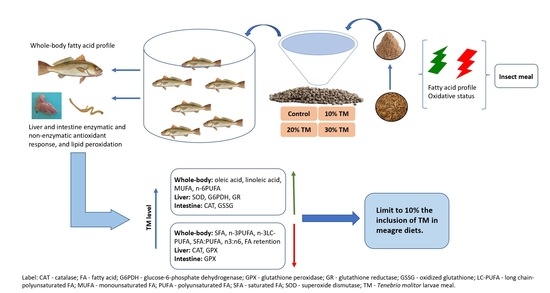
Feeding Yellow Worms to Meagre: Effects on Whole-Body Fatty Acid Profile and Hepatic and Intestine Oxidative Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets and Growth Trial
2.2. Sampling and Proximate Analysis
2.3. Enzymes Activities, Lipid Peroxidation Determination, and Glutathione
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Regulation (EU), No. 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R2283 (accessed on 27 February 2023).
- Regulation (EU), No. 2017/893 of the European Parliament and of the Council of 24 May 2017 as Regards the Provisions on Processed Animal Proteins (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0893&from=NL (accessed on 27 February 2023).
- Regulation (EU), No. 2021/1372 of the European Parliament and of the Council of 17 August 2021 as Regards the Prohibition to Feed Non-Ruminant Farmed Animals, Other Than Fur Animals, with Protein Derived from Animals (Text with EEA Relevance). Available online: https://www.stradalex.eu/en/se_src_publ_leg_eur_jo/toc/leg_eur_jo_3_20210818_295/doc/ojeu_2021.295.01.0001.01 (accessed on 27 February 2023).
- Errico, S.; Spagnoletta, A.; Verardi, A.; Moliterni, S.; Dimatteo, S.; Sangiorgio, P. Tenebrio molitor as a source of interesting natural compounds, their recovery processes, biological effects, and safety aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 148–197. [Google Scholar] [CrossRef]
- Shafique, L.; Abdel-Latif, H.M.; Hassan, F.-U.; Alagawany, M.; Naiel, M.A.E.; Dawood, M.A.O.; Yilmaz, S.; Liu, Q. The feasibility of using yellow mealworms (Tenebrio molitor): Towards a sustainable aquafeed industry. Animals 2021, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Maulu, S.; Langi, S.; Hasimuna, O.J.; Missinhoun, D.; Munganga, B.P.; Hampuwo, B.M.; Gabriel, N.N.; Elsabagh, M.; Doan, H.V.; Kari, Z.A.; et al. Recent advances in the utilization of insects as an ingredient in aquafeeds: A review. Anim. Nutr. 2022, 11, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Alfiko, Y.; Xie, D.; Astuti, R.T.; Wong, J.; Wang, L. Insects as a feed ingredient for fish culture: Status and trends. Aquac. Fish. 2022, 7, 166–178. [Google Scholar] [CrossRef]
- Sankian, Z.; Khosravi, S.; Kim, Y.O.; Lee, S.M. Effects of dietary inclusion of yellow mealworm (Tenebrio molitor) meal on growth performance, feed utilization, body composition, plasma biochemical indices, selected immune parameters and antioxidant enzyme activities of mandarin fish (Siniperca scherzeri) juveniles. Aquaculture 2018, 496, 79–87. [Google Scholar] [CrossRef]
- Guerreiro, I.; Castro, C.; Antunes, B.; Coutinho, F.; Rangel, F.; Couto, A.; Serra, C.R.; Peres, H.; Pousão-Ferreira, P.; Matos, E.; et al. Catching black soldier fly for meagre: Growth, whole-body fatty acid profile and metabolic responses. Aquaculture 2020, 516, 734613. [Google Scholar] [CrossRef]
- Mastoraki, M.; Ferrándiz, P.M.; Vardali, S.C.; Kontodimas, D.C.; Kotzamanis, Y.P.; Gasco, L.; Chatzifotis, S.; Antonopoulou, E. A comparative study on the effect of fish meal substitution with three different insect meals on growth, body composition and metabolism of European sea bass (Dicentrarchus labrax L.). Aquaculture 2020, 528, 735511. [Google Scholar] [CrossRef]
- Mastoraki, M.; Katsika, L.; Enes, P.; Guerreiro, I.; Kotzamanis, Y.P.; Gasco, L.; Chatzifotis, S.; Antonopoulou, E. Insect meals in feeds for juvenile gilthead seabream (Sparus aurata): Effects on growth, blood chemistry, hepatic metabolic enzymes, body composition and nutrient utilization. Aquaculture 2022, 561, 738674. [Google Scholar] [CrossRef]
