Cocoa Bean Shell: A By-Product with High Potential for Nutritional and Biotechnological Applications
Abstract
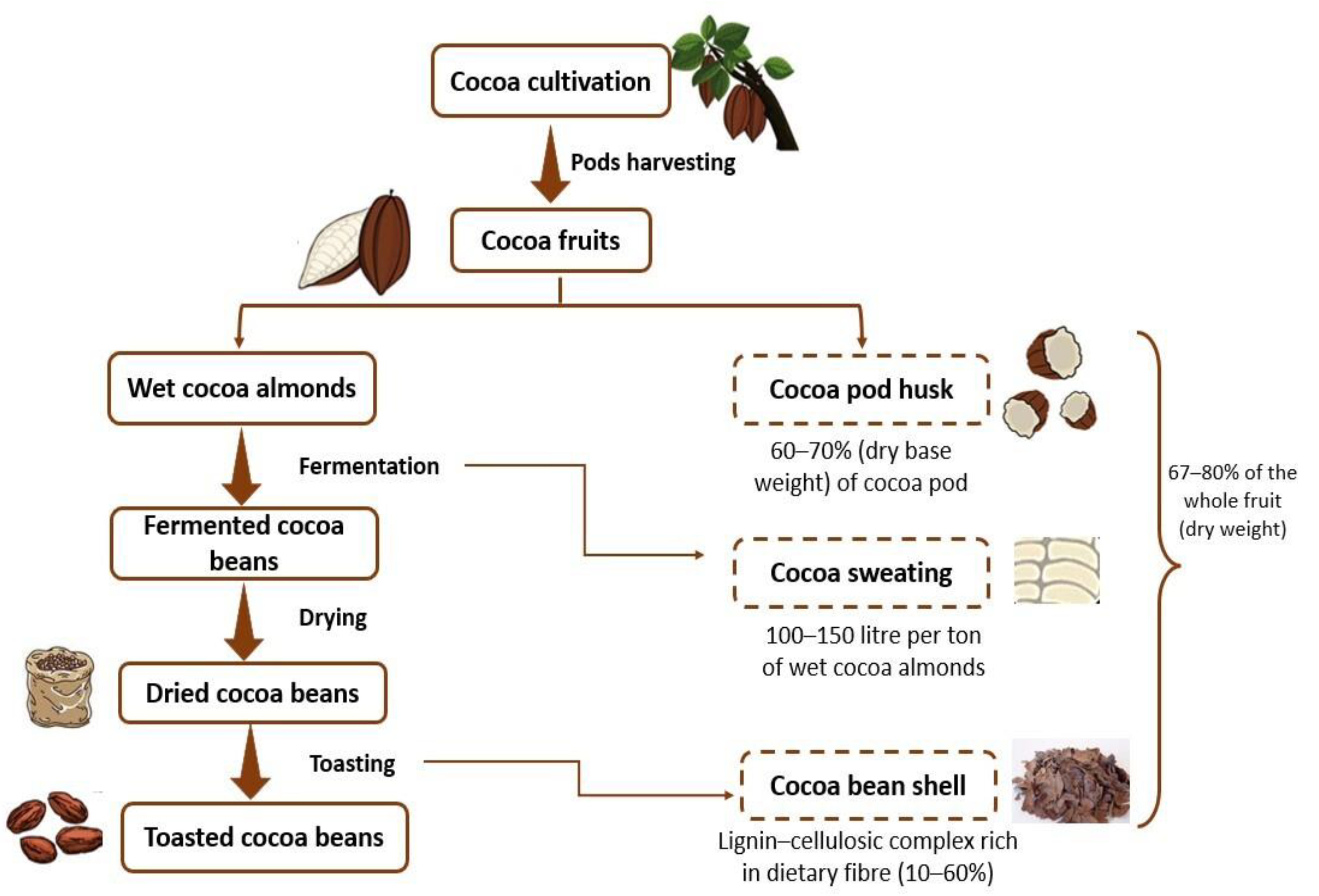
1. Introduction
2. Chemical and Nutritional Composition of CBS
3. CBS Valorization
3.1. Bioactive Compounds
3.1.1. Antioxidant Compounds
3.1.2. Antimicrobial Compounds
3.1.3. Antiviral Compounds
3.2. Biomaterials
3.3. Adsorbent
3.4. Biofuels
3.5. Corrosion Inhibitor
3.6. Food Ingredient
3.7. Animal Feed
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/home/en/ (accessed on 24 February 2023).
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Value-added Products from Fruit and Vegetable Wastes: A Review. CLEAN—Soil Air Water 2021, 49, 2000376. [Google Scholar] [CrossRef]
- Amicarelli, V.; Tricase, C.; Spada, A.; Bux, C. Households’ Food Waste Behavior at Local Scale: A Cluster Analysis after the COVID-19 Lockdown. Sustainability 2021, 13, 3283. [Google Scholar] [CrossRef]
- Despoudi, S.; Bucatariu, C.; Otles, S.; Kartal, C. Food Waste Management, Valorization, and Sustainability in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128205631. [Google Scholar]
- United Nations. 2023. Available online: https://www.un.org/en/ (accessed on 3 March 2023).
