of polysaccharides produced by Chaetomium globosum CGMCC 6882. Int. J. Biol. Macromol. 2021, 188, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Cho, Y.N.; Patil, M.P.; Cho, Y.J.; Kim, G.D.; Park, Y.B.; Chun, B.S. Hydrothermal degradation of seaweed polysaccharide: Characterization and biological activities. Food Chem. 2018, 268, 179–187. [Google Scholar] [CrossRef]
- Tao, Y.; Ma, J.; Huang, C.; Lai, C.; Ling, Z.; Yong, Q. The immunomodulatory activity of degradation products of Sesbania cannabina galactomannan with different molecular weights. Int. J. Biol. Macromol. 2022, 205, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Guo, D.; Sun, X.Y.; Wang, J.M.; Ouyang, J.M.; Gui, B.S. Repair effects of astragalus polysaccharides with different molecular weights on oxidatively damaged HK-2 cells. Sci. Rep. 2019, 9, 9871. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Li, X.; Chang, Y.; Duan, G.; Yu, L.; Zheng, R.; Tang, Q. Dietary fucoidan of acaudina molpadioides and its enzymatically degraded fragments could prevent intestinal mucositis induced by chemotherapy in mice. Food Funct. 2015, 6, 415–422. [Google Scholar] [CrossRef]
- Lieske, J.C.; Norris, R.; Swift, H.; Toback, F.G. Adhesion, internalization and metabolism of calcium oxalate monohydrate crystals by renal epithelial cells. Kidney Int. 1997, 52, 1291–1301. [Google Scholar] [CrossRef]
- Yu, H.D.; Chun, C.; Xiong, F.; Rui, H.L. Study on the pharmacokinetics of mulberry fruit polysaccharides through fluorescence labeling. Int. J. Boil. Macromol. 2021, 186, 462–471. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.; Zhao, J.; Zhang, L.; Li, Q. In vivo pharmacokinetic study of a Cucurbita moschata polysaccharide after oral administration. Int. J. Boil. Macromol. 2022, 203, 19–28. [Google Scholar] [CrossRef]
- Xia, H.; Yang, C.; Zhou, B.; Tang, H.; Yang, L.; Liao, W.; Sun, G. Pharmacokinetics and Excretion Study of Lycium barbarum Polysaccharides in Rats by FITC-Fluorescence Labeling. Foods 2021, 10, 2851. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Z.; Sun, G.; Shen, L.; Xu, D.; Feng, Y. A sensitive and specific HPGPC-FD method for the study of pharmacokinetics and tissue distribution of Radix Ophiopogonis polysaccharide in rats. Biomed. Chromatogr. 2010, 24, 820–825. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-W.; Zheng, Y.-Y.; Ouyang, J.-M. Sulfated Undaria pinnatifida Polysaccharide Promotes Endocytosis of Nano-Calcium Oxalate Dihydrate by Repairing Subcellular Organelles in HK-2 Cells. Antioxidants 2023, 12, 1015. https://doi.org/10.3390/antiox12051015
Chen X-W, Zheng Y-Y, Ouyang J-M. Sulfated Undaria pinnatifida Polysaccharide Promotes Endocytosis of Nano-Calcium Oxalate Dihydrate by Repairing Subcellular Organelles in HK-2 Cells. Antioxidants. 2023; 12(5):1015. https://doi.org/10.3390/antiox12051015
Chicago/Turabian StyleChen, Xue-Wu, Yu-Yun Zheng, and Jian-Ming Ouyang. 2023. "Sulfated Undaria pinnatifida Polysaccharide Promotes Endocytosis of Nano-Calcium Oxalate Dihydrate by Repairing Subcellular Organelles in HK-2 Cells" Antioxidants 12, no. 5: 1015. https://doi.org/10.3390/antiox12051015




