Continuous Monochromatic Blue Light Exacerbates High-Fat Diet-Induced Kidney Injury via Corticosterone-Mediated Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Light Patterns
2.2. Blood Biochemical Analysis
2.3. Urine Examination
2.4. Histological Analysis
2.5. Immunohistochemistry (IHC) Staining and Immunofluorescence (IF)
2.6. RNA Isolation and Quantitative Real-Time (RT)-PCR
2.7. Western Blotting
2.8. Measurements of Antioxidant Activity and Lipid Peroxidation
2.9. Enzyme-linked Immunosorbent Assay
2.10. Cell Culture
2.11. Statistical Analyses
3. Results
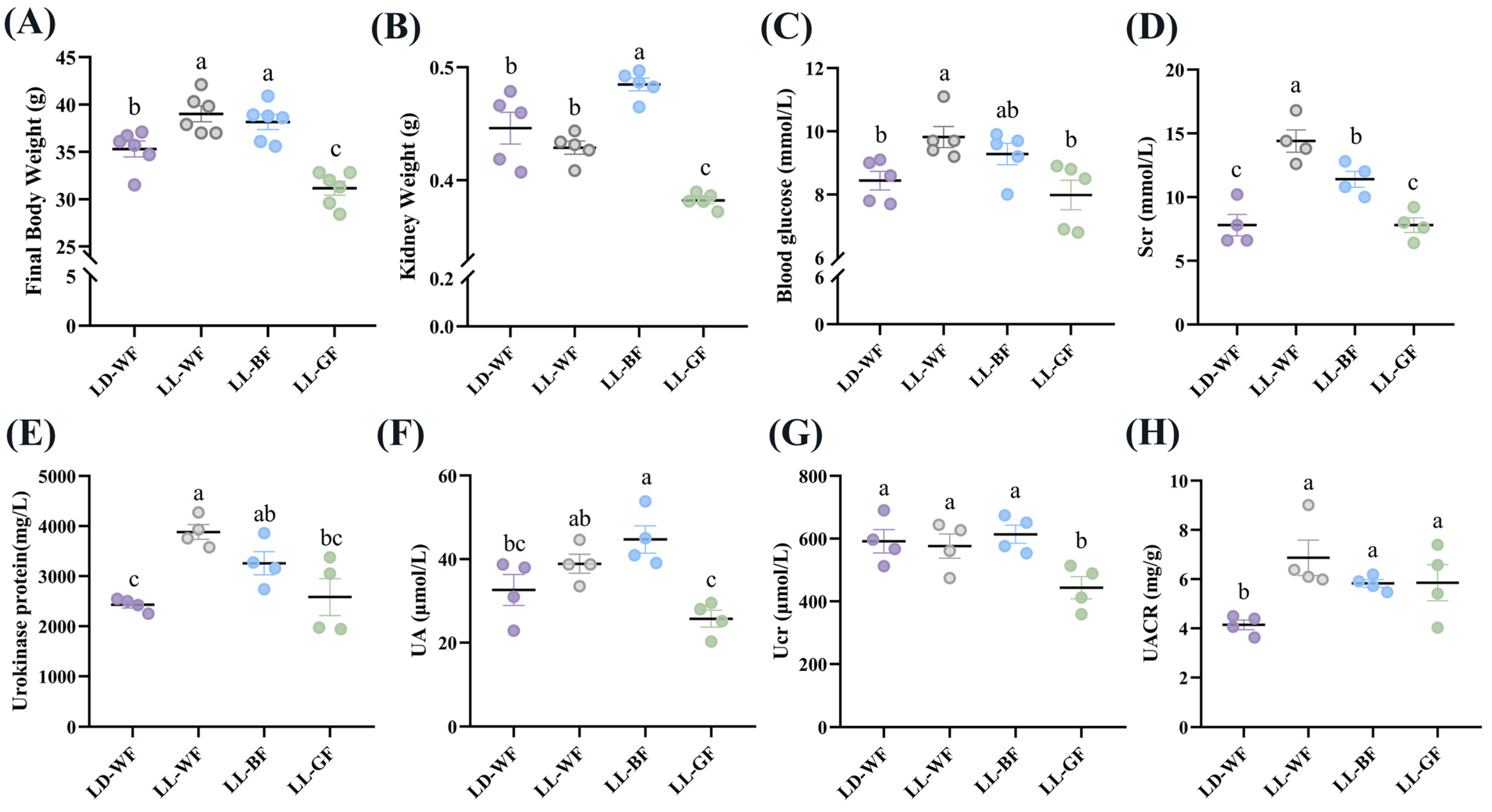
3.1. Renal Function Is Significantly Worse in HFD Mice under Continuous Monochromatic Blue Light Exposure
3.2. Continuous Monochromatic Blue Light Aggravated Renal Histological Injury and Apoptosis in the LD-WF Mice
3.3. Continuous Monochromatic Blue Light Increased Renal Fibrosis Induced by HFD
3.4. Continuous Monochromatic Blue Light Increased Expression of Kidney Injury-Related Genes in the LD-WF Mice
3.5. Effect of Continuous Monochromatic Blue Light on the Antioxidant Capacity and Inflammation of the Kidney in HFD-Mice
3.6. Continuous Monochromatic Blue Light Affected Plasma CORT Concentration and Glucocorticoid Receptors Expression of the Kidney in HFD Mice
3.7. Corticosterone Induced Oxidative Stress and Inflammation in HK-2 Cells via Glucocorticoid Receptors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Margolis, G.; Elbaz-Greener, G.; Ruskin, J.N.; Roguin, A.; Amir, O.; Rozen, G. The impact of obesity on sudden cardiac death risk. Curr. Cardiol. Rep. 2022, 24, 497–504. [Google Scholar] [CrossRef]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef]
- Maric-Bilkan, C. Obesity and diabetic kidney disease. Med. Clin. N. Am. 2013, 97, 59–74. [Google Scholar] [CrossRef]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Xu, X.; Nie, S.; Ding, H.; Hou, F.F. Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 2018, 14, 313–324. [Google Scholar] [CrossRef]
- Dibner, C. The importance of being rhythmic: Living in harmony with your body clocks. Acta Physiol. 2020, 228, e13281. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Fonken, L.K.; Walton, J.C.; Nelson, R.J. Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol. Lett. 2011, 7, 468–471. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lin, Y.J.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal melatonin or agomelatine therapy prevents programmed hypertension in male offspring of mother exposed to continuous light. Biol. Reprod. 2017, 97, 636–643. [Google Scholar] [CrossRef]
- Paixao, A.D.; Alexander, B.T. How the kidney is impacted by the perinatal maternal environment to develop hypertension. Biol. Reprod. 2013, 89, 144. [Google Scholar] [CrossRef]