- Majerska, J.; Michalska, A.; Figiel, A. A Review of New Directions in Managing Fruit and Vegetable Processing By-Products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Cinar, Z.Ö.; Atanassova, M.; Tumer, T.B.; Caruso, G.; Antika, G.; Sharma, S.; Sharifi-Rad, J.; Pezzani, R. Cocoa and Cocoa Bean Shells Role in Human Health: An Updated Review. J. Food Compos. Anal. 2021, 103, 104115. [Google Scholar] [CrossRef]
- Mariatti, F.; Gunjević, V.; Boffa, L.; Cravotto, G. Process Intensification Technologies for the Recovery of Valuable Compounds from Cocoa By-Products. Innov. Food Sci. Emerg. Technol. 2021, 68, 102601. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Alongi, J.; Frache, A. Plasticizers, Antioxidants and Reinforcement Fillers from Hazelnut Skin and Cocoa by-Products: Extraction and Use in PLA and PP. Polym. Degrad. Stab. 2014, 108, 297–306. [Google Scholar] [CrossRef]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Cocoa Bean Shell as Promising Feedstock for the Production of Poly(3-Hydroxybutyrate) (PHB). Appl. Sci. 2023, 13, 975. [Google Scholar] [CrossRef]
- Porto de Souza, L.; Kley Valladares-Diestra, K.; Amaro Bittencourt, G.; Fátima Murawski de Mello, A.; Sarmiento Vásquez, Z.; Zwiercheczewski de Oliveira, P.; Vinícius de Melo Pereira, G.; Ricardo Soccol, C. Added-Value Biomolecules’ Production from Cocoa Pod Husks: A Review. Bioresour. Technol. 2022, 344, 126252. [Google Scholar] [CrossRef]
- Oddoye, E.; Agyente-Badu, C.; Gyedu-Akoto, E. Cocoa and Its By-Products: Identification and Utilization. Choc. Health Nutr. 2013, 7, 23–37. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) Pod Husk: Renewable Source of Bioactive Compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Lu, F.; Rodriguez-Garcia, J.; Van Damme, I.; Westwood, N.J.; Shaw, L.; Robinson, J.S.; Warren, G.; Chatzifragkou, A.; Mason, S.M.; Gomez, L.; et al. Valorisation Strategies for Cocoa Pod Husk and Its Fractions. Curr. Opin. Green Sustain. Chem. 2018, 14, 80–88. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Lucas-González, R.; Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Martuscelli, M.; Chaves-López, C. Bioactive Compounds and Techno-Functional Properties of High-Fiber Co-Products of the Cacao Agro-Industrial Chain. Heliyon 2021, 7, e06799. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; de Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological Approaches for Cocoa Waste Management: A Review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef]
- Balentić, J.P.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef][Green Version]
- Cooper, K.A.; Donovan, J.L.; Waterhouse, A.I.; Williamson, G. Cocoa and Health: A Decade of Research. Br. J. Nutr. 2008, 99, 1–11. [Google Scholar] [CrossRef][Green Version]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa Shell and Its Compounds: Applications in the Food Industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Rojo-poveda, O.; Barbosa-pereira, L.; Zeppa, G.; St, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef][Green Version]
- Picchioni, F.; Warren, G.P.; Lambert, S.; Balcombe, K.; Robinson, J.S.; Srinivasan, C.; Gomez, L.D.; Faas, L.; Westwood, N.J.; Chatzifragkou, A.; et al. Valorisation of Natural Resources and the Need for Economic and Sustainability Assessment: The Case of Cocoa Pod Husk in Indonesia. Sustainability 2020, 12, 8962. [Google Scholar] [CrossRef]
- Hashimoto, J.C.; Lima, J.C.; Celeghini, R.M.S.; Nogueira, A.B.; Efraim, P.; Poppi, R.J.; Pallone, J.A.L. Quality Control of Commercial Cocoa Beans (Theobroma cacao L.) by Near-Infrared Spectroscopy. Food Anal. Methods 2018, 11, 1510–1517. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Zhao, J.; Fleet, G. Yeasts Are Essential for Cocoa Bean Fermentation. Int. J. Food Microbiol. 2014, 174, 72–87. [Google Scholar] [CrossRef]
- Afrane, G.; Ntiamoah, A. Use of Pesticides in the Cocoa Industry and Their Impact on the Environment and the Food Chain. In Pesticides in the Modern World-Risks and Benefit; InTechOpen: London, UK, 2011. [Google Scholar] [CrossRef][Green Version]
- Botella-Martínez, C.; Lucas-Gonzalez, R.; Ballester-Costa, C.; Pérez-álvarez, J.Á.; Fernández-López, J.; Delgado-Ospina, J.; Chaves-López, C.; Viuda-Martos, M. Ghanaian Cocoa (Theobroma cacao L.) Bean Shells Coproducts: Effect of Particle Size on Chemical Composition, Bioactive Compound Content and Antioxidant Activity. Agronomy 2021, 11, 401. [Google Scholar] [CrossRef]
- Cantele, C.; Rojo-Poveda, O.; Bertolino, M.; Ghirardello, D.; Cardenia, V.; Barbosa-Pereira, L.; Zeppa, G. In Vitro Bioaccessibility and Functional Properties of Phenolic Compounds from Enriched Beverages Based on Cocoa Bean Shell. Foods 2020, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Mateus-Reguengo, L.; Bertolino, M.; Stévigny, C.; Zeppa, G. Effects of Particle Size and Extraction Methods on Cocoa Bean Shell Functional Beverage. Nutrients 2019, 11, 867. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Optimization of Microwave-Assisted Extraction of Cocoa Bean Shell Waste and Evaluation of Its Antioxidant, Physicochemical and Functional Properties. LWT 2020, 127, 109361. [Google Scholar] [CrossRef]
- Agus, B.A.P.; Mohamad, N.N.; Hussain, N. Composition of Unfermented, Unroasted, Roasted Cocoa Beans and Cocoa Shells from Peninsular Malaysia. J. Food Meas. Charact. 2018, 12, 2581–2589. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa Bean Shell Waste Valorisation; Extraction from Lab to Pilot-Scale Cavitational Reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef]
- Adamafio, N.A. Theobromine Toxicity and Remediation of Cocoa Byproducts: An Overview. J. Biol. Sci. 2013, 7, 570–576. [Google Scholar] [CrossRef][Green Version]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, Technological and in Vitro Antioxidant Properties of Cocoa (Theobroma cacao L.) Co-Products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Vojvodić, A.; Komes, D.; Vovk, I.; Belščak-Cvitanović, A.; Bušić, A. Compositional Evaluation of Selected Agro-Industrial Wastes as Valuable Sources for the Recovery of Complex Carbohydrates. Food Res. Int. 2016, 89, 565–573. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Anaerobic Digestion of Lignocellulosic Materials Using Ethanol-Organosolv Pretreatment. Environ. Eng. Sci. 2018, 35, 953–960. [Google Scholar] [CrossRef]
- Fakhlaei, R.; Rozzamri, A.; Hussain, N. Composition, Color and Antioxidant Properties of Cocoa Shell at Different Roasting Temperatures. Food Res. Int. 2020, 4, 585–593. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Vojvodić, A.; Bušić, A.; Keppler, J.; Steffen-Heins, A.; Komes, D. Encapsulation Templated Approach to Valorization of Cocoa Husk, Poppy and Hemp Macrostructural and Bioactive Constituents. Ind. Crops Prod. 2018, 112, 402–411. [Google Scholar] [CrossRef]
- Sandoval, A.J.; Barreiro, J.A.; De Sousa, A.; Valera, D.; López, J.V.; Müller, A.J. Composition and Thermogravimetric Characterization of Components Ov Venezuelan Fermented and Dry Trinitario Cocoa Beans (Theobroma cacao L.): Whole Beans, Peeled Beans and Shells. Rev. Técnica La Fac. Ing. 2019, 42, 38–47. [Google Scholar]
- Becerra, L.D.; Quintanilla-Carvajal, M.X.; Escobar, S.; Ruiz, R.Y. Correlation between Color Parameters and Bioactive Compound Content during Cocoa Seed Transformation under Controlled Process Conditions. Food Biosci. 2023, 53, 102526. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J.; Bangoura, M.L.; Lagnika, C. Quantification of Total Polyphenolic Content and Antimicrobial Activity of Cocoa (Thebroma cacao L.) Bean Shells. Pakistan J. Nutr. 2012, 11, 672–677. [Google Scholar] [CrossRef][Green Version]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Brezo-Borjan, T.; Dzedik, V.; Rodrigues, F.; Morais, S.; Delerue-Matos, C. ESG Approach in the Valorization of Cocoa (Theobroma cacao) by-Products by Subcritical Water: Application in the Cosmetic Industry. Sustain. Chem. Pharm. 2023, 31, 100908. [Google Scholar] [CrossRef]
- Jokić, S.; Nastić, N.; Vidović, S.; Flanjak, I.; Aladić, K.; Vladić, J. An Approach to Value Cocoa Bean By-Product Based on Subcritical Water Extraction and Spray Drying Using Different Carriers. Sustainability 2020, 12, 2174. [Google Scholar] [CrossRef][Green Version]
- Yusof, A.H.M.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E.; Zainudin, B.H. Optimization of an Ultrasound-Assisted Extraction Condition for Flavonoid Compounds from Cocoa Shells (Theobroma cacao) Using Response Surface Methodology. Molecules 2019, 24, 711. [Google Scholar] [CrossRef][Green Version]
- Aranaz, P.; Romo-Hualde, A.; Navarro-Herrera, D.; Zabala, M.; López-Yoldi, M.; González-Ferrero, C.; Gil, A.G.; Martínez, J.A.; Vizmanos, J.L.; Milagro, F.I.; et al. Low Doses of Cocoa Extract Supplementation Ameliorate Diet-Induced Obesity and Insulin Resistance in Rats. Food Funct. 2019, 10, 4811–4822. [Google Scholar] [CrossRef]
- Lemarcq, V.; Monterde, V.; Tuenter, E.; Van de Walle, D.; Pieters, L.; Sioriki, E.; Dewettinck, K. Flavor Diversification of Dark Chocolate Produced through Microwave Roasting of Cocoa Beans. LWT 2022, 159, 113198. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Ortiz-Moreno, A.; Ceballos-Reyes, G.; Mendiola, J.A.; Ibáñez, E. Valorization of Cacao Pod Husk through Supercritical Fluid Extraction of Phenolic Compounds. J. Supercrit. Fluids 2018, 131, 99–105. [Google Scholar] [CrossRef]
- Caprioli, G.; Fiorini, D.; Maggi, F.; Nicoletti, M.; Ricciutelli, M.; Toniolo, C.; Prosper, B.; Vittori, S.; Sagratini, G. Nutritional Composition, Bioactive Compounds and Volatile Profile of Cocoa Beans from Different Regions of Cameroon. Int. J. Food Sci. Nutr. 2016, 67, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Papillo, V.A.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Garino, C.; Coïsson, J.D.; Arlorio, M. Cocoa Hulls Polyphenols Stabilized by Microencapsulation as Functional Ingredient for Bakery Applications. Food Res. Int. 2019, 115, 511–518. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.B.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E.C. Pressurized Liquid Extraction of Flavanols and Alkaloids from Cocoa Bean Shell Using Ethanol as Solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef]
- Pavlović, N.; Jokić, S.; Jakovljević, M.; Blažić, M.; Molnar, M. Green Extraction Methods for Active Compounds from Food Waste—Cocoa Bean Shell. Foods 2020, 9, 140. [Google Scholar] [CrossRef][Green Version]
- Bartella, L.; Di Donna, L.; Napoli, A.; Siciliano, C.; Sindona, G.; Mazzotti, F. A Rapid Method for the Assay of Methylxanthines Alkaloids: Theobromine, Theophylline and Caffeine, in Cocoa Products and Drugs by Paper Spray Tandem Mass Spectrometry. Food Chem. 2019, 278, 261–266. [Google Scholar] [CrossRef]
- Boluda-Aguilar, M.; García-Vidal, L.; del Pilar González-Castañeda, F.; López-Gómez, A. Mandarin Peel Wastes Pretreatment with Steam Explosion for Bioethanol Production. Bioresour. Technol. 2010, 101, 3506–3513. [Google Scholar] [CrossRef]
- Younes, A.; Li, M.; Karboune, S. Cocoa Bean Shells: A Review into the Chemical Profile, the Bioactivity and the Biotransformation to Enhance Their Potential Applications in Foods. Crit. Rev. Food Sci. Nutr. 2022, 2, 1–25. [Google Scholar] [CrossRef]
- Osundahunsi, O.F.; Bolade, M.K.; Akinbinu, A.A. Effect of Cocoa Shell Ash as an Alkalizing Agent on Cocoa Products. J. Appl. Sci. 2007, 7, 1674–1678. [Google Scholar] [CrossRef][Green Version]
- González-Alejo, F.A.; Barajas-Fernández, J.; Olán-Acosta, M.D.L.Á.; Lagunes-Gálvez, L.M.; García-Alamilla, P. Supercritical Fluid Extraction of Fat and Caffeine with Theobromine Retention in the Cocoa Shell. Processes 2019, 7, 385. [Google Scholar] [CrossRef][Green Version]
- Bentil, J.A.; Dzogbefia, V.P.; Alemawor, F. Enhancement of the Nutritive Value of Cocoa (Theobroma cacao) Bean Shells for Use as Feed for Animals through a Two-Stage Solid State Fermentation with Pleurotus Ostreatus and Aspergillus Niger. Int. J. Appl. Miicrobiology Biotechnol. Res. 2015, 3, 20–30. [Google Scholar]