- Wirz-Justice, A.; Skene, D.J.; Munch, M. The relevance of daylight for humans. Biochem. Pharmacol. 2021, 191, 114304. [Google Scholar] [CrossRef]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef]
- Romeo, S.; Viaggi, C.; Di Camillo, D.; Willis, A.W.; Lozzi, L.; Rocchi, C.; Capannolo, M.; Aloisi, G.; Vaglini, F.; Maccarone, R.; et al. Bright light exposure reduces TH-positive dopamine neurons: Implications of light pollution in Parkinson’s disease epidemiology. Sci. Rep. 2013, 3, 1395. [Google Scholar] [CrossRef]
- Tosini, G.; Ferguson, I.; Tsubota, K. Effects of blue light on the circadian system and eye physiology. Mol. Vis. 2016, 22, 61–72. [Google Scholar]
- Liu, Q.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Dim blue light at night induces spatial memory impairment in mice by hippocampal neuroinflammation and Oxidative Stress. Antioxidants 2022, 11, 1218. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Monochromatic blue light not green light exposure is associated with continuous light-induced hepatic steatosis in high fat diet fed-mice via oxidative stress. Ecotoxicol. Environ. Saf. 2022, 239, 113625. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef]
- Tsuji, T.; Allchorne, A.J.; Zhang, M.; Tsuji, C.; Tobin, V.A.; Pineda, R.; Raftogianni, A.; Stern, J.E.; Grinevich, V.; Leng, G.; et al. Vasopressin casts light on the suprachiasmatic nucleus. J. Physiol. 2017, 595, 3497–3514. [Google Scholar] [CrossRef]
- Firsov, D.; Bonny, O. Circadian rhythms and the kidney. Nat. Rev. Nephrol. 2018, 14, 626–635. [Google Scholar] [CrossRef]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef]
- Firsov, D.; Bonny, O. Circadian regulation of renal function. Kidney Int. 2010, 78, 640–645. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takabatake, Y.; Takahashi, A.; Kimura, T.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.Y.; Matsui, I.; Matsusaka, T.; et al. High-fat diet-induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. J. Am. Soc. Nephrol. 2017, 28, 1534–1551. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.A.; Fu, X.; Wang, Z. Lipid accumulation and chronic kidney disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef]
- Okuliarova, M.; Mazgutova, N.; Majzunova, M.; Rumanova, V.S.; Zeman, M. Dim light at night impairs daily variation of circulating immune cells and renal immune homeostasis. Front. Immunol. 2020, 11, 614960. [Google Scholar] [CrossRef]
- Du, X.G.; Ruan, X.Z. Lipid metabolism disorder and renal fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 525–541. [Google Scholar] [CrossRef]
- Han, Y.C.; Tang, S.Q.; Liu, Y.T.; Li, A.M.; Zhan, M.; Yang, M.; Song, N.; Zhang, W.; Wu, X.Q.; Peng, C.H.; et al. AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice. Cell Death Dis. 2021, 12, 925. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic kidney disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Deng, H.F.; Yue, L.X.; Wang, N.N.; Zhou, Y.Q.; Zhou, W.; Liu, X.; Ni, Y.H.; Huang, C.S.; Qiu, L.Z.; Liu, H.; et al. Mitochondrial iron overload-mediated inhibition of Nrf2-HO-1/GPX4 assisted ALI-induced nephrotoxicity. Front. Pharmacol. 2020, 11, 624529. [Google Scholar] [CrossRef]
- Furfaro, A.L.; Traverso, N.; Domenicotti, C.; Piras, S.; Moretta, L.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. The Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid. Med. Cell Longev. 2016, 2016, 1958174. [Google Scholar] [CrossRef]
- Lan, H.Y. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef]
- Wang, J.; Xu, G.; Jin, H.; Chai, Y.; Yang, X.; Liu, Z.; Hou, S.; Fan, H. Ulinastatin alleviates rhabdomyolysis-induced acute kidney injury by suppressing inflammation and apoptosis via inhibiting TLR4/NF-kappaB signaling pathway. Inflammation 2022, 45, 2052–2065. [Google Scholar] [CrossRef]
- Sage, D.; Maurel, D.; Bosler, O. Corticosterone-dependent driving influence of the suprachiasmatic nucleus on adrenal sensitivity to ACTH. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E458–E465. [Google Scholar] [CrossRef]
- Przybycinski, J.; Drozdzal, S.; Domanski, L.; Dziedziejko, V.; Pawlik, A. Role of endothelial glucocorticoid receptor in the pathogenesis of kidney diseases. Int. J. Mol. Sci. 2021, 22, 13295. [Google Scholar] [CrossRef]
- Van Ginhoven, T.M.; Van Den Berg, J.W.; Dik, W.A.; Ijzermans, J.N.; De Bruin, R.W. Preoperative fasting induces protection against renal ischemia/reperfusion injury by a corticosterone-independent mechanism. Transpl. Int. 2010, 23, 1171–1178. [Google Scholar] [CrossRef]
- Herman, J.P.; Figueiredo, H.; Mueller, N.K.; Ulrich-Lai, Y.; Ostrander, M.M.; Choi, D.C.; Cullinan, W.E. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 2003, 24, 151–180. [Google Scholar] [CrossRef]
- Noddings, C.M.; Wang, R.Y.; Johnson, J.L.; Agard, D.A. Structure of Hsp90-p23-GR reveals the Hsp90 client-remodelling mechanism. Nature 2022, 601, 465–469. [Google Scholar] [CrossRef]
- Alam, M.M.; Okazaki, K.; Nguyen, L.T.T.; Ota, N.; Kitamura, H.; Murakami, S.; Shima, H.; Igarashi, K.; Sekine, H.; Motohashi, H. Glucocorticoid receptor signaling represses the antioxidant response by inhibiting histone acetylation mediated by the transcriptional activator NRF2. J. Biol. Chem. 2017, 292, 7519–7530. [Google Scholar] [CrossRef]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 2013, 34, 518–530. [Google Scholar] [CrossRef]