- Redgwell, R.; Trovato, V.; Merinat, S.; Curti, D.; Hediger, S.; Manez, A. Dietary Fibre in Cocoa Shell: Characterisation of Component Polysaccharides. Food Chem. 2003, 81, 103–112. [Google Scholar] [CrossRef]
- Soares, T.F.; Oliveira, M.B.P.P. Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects. Molecules 2022, 27, 1625. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The Inhibitory Effects of Flavonoids on α-Amylase and α-Glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef]
- Jokić, S.; Gagić, T.; Knez, E.; Ubarić, D.; Kerget, M. Separation of Active Compounds from Food By-Product (Cocoa Shell) Using Subcritical Water Extraction. Molecules 2018, 23, 1408. [Google Scholar] [CrossRef][Green Version]
- Hernández-Hernández, C.; Morales-Sillero, A.; Fernández-Bolaños, J.; Bermúdez-Oria, A.; Morales, A.A.; Rodríguez-Gutiérrez, G. Cocoa Bean Husk: Industrial Source of Antioxidant Phenolic Extract. J. Sci. Food Agric. 2019, 99, 325–333. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products-An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Neshatdoust, S.; Saunders, C.; Castle, S.M.; Vauzour, D.; Williams, C.; Butler, L.; Lovegrove, J.A.; Spencer, J.P.E. High-Flavonoid Intake Induces Cognitive Improvements Linked to Changes in Serum Brain-Derived Neurotrophic Factor: Two Randomised, Controlled Trials. Nutr. Health Aging 2016, 4, 81–93. [Google Scholar] [CrossRef][Green Version]
- Pavlović, N.; Miškulin, M.; Aladić, K.; Jokić, S. Cocoa Bean Shell—A Promising By-Product Rich in Bioactive Compounds. Food Health Dis. 2019, 8, 116–122. [Google Scholar]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Towards Food Circular Economy: Hydrothermal Treatment of Mixed Vegetable and Fruit Wastes to Obtain Fermentable Sugars and Bioactive Compounds. Environ. Sci. Pollut. Res. 2022, 30, 3901–3917. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.; Moulay, L.; Muguerza, B.; Quiñones, M.; Miguel, M.; Aleixandre, A. Effect of a Soluble Cocoa Fiber-Enriched Diet in Zucker Fatty Rats. J. Med. Food 2010, 13, 621–628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Domínguez-Pérez, L.A.; Beltrán-Barrientos, L.M.; González-Córdova, A.F.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Artisanal Cocoa Bean Fermentation: From Cocoa Bean Proteins to Bioactive Peptides with Potential Health Benefits. J. Funct. Foods 2020, 73, 104134. [Google Scholar] [CrossRef]
- Andre, C.; Larondelle, Y.; Evers, D. Dietary Antioxidants and Oxidative Stress from a Human and Plant Perspective: A Review. Curr. Nutr. Food Sci. 2010, 6, 2–12. [Google Scholar] [CrossRef]
- Ćetković, G.; Savatović, S.; Čanadanović-Brunet, J.; Djilas, S.; Vulić, J.; Mandić, A.; Četojević-Simin, D. Valorisation of Phenolic Composition, Antioxidant and Cell Growth Activities of Tomato Waste. Food Chem. 2012, 133, 938–945. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; García-Villalba, R.; Martínez-Díaz, F.; Ocaña-Castillo, B.; Monedero-Saiz, T.; Torrecillas-Sánchez, A.; Abellán, B.; González-Sarrías, A.; Espín, J.C. Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol. Nutr. Food Res. 2019, 63, 1801239. [Google Scholar] [CrossRef]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Sottero, B.; Danzero, A.C.; Poli, G.; Zeppa, G.; Biasi, F. Protective Effect of Cocoa Bean Shell against Intestinal Damage: An Example of Byproduct Valorization. Antioxidants 2021, 10, 280. [Google Scholar] [CrossRef]
- Martín, M.Á.; Ramos, S.; Cordero-Herrero, I.; Bravo, L.; Goya, L. Cocoa Phenolic Extract Protects Pancreatic Beta Cells against Oxidative Stress. Nutrients 2013, 5, 2955–2968. [Google Scholar] [CrossRef][Green Version]
- Martín, M.A.; Ramos, S. Cocoa Polyphenols in Oxidative Stress: Potential Health Implications. J. Funct. Foods 2016, 27, 570–588. [Google Scholar] [CrossRef][Green Version]
- Kerimi, A.; Williamson, G. The Cardiovascular Benefits of Dark Chocolate. Vasc. Pharmacol. 2015, 71, 11–15. [Google Scholar] [CrossRef][Green Version]
- Baharum, Z.; Akim, A.M.; Taufiq-Yap, Y.H.; Hamid, R.A.; Kasran, R. In Vitro Antioxidant and Antiproliferative Activities of Methanolic Plant Part Extracts of Theobroma cacao. Molecules 2014, 19, 18317–18331. [Google Scholar] [CrossRef][Green Version]
- Zainal, B.; Abdah, M.A.; Taufiq Yap, Y.H.; Roslida, A.H.; Mohd Redzuan, S.; Kasran, R. Bioactivity-Guided Fractionation of Potent Anti-Cancer Properties from Non-Edible Tissues of Theobroma cacao. Malays. Cocoa J. 2016, 9, 170–181. [Google Scholar]
- Zainal, B.; Abdah, M.; Taufiq-Yap, Y.; Roslida, A.; Rosmin, K. Anticancer Agents from Non-Edible Parts of Theobroma cacao. Nat. Prod. Chem. Res. 2014, 2, 4. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Belviso, S.; Ferrocino, I.; Rojo-Poveda, O.; Zeppa, G. Characterization and Classification of Cocoa Bean Shells from Different Regions of Venezuela Using Hplc-Pda-Ms/Ms and Spectrophotometric Techniques Coupled to Chemometric Analysis. Foods 2021, 10, 1791. [Google Scholar] [CrossRef]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Testa, G.; Sottero, B.; Poli, G.; Zeppa, G.; Biasi, F. A Dietary Mixture of Oxysterols Induces in Vitro Intestinal Inflammation through TLR2/4 Activation: The Protective Effect of Cocoa Bean Shells. Antioxidants 2019, 8, 151. [Google Scholar] [CrossRef][Green Version]
- Manzano, P.; Hernández, J.; Quijano-Avilés, M.; Barragán, A.; Chóez-Guaranda, I.; Viteri, R.; Valle, O. Polyphenols Extracted from Theobroma cacao Waste and Its Utility as Antioxidant. Emir. J. Food Agric. 2017, 29, 45–50. [Google Scholar] [CrossRef][Green Version]
- Ferreira-Santos, P.; Genisheva, Z.; Pereira, R.N.; Teixeira, J.A.; Rocha, C. Moderate Electric Fields as a Potential Tool for Sustainable Recovery of Phenolic Compounds from Pinus Pinaster Bark. ACS Sustain. Chem. Eng. 2019, 7, 8816–8826. [Google Scholar] [CrossRef][Green Version]
- Jesus, M.S.; Ballesteros, L.F.; Pereira, R.N.; Genisheva, Z.; Carvalho, A.C.; Pereira-wilson, C.; Teixeira, J.A.; Domingues, L. Ohmic Heating Polyphenolic Extracts from Vine Pruning Residue with Enhanced Biological Activity. Food Chem. 2020, 316, 126298. [Google Scholar] [CrossRef][Green Version]
- Agudelo, C.; Bravo, K.; Ramírez-Atehortúa, A.; Torres, D.; Osorio, E.; Carrillo-Hormaza, L. Chemical and Skincare Property Characterization of the Main Cocoa Byproducts: Extraction Optimization by Rsm Approach for Development of Sustainable Ingredients. Molecules 2021, 26, 7429. [Google Scholar] [CrossRef]
- Carpentieri, S.; Režek Jambrak, A.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds from Aromatic Plants and Food By-Products. Front. Nutr. 2022, 8, 1355. [Google Scholar] [CrossRef]
- Jensch, C.; Schmidt, A.; Strube, J. Versatile Green Processing for Recovery of Phenolic Compounds from Natural Product Extracts towards Bioeconomy and Cascade Utilization for Waste Valorization on the Example of Cocoa Bean Shell (CBS). Sustainability 2022, 14, 3126. [Google Scholar] [CrossRef]
- Leal, C.; Santos, R.A.; Pinto, R.; Queiroz, M.; Rodrigues, M.; José Saavedra, M.; Barros, A.; Gouvinhas, I. Recovery of Bioactive Compounds from White Grape (Vitis vinifera L.) Stems as Potential Antimicrobial Agents for Human Health. Saudi J. Biol. Sci. 2020, 27, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Ma, Y.; Daliri, E.B.M.; Koseki, S.; Wei, S.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Interplay of Antibiotic Resistance and Food-Associated Stress Tolerance in Foodborne Pathogens. Trends Food Sci. Technol. 2020, 95, 97–106. [Google Scholar] [CrossRef]
- Kayaputri, I.L.; Djali, M.; Sukri, N.; Fazaryasti, R.H. The Antimicrobial Effectiveness of Cacao Shell and Cacao Husk Combination on Inhibition of Pathogenic Bacteria in Food Products. IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 012077. [Google Scholar] [CrossRef]
- Faradilla, R.H.F.; Ibrahim, M.N.; Rejeki, S.; Ufrianto, N.; Cahyani, D.R. Understanding the Heat Stability and Solubility of Cocoa Bean Shell Extract as Antioxidant and Antibacterial Functional Ingredients. Int. Food Res. J. 2020, 27, 660–665. [Google Scholar]
- Rojo-Poveda, O.; Ribeiro, S.O.; Anton-Sales, C.; Keymeulen, F.; Barbosa-Pereira, L.; Delporte, C.; Zeppa, G.; Stévigny, C. Evaluation of Cocoa Bean Shell Antimicrobial Activity: A Tentative Assay Using a Metabolomic Approach for Active Compound Identification. Planta Med. 2021, 87, 841–849. [Google Scholar] [CrossRef]
- Babu, N.S.V.; Vivek, D.K.; Ambika, G. Comparative Evaluation of Chlorhexidine Mouthrinse versus Cacao Bean Husk Extract Mouthrinse as Antimicrobial Agents in Children. Eur. Arch. Paediatr. Dent. 2011, 12, 245–249. [Google Scholar] [CrossRef]
- Marotta, M.; Martino, A.; De Rosa, A.; Farina, E.; Cartenı, M.; De Rosa, M. Degradation of Dental Plaque Glucans and Prevention of Glucan formation using commercial enzymes. Process Biochem. 2002, 38, 101–108. [Google Scholar] [CrossRef]
- Percival, R.S.; Devine, D.A.; Duggal, M.S.; Chartron, S.; Marsh, P.D. The Effect of Cocoa Polyphenols on the Growth, Metabolism, and Biofilm Formation by Streptococcus Mutans and Streptococcus Sanguinis. Eur. J. Oral Sci. 2006, 114, 343–348. [Google Scholar] [CrossRef]
- Osawa, K.; Miyazaki, K.; Shimura, S.; Okuda, J.; Matsumoto, M.; Ooshima, T. Identification of Cariostatic Substances in the Cacao Bean Husk: Their Anti-Glucosyltransferase and Antibacterial Activities. J. Dent. Res. 2001, 80, 2000–2004. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tsuji, M.; Okuda, J.; Sasaki, H.; Nakano, K.; Osawa, K.; Shimura, S.; Ooshima, T. Inhibitory Effects of Cacao Bean Husk Extract on Plaque Formation in Vitro and in Vivo. Eur. J. Oral Sci. 2004, 112, 249–252. [Google Scholar] [CrossRef]
- Nieto-Figueroa, K.H.; Mendoza-García, N.V.; Campos-Vega, R. Cocoa By-Products. In Food Wastes and By-Products: Nutraceutical and Health Potential; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 373–410. [Google Scholar]
- Sakagami, H.; Matsuta, T. Biological Activity of Cacao Husk and Mass Lignin-Carbohydrate Complexes. In Chocolate in Health and Nutrition; Humana: Totowa, NJ, USA, 2013; pp. 247–262. ISBN 9781617798030. [Google Scholar]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Teleky, B.E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Călinoiu, L.F.; Pascuta, M.S.; Varvara, R.A.; Szabo, K.; Vajda, P.; Szekely, C.; et al. Development of Pectin and Poly(Vinyl Alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. [Google Scholar] [CrossRef]
- Kirwan, M.J.; Plant, S.; Strawbridge, J.W. Plastics in Food Packaging. In Food and Beverage Packaging Technology: Second Edition; Wiley: Hoboken, NJ, USA, 2011; pp. 157–212. ISBN 9781405189101. [Google Scholar]
- Nguyen, N.A.; Bowland, C.C.; Naskar, A.K. A General Method to Improve 3D-Printability and Inter-Layer Adhesion in Lignin-Based Composites. Appl. Mater. Today 2018, 12, 138–152. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and Characterization of Plasticized PLA/PHB Blends for Biodegradable Multiphase Systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Somdee, P.; Hasook, A. Effect of Modified Eggshell Powder on Physical Properties of Poly(Lactic Acid) and Natural Rubber Composites. Mater. Today Proc. 2017, 4, 6502–6511. [Google Scholar] [CrossRef]
- Papadopoulou, E.L.; Paul, U.C.; Tran, T.N.; Suarato, G.; Ceseracciu, L.; Marras, S.; D’arcy, R.; Athanassiou, A. Sustainable Active Food Packaging from Poly(Lactic Acid) and Cocoa Bean Shells. ACS Appl. Mater. Interfaces 2019, 11, 31317–31327. [Google Scholar] [CrossRef]
- Puglia, D.; Dominici, F.; Badalotti, M.; Santulli, C.; Kenny, J.M. Tensile, Thermal and Morphological Characterization of Cocoa Bean Shells (CBS)/Polycaprolactone-Based Composites. J. Renew. Mater. 2016, 4, 199–205. [Google Scholar] [CrossRef]