| Gene | Product Size | Primer Sequences (5′ to 3′) | Accession No. |
|---|---|---|---|
| Kim-1 | 114 | F: ACATATCGTGGAATCACAACGAC R: ACTGCTCTTCTGATAGGTGACA | XM_011248784.3 |
| Lcn2 | 418 | F: TGCAGGTGGTACGTTGTGG R: TGTTGTCGTCCTTGAGGC | NM_008491.1 |
| Nephrin | 213 | F: CCCCTCTATGATGAAGTACAAATGGA R: GTACGGATTTCCTCAGGTCTTCT | XM_011250647.3 |
| Podocin | 232 | F: GTGTCCAAAGCCATCCAGTT R: GTCTTTGTGCCTCAGCTTCC | XM_006496684.3 |
| Cd2ap | 200 | F: AGGAATTCAGCCACATCCAC R: TTGAGGGAAACAGTCCCAAC | NM_009847.4 |
| α-Actinin-4 | 189 | F: GCCATCCAGGACATCTCTGT R: CCGCAGCTTGTCATACTCAA | XM_006540257.4 |
| Acta2 | 102 | F: GTCCCAGACATCAGGGAGTAA R: TCGGATACTTCAGCGTCAGGA | XM_006526606.2 |
| Fibronectin | 117 | F: TCCACAGCCATTCCTGCGCC R: GTTCACCCGCACCCGGTAGC | XM_006495700.4 |
| Il-6 | 200 | F: ATAGTCCTTCCTACCCCAATTTCC R: CTGACCACAGTGAGGAATGTCCAC | NM_031168.2 |
| Il-1β | 116 | F: GAAATGCCACCTTTTGACAGTG R: TGGATGCTCTCATCAGGACAG | NM_008361.4 |
| Tnf-α | 177 | F: TCAGCCTCTTCTCATTCCTG R: CAGGCTTGTCACTCGAATTT | NM_013693.3 |
| Mcp-1 | 175 | F: CAAGAAGGAATGGGTCCAGA R: TGAGGTGGTTGTGGAAAAGG | NM_011333.3 |
| Il-4 | 102 | F: GGTCTCAACCCCCAGCTAGT R: GCCGATGATCTCTCTCAAGTGAT | NC_000077.7 |
| Tgf-β1 | 133 | F: CTCCCGTGGCTTCTAGTGC R: GCCTTAGTTTGGACAGGATCTG | XM_036152883.1 |
| Hsp90 | 116 | F: TGTTGCGGTACTACACATCTGC R: GTCCTTGGTCTCACCTGTGATA | NM_010480.5 |
| Hsp70 | 219 | F: TGGTGCAGTCCGACATGAAG R:GCTGAGAGTCGTTGAAGTAGGC | NM_010479.2 |
| P23 | 120 | F: GTTCTTCGGAGAGCACCTGTT R: GAGAGTCCGGTGTCAATCCAG | XM_036154592.1 |
| Gapdh | 232 | F: CCGAGAATGGGAAGCTTGTC R: TTCTCGTGGTTCACACCCATC | XM_036165840.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, W.; Wang, Z.; Cao, J.; Dong, Y.; Wang, T.; Chen, Y. Continuous Monochromatic Blue Light Exacerbates High-Fat Diet-Induced Kidney Injury via Corticosterone-Mediated Oxidative Stress. Antioxidants 2023, 12, 1018. https://doi.org/10.3390/antiox12051018
Ren W, Wang Z, Cao J, Dong Y, Wang T, Chen Y. Continuous Monochromatic Blue Light Exacerbates High-Fat Diet-Induced Kidney Injury via Corticosterone-Mediated Oxidative Stress. Antioxidants. 2023; 12(5):1018. https://doi.org/10.3390/antiox12051018
Chicago/Turabian StyleRen, Wenji, Zixu Wang, Jing Cao, Yulan Dong, Tuanjie Wang, and Yaoxing Chen. 2023. "Continuous Monochromatic Blue Light Exacerbates High-Fat Diet-Induced Kidney Injury via Corticosterone-Mediated Oxidative Stress" Antioxidants 12, no. 5: 1018. https://doi.org/10.3390/antiox12051018
APA StyleRen, W., Wang, Z., Cao, J., Dong, Y., Wang, T., & Chen, Y. (2023). Continuous Monochromatic Blue Light Exacerbates High-Fat Diet-Induced Kidney Injury via Corticosterone-Mediated Oxidative Stress. Antioxidants, 12(5), 1018. https://doi.org/10.3390/antiox12051018