- Garcia-Brand, A.J.; Morales, M.A.; Hozman, A.S.; Ramirez, A.C.; Cruz, L.J.; Maranon, A.; Muñoz-Camargo, C.; Cruz, J.C.; Porras, A. Bioactive Poly(Lactic Acid)–Cocoa Bean Shell Composites for Biomaterial Formulation: Preparation and Preliminary in Vitro Characterization. Polymers 2021, 13, 3707. [Google Scholar] [CrossRef]
- Murthy, G.S.; Kumar, D.; Strauss, S.; Dalton, D.; Vionocur, J. Feasibility Analysis of Poly-β-Hydroxybutyrate (PHB) Extraction from Hybrid Poplar Leaves. Am. Soc. Agric. Biol. Eng. 2010, 6, 4461–4471. [Google Scholar]
- Mohanrasu, K.; Premnath, N.; Siva Prakash, G.; Sudhakar, M.; Boobalan, T.; Arun, A. Exploring Multi Potential Uses of Marine Bacteria; an Integrated Approach for PHB Production, PAHs and Polyethylene Biodegradation. J. Photochem. Photobiol. B Biol. 2018, 185, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Silviya, N.; Binod, P.; Pandey, A. Pentose-Rich Hydrolysate from Acid Pretreated Rice Straw as a Carbon Source for the Production of Poly-3-Hydroxybutyrate. Biochem. Eng. J. 2013, 78, 67–72. [Google Scholar] [CrossRef]
- Balakrishna Pillai, A.; Jaya Kumar, A.; Kumarapillai, H. Enhanced Production of Poly(3-Hydroxybutyrate) in Recombinant Escherichia Coli and EDTA–Microwave-Assisted Cell Lysis for Polymer Recovery. AMB Express 2018, 8, 142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boyd, G.R.; Zhang, S.; Grimm, D.A. Naproxen Removal from Water by Chlorination and Biofilm Processes. Water Res. 2005, 39, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Zabczynski, S.; Göbel, A.; Hoffmann, B.; Löffler, D.; McArdell, C.S.; Ternes, T.A.; Thomsen, A.; Siegrist, H. Biological Degradation of Pharmaceuticals in Municipal Wastewater Treatment: Proposing a Classification Scheme. Water Res. 2006, 40, 1686–1696. [Google Scholar] [CrossRef]
- Esplugas, S.; Bila, D.M.; Krause, L.G.T.; Dezotti, M. Ozonation and Advanced Oxidation Technologies to Remove Endocrine Disrupting Chemicals (EDCs) and Pharmaceuticals and Personal Care Products (PPCPs) in Water Effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar] [CrossRef]
- Sol, D.; Menéndez-Manjón, A.; Arias-García, P.; Laca, A.; Laca, A.; Rancaño, A.; Díaz, M. Occurrence of Selected Emerging Contaminants in Southern Europe WWTPs: Comparison of Simulations and Real Data. Processes 2022, 10, 2491. [Google Scholar] [CrossRef]
- Solís-Balbín, C.; Sol, D.; Laca, A.; Laca, A.; Díaz, M. Destruction and Entrainment of Microplastics in Ozonation and Wet Oxidation Processes. J. Water Process. Eng. 2023, 51, 103456. [Google Scholar] [CrossRef]
- Al-yousef, H.A.; Alotaibi, B.M.; Aouaini, F.; Bonilla-petriciolet, A. Adsorption of Ibuprofen on Cocoa Shell Biomass-Based Adsorbents: Interpretation of the Adsorption Equilibrium via Statistical Physics Theory. J. Mol. Liq. 2021, 331, 115697. [Google Scholar] [CrossRef]
- Belibi, J.-R.; Takam, B.; Dalhtou, S.; Tarkwa, J.-B.; Sop, B.; Acayanka, E.; Kamgang, G.; Laminsi, S. Multi-Functionalized Cellulosic Biomass by Plasma-Assisted Bonding of α-Amino Carboxylic Acid to Enhance the Removal of Ibuprofen in Aqueous Solution. J. Polym. Environ. 2021, 29, 1176–1191. [Google Scholar] [CrossRef]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent Advances in Applications of Activated Carbon from Biowaste for Wastewater Treatment: A Short Review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Oulego, P.; Laca, A.; Calvo, S.; Díaz, M. Eggshell-Supported Catalysts for the Advanced Oxidation Treatment of Humic Acid Polluted Wastewaters. Water 2020, 12, 100. [Google Scholar] [CrossRef][Green Version]
- Salman, T.; Temel, F.A.; Turan, N.G.; Ardali, Y. Adsorption of Lead (II) Ions onto Diatomite from Aqueous Solutions: Mechanism, Isotherm and Kinetic Studies. Glob. Nest J. 2016, 18, 1–10. [Google Scholar] [CrossRef]
- Rodriguez-Arellano, G.; Barajas-Fernández, J.; García-Alamilla, R.; Lagunes-Gálvez, L.; Lara-Rivera, A.H.; García-Alamilla, P. Evaluation of Cocoa Beans Shell Powder as a Bioadsorbent of Congo Red Dye Aqueous Solutions. Materials 2021, 14, 2763. [Google Scholar] [CrossRef]
- Ribas, M.C.; Adebayo, M.A.; Prola, L.D.T.; Lima, E.C.; Cataluña, R.; Feris, L.A.; Puchana-Rosero, M.J.; Machado, F.M.; Pavan, F.A.; Calvete, T. Comparison of a Homemade Cocoa Shell Activated Carbon with Commercial Activated Carbon for the Removal of Reactive Violet 5 Dye from Aqueous Solutions. Chem. Eng. J. 2014, 248, 315–326. [Google Scholar] [CrossRef]
- El Achaby, M.; Fayoud, N.; Figueroa-Espinoza, M.C.; Ben Youcef, H.; Aboulkas, A. New Highly Hydrated Cellulose Microfibrils with a Tendril Helical Morphology Extracted from Agro-Waste Material: Application to Removal of Dyes from Waste Water. RSC Adv. 2018, 8, 5212–5224. [Google Scholar] [CrossRef][Green Version]
- Kalaivani, S.S.; Vidhyadevi, T.; Murugesan, A.; Baskaralingam, P.; Anuradha, C.D.; Ravikumar, L.; Sivanesan, S. Equilibrium and Kinetic Studies on the Adsorption of Ni(II) Ion from an Aqueous Solution Using Activated Carbon Prepared from Theobroma cacao (Cocoa) Shell. Desalination Water Treat. 2015, 54, 1629–1641. [Google Scholar] [CrossRef]
- Kamp, A.; Østergård, H. Environmental Sustainability Assessment of Fruit Cultivation and Processing Using Fruit and Cocoa Residues for Bioenergy and Compost. Case Study from Ghana. J. Clean. Prod. 2016, 129, 329–340. [Google Scholar] [CrossRef]
- Awolu, O.; Oyeyemi; Oyetuji, S. Optimization of Bioethanol Production from Cocoa (Theobroma cacao) Bean Shell. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 506–514. [Google Scholar]
- Shet, V.B.; Shetty, V.C.; Siddik, A.; Rakshith, K.G.; Shetty, N.J.; Goveas, L.C.; D’Mello, G.; Rao, C.V.; Ujwal, P.; Aparna, A. Optimization of Microwave Assisted H2SO4 Hydrolysis of Cocoa Pod Shells: Comparison between Response Surface Methodology and Artificial Neural Network and Production of Bioethanol Thereof. J. Microbiol. Biotechnol. Food Sci. 2018, 7, 473–477. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Effect of N-Methylmorpholine-N-Oxide Pretreatment on Biogas Production from Rice Straw, Cocoa Shell, and Hazelnut Skin. Environ. Eng. Sci. 2016, 33, 843–850. [Google Scholar] [CrossRef]
- Thompson, S.O.; Rough, S.L. The Densification of Cocoa Bean Shells for Bioenergy Purposes. Biomass Bioenergy 2021, 148, 106057. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.H.; Hemapriya, V.; Chung, I.M. Tragia Plukenetii Extract as an Eco-Friendly Inhibitor for Mild Steel Corrosion in HCl 1 M Acidic Medium. Res. Chem. Intermed. 2016, 42, 3703–3719. [Google Scholar] [CrossRef]
- Anupama, K.K.; Ramya, K.; Joseph, A. Electrochemical Measurements and Theoretical Calculations on the Inhibitive Interaction of Plectranthus amboinicus Leaf Extract with Mild Steel in Hydrochloric Acid. Measurement 2017, 95, 297–305. [Google Scholar] [CrossRef]
- Da Rocha, J.C.; Da Cunha Ponciano Gomes, J.A.; D’Elia, E. Aqueous Extracts of Mango and Orange Peel as Green Inhibitors for Carbon Steel in Hydrochloric Acid Solution. Mater. Res. 2014, 17, 1581–1587. [Google Scholar] [CrossRef][Green Version]
- Singh, A.; Ebenso, E.E.; Quraishi, M.A. Corrosion Inhibition of Carbon Steel in HCl Solution by Some Plant Extracts. Int. J. Corros. 2012, 2012, 1–20. [Google Scholar] [CrossRef][Green Version]
- Rani, B.E.A.; Basu, B.B.J. Green Inhibitors for Corrosion Protection of Metals and Alloys: An Overview. Int. J. Corros. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- de Carvalho, M.C.F.; E Silva, I.M.F.C.R.; Macedo, P.L.A.; Tokumoto, M.S.; da Cruz, R.S.; Capelossi, V.R. Assessment of the Hydroalcoholic Extract and Powder Cocoa Bean Shell as Corrosion Inhibitors for Carbon Steel in Sodium Chloride Solution. Rev. Mater. 2021, 26, 1–17. [Google Scholar] [CrossRef]
- De Carvalho, M.C.F.; De Almeida, N.M.S.; Silva, I.M.F.C.R.; Cotting, F.; Aoki, I.V.; Capelossi, V.R. Electrochemical and Economic Evaluation of the Cocoa Bean Shell as a Corrosion Inhibitor in Acidic Medium. Mater. Res. 2022, 25, 1–13. [Google Scholar] [CrossRef]
- Martínez-Cervera, S.; Salvador, A.; Muguerza, B.; Moulay, L.; Fiszman, S.M. Cocoa Fibre and Its Application as a Fat Replacer in Chocolate Muffins. LWT 2011, 44, 729–736. [Google Scholar] [CrossRef]
- Öztürk, E.; Ova, G. Evaluation of Cocoa Bean Hulls as a Fat Replacer on Functional Cake Production. Turk. J. Agric.—Food Sci. Technol. 2018, 6, 1043. [Google Scholar] [CrossRef][Green Version]
- Collar, C.; Rosell, C.M.; Muguerza, B.; Moulay, L. Breadmaking Performance and Keeping Behavior of Cocoa-Soluble Fiber-Enriched Wheat Breads. Food Sci. Technol. Int. 2009, 15, 79–87. [Google Scholar] [CrossRef]
- Rinaldi, M.; Littardi, P.; Paciulli, M.; Caligiani, A.; Chiavaro, E. Effect of Cocoa Bean Shells Granulometries on Qualitative Properties of Gluten-Free Bread during Storage. Eur. Food Res. Technol. 2020, 246, 1583–1590. [Google Scholar] [CrossRef]
- Karklina, D.; Gedrovica, I.; Reca, M.; Kronberga, M. Production of Biscuits with Higher Nutritional Value. Proc. Latv. Acad. Sciences. Sect. B Nat. Exact Appl. Sci. 2012, 66, 113–116. [Google Scholar] [CrossRef][Green Version]
- Nogueira, F.; Rocha Vieira, S.; Leopoldina Lamounier Campidelli, M.; Abadia Reis Rocha, R.; Milani Avelar Rodrigues, L.; Henrique Santos, P.; de Deus Souza Carneiro, J.; Maria de Carvalho Tavares, I.; Patrícia de Oliveira, C. Impact of Using Cocoa Bean Shell Powder as a Substitute for Wheat Flour on Some of Chocolate Cake Properties. Food Chem. 2022, 381, 132215. [Google Scholar] [CrossRef]
- Barišić, V.; Petrović, J.; Lončarević, I.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Doko, K.; Blažić, M.; Ačkar, Ð. Physical Properties of Chocolates Enriched with Untreated Cocoa Bean Shells and Cocoa Bean Shells Treated with High-Voltage Electrical Discharge. Sustainability 2021, 13, 2620. [Google Scholar] [CrossRef]
- Fernandes, A.; Mateus, N.; de Freitas, V. Polyphenol-Dietary Fiber Conjugates from Fruits and Vegetables: Nature and Biological Fate in a Food and Nutrition Perspective. Foods 2023, 12, 1052. [Google Scholar] [CrossRef]
- Costa, R.D.S.; de Almeida, S.S.; Cavalcanti, E.D.C.; Freire, D.M.G.; Moura-Nunes, N.; Monteiro, M.; Perrone, D. Enzymes Produced by Solid State Fermentation of Agro-Industrial by-Products Release Ferulic Acid in Bioprocessed Whole-Wheat Breads. Food Res. Int. 2021, 140, 09843. [Google Scholar] [CrossRef]
- Grassia, M.; Messia, M.C.; Marconi, E.; Demirkol, Ş.; Erdoğdu, F.; Sarghini, F.; Cinquanta, L.; Corona, O.; Planeta, D. Microencapsulation of Phenolic Extracts from Cocoa Shells to Enrich Chocolate Bars. Plant Foods Hum. Nutr. 2021, 76, 449–457. [Google Scholar] [CrossRef]
- Altin, G.; Gültekin-Özgüven, M.; Ozcelik, B. Liposomal Dispersion and Powder Systems for Delivery of Cocoa Hull Waste Phenolics via Ayran (Drinking Yoghurt): Comparative Studies on in-Vitro Bioaccessibility and Antioxidant Capacity. Food Hydrocoll. 2018, 81, 364–370. [Google Scholar] [CrossRef]
- Quijano-Aviles, M.F.; Franco-Agurto, G.L.; Suárez-Quirumbay, K.B.; Barragán-Lucas, A.D.; Manzano-Santana, P.I. Linear Programming Formulation of a Dairy Drink Made of Cocoa, Coffee and Orange by-Products. Emir. J. Food Agric. 2016, 28, 554–559. [Google Scholar] [CrossRef][Green Version]
- Siow, C.S.; Chan, E.W.C.; Wong, C.W.; Ng, C.W. Antioxidant and Sensory Evaluation of Cocoa (Theobroma cacao L.) Tea Formulated with Cocoa Bean Hull of Different Origins. Futur. Foods 2022, 5, 100108. [Google Scholar] [CrossRef]
- Dos Anjos, S.M.; Martins, M.V.; de Souza, V.B.; Tulini, F.L. Evaluation of the Nutritional Composition of Cocoa Bean Shell Waste (Theobroma cacao) and Application in the Production of a Phenolic-Rich Iced Tea. J. Culin. Sci. Technol. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Bernaert, H.; Ruysscher, I. Process, Use, and Product. US Patent 2016135478A1, May 2016. [Google Scholar]
- Copetti, M.V.; Iamanaka, B.T.; Nester, M.A.; Efraim, P.; Taniwaki, M.H. Occurrence of Ochratoxin A in Cocoa By-Products and Determination of Its Reduction during Chocolate Manufacture. Food Chem. 2013, 136, 100–104. [Google Scholar] [CrossRef][Green Version]
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Milicević, B.; Doko, K.; Ackar, D. Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution. Sustainability 2020, 12, 3981. [Google Scholar] [CrossRef]
- Ciecierska, M. Cocoa Beans of Different Origins and Varieties and Their Derived Products Contamination with Polycyclic Aromatic Hydrocarbons. Food Chem. 2020, 317, 126408. [Google Scholar] [CrossRef]
- Assa, A.; Noor, A.; Yunus, M.R.; Misnawi; Djide, M.N. Heavy Metal Concentrations in Cocoa Beans (Theobroma cacao L.) Originating from East Luwu, South Sulawesi, Indonesia. J. Phys. Conf. Ser. 2018, 979, 012011. [Google Scholar] [CrossRef]
- Meunier, N.; Blais, J.F.; Tyagi, R.D. Removal of Heavy Metals from Acid Soil Leachate Using Cocoa Shells in a Batch Counter-Current Sorption Process. Hydrometallurgy 2004, 73, 225–235. [Google Scholar] [CrossRef]
- Renna, M.; Lussiana, C.; Colonna, L.; Malfatto, V.M.; Mimosi, A.; Cornale, P. Inclusion of Cocoa Bean Shell in the Diet of Dairy Goats: Effects on Milk Production Performance and Milk Fatty Acid Profile. Front. Vet. Sci. 2022, 9, 1–15. [Google Scholar] [CrossRef]
- Emiola, I.A.; Ojebiyi, O.O.; Akande, T.O. Performance and Organ Weights of Paying Hens Fed Diets Containing Graded Levels of Sun-Dried Cocoa Beal Shell (CBS). Int. J. Poult. Sci. 2011, 10, 987–990. [Google Scholar] [CrossRef][Green Version]
- Giamouri, E.; Pappas, A.C.; Papadomichelakis, G.; Simitzis, P.E.; Manios, T.; Zentek, J.; Lasaridi, K.; Tsiplakou, E.; Zervas, G. The Food for Feed Concept: Redefining the Use of Hotel Food Residues in Broiler Diets. Sustainability 2022, 14, 3659. [Google Scholar] [CrossRef]
- Olubamiwa, O.; Ikyo, S.M.; Adeboale, B.A.; Omojola, A.B.; Hamzat, R.A. Effect of Boligin Time on the Utilization of Cocoa Ban Shell in Laying Hen Feeds. Int. J. Poult. Sci. 2006, 5, 1137–1139. [Google Scholar]
- Ayinde, O.E.; Ojo, V.; Adeyina, A.A.; Adesoye, O. Economics of Using Cocoa Bean Shell as Feed Supplement of Rabbits. Pak. J. Nutr. 2010, 9, 195–197. [Google Scholar] [CrossRef][Green Version]
- Hikmah, H.; Alam, G.; Syamsu, J.A.; Salengke, S.; Nahariah, N. The Digestive and Physiological Visceral Organs of Male Bali Cattle Were Fed with Cocoa Bean Shell. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012063. [Google Scholar] [CrossRef]
- Campione, A.; Pauselli, M.; Natalello, A.; Valenti, B.; Pomente, C.; Avondo, M.; Luciano, G.; Caccamo, M.; Morbidini, L. Inclusion of Cocoa By-Product in the Diet of Dairy Sheep: Effect on the Fatty Acid Profile of Ruminal Content and on the Composition of Milk and Cheese. Animal 2021, 15, 100243. [Google Scholar] [CrossRef]

| Content | References | |
|---|---|---|
| Moisture | 4–13.1 g/100 g | [25,27,28,29] |
| Ash | 6.0–9.1 g/100 g | [27,28,30] |
| Carbohydrates | 13.2–70.3 g/100 g | [25,29,31,32,33] |
| Proteins | 18.2–27.4 g/100 g | [27,29,33,34,35] |
| Lipids | 2.3–6.5 g/100 g | [25,27,36,37] |
| Dietary fibres | 13.8–65.6 g/100 g | [25,27,29] |
| Total phenolic content | 22–100 mg GAE/g | [28,30,38,39,40] |
| Total flavonoid content | 7.5–21.8 mg RU/g1.6–43.9 mg CE/g | [39,40,41,42,43] |
| Total tannin content | 2.3–25.3 mg CE/g | [39,40] |
| Flavanols | ||
| Catechin | 0.8–5.7 mg/g | [25,44,45,46] |
| Epicatechin | 0.6–30 mg/g | [25,44,45,46,47] |
| Procyanidin B1 | 0.5–0.8 mg/g | [48] |
| Procyanidin B2 | 0.2–1.4 mg/g | [48,49] |
| Methylxanthines | ||
| Theobromine | 0.6–13.5 mg/g | [25,30,49,50] |
| Caffeine | 0.1–1.1 mg/g | [30,51] |
| Theophylline | 0.1–0.3 mg/g | [51] |
| Extraction Method | Value | References | |
|---|---|---|---|
| Antioxidant activity | Ethanol, methanol–acetone, water, UAE, MAE | 2.5–218 µM TE/g CBS | [28,30,39,48] |
| Total phenolic content | Ethanol, methanol–acetone, water, methanol, acetone, UAE, SWE, PEF, MAE | 5.8–154.4 mg GAE/g CBS | [30,32,40,61,63,79,80] |
| Total flavonoid content | Ethanol, acetone, methanol, PEF | 1.6–43.9 mg CE/g CBS | [39,40] |
| Total tannin content | Ethanol, acetone, methanol, PEF | 0.8–25.3 mg CE/g CBS | [39,40] |
| Total dietary fibre | Ethanol, acetone, methanol | 51.8–56.7 g/100 g CBS | [32] |
| Soluble dietary fibre | Ethanol, acetone, methanol | 14.5–16.2 g/100 g CBS | [32] |
| Insoluble dietary fibre | Ethanol, acetone, methanol | 35.6–42.1 g/100 g CBS | [32] |
| Theobromine | Ethanol, methanol, water, UAE, SWE, PEF, MAE | 1.3–11.6 mg/g CBS | [28,30,40,42,79,80,83] |
| Caffeine | UAE, SWE, PEF, MAE | 0.1–4.2 mg/g CBS | [30,40,42,50] |
| Theophylline | SWE | Traces-0.2 mg/g CBS | [42] |
| Catechin | Ethanol, water, methanol, PHWE | 0.2–6.1 mg/g CBS | [49,61,83,85] |
| Epicatechin | Ethanol, methanol, water, PEF, MAE, PHWE | 0.3–17.7 mg/g CBS | [28,40,48,61,83] |
| Procyanidin B1 | Ethanol, water | 0.5–0.8 mg/g CBS | [48] |
| Procyanidin B2 | Ethanol, water | 0.2–0.9 mg/g CBS | [48] |
| Protocatechuic acid | Ethanol, water, MAE | 0.9–2.1 mg/g CBS | [28,48] |
| Caffeic acid | Ethanol, water, methanol, MAE | Traces-0.9 mg/g CBS | [28,61] |
| Ferulic acid | MAE | 0.3–0.5 mg/g CBS | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Cocoa Bean Shell: A By-Product with High Potential for Nutritional and Biotechnological Applications. Antioxidants 2023, 12, 1028. https://doi.org/10.3390/antiox12051028
Sánchez M, Laca A, Laca A, Díaz M. Cocoa Bean Shell: A By-Product with High Potential for Nutritional and Biotechnological Applications. Antioxidants. 2023; 12(5):1028. https://doi.org/10.3390/antiox12051028
Chicago/Turabian StyleSánchez, Marta, Amanda Laca, Adriana Laca, and Mario Díaz. 2023. "Cocoa Bean Shell: A By-Product with High Potential for Nutritional and Biotechnological Applications" Antioxidants 12, no. 5: 1028. https://doi.org/10.3390/antiox12051028
APA StyleSánchez, M., Laca, A., Laca, A., & Díaz, M. (2023). Cocoa Bean Shell: A By-Product with High Potential for Nutritional and Biotechnological Applications. Antioxidants, 12(5), 1028. https://doi.org/10.3390/antiox12051028