- Mourente, G.; Bell, J.G.; Tocher, D.R. Does dietary tocopherol level affect fatty acid metabolism in fish? Fish Physiol. Biochem. 2007, 33, 269–280. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Giordano, E.; Visioli, F. Long-chain omega 3 fatty acids: Molecular bases of potential antioxidant actions. Prostaglandins Leukot. Essent. Fatty Acids 2014, 90, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Kim, S.K. Antioxidant effects of chitin, chitosan and their derivatives. Adv. Food Nutr. Res. 2014, 73, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Gong, Y.; Cao, S.; Lu, F.; Han, D.; Liu, H.; Jin, J.; Yang, Y.; Zhu, X.; Xie, S. Effects of dietary Tenebrio molitor meal on the growth performance, immune response and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2017, 69, 59–66. [Google Scholar] [CrossRef]
- Henry, M.A.; Gai, F.; Enes, P.; Peréz-Jiménez, A.; Gasco, L. Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 83, 308–313. [Google Scholar] [CrossRef]
- Hidalgo, M.C.; Morales, A.E.; Pula, H.J.; Tomás-Almenar, C.; Sánchez-Muros, M.J.; Melenchón, F.; Fabrikov, D.; Cardenete, G. Oxidative metabolism of gut and innate immune status in skin and blood of tench (Tinca tinca) fed with different insect meals (Hermetia illucens and Tenebrio molitor). Aquaculture 2022, 558, 738384. [Google Scholar] [CrossRef]
- Gu, J.; Liang, H.; Ge, X.; Xia, D.; Pan, L.; Mi, H.; Ren, M. A study of the potential effect of yellow mealworm (Tenebrio molitor) substitution for fish meal on growth, immune and antioxidant capacity in juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2022, 120, 214–221. [Google Scholar] [CrossRef]
- Song, S.-G.; Chi, S.-Y.; Tan, B.-P.; Liang, G.-L.; Lu, B.-Q.; Dong, X.-H.; Yang, Q.-H.; Liu, H.-Y.; Zhang, S. Effects of fishmeal replacement by Tenebrio molitor meal on growth performance, antioxidant enzyme activities and disease resistance of the juvenile pearl gentian grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀). Aquac. Res. 2018, 49, 2210–2217. [Google Scholar] [CrossRef]
- Bousdras, T.; Feidantsis, K.; Panteli, N.; Chatzifotis, S.; Piccolo, G.; Gasco, L.; Gai, F.; Antonopoulou, E. Dietary Tenebrio molitor larvae meal inclusion exerts tissue-specific effects on cellular, metabolic, and antioxidant status in European sea bass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata). Aquac. Nutr. 2022, 2022, 9858983. [Google Scholar] [CrossRef]
- Coutinho, F.; Castro, C.; Guerreiro, I.; Rangel, F.; Couto, A.; Serra, C.R.; Peres, H.; Pousão-Ferreira, P.; Rawski, M.; Oliva-Teles, A.; et al. Mealworm larvae meal in diets for meagre juveniles: Growth, nutrient digestibility and digestive enzymes activity. Aquaculture 2021, 535, 736362. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Santha, N.C.; Ackman, R.G. Nervonic acid versus tricosanoic acid as internal standards in quantitative gas chromatographic analyses of fish oil longer-chain n−3 polyunsaturated fatty acid methyl esters. J. Chromatogr. B Biomed. Appl. 1990, 533, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Corraze, G.; Panserat, S.; Oliva-Teles, A. Effects of fish oil replacement by a vegetable oil blend on digestibility, postprandial serum metabolite profile, lipid and glucose metabolism of European sea bass (Dicentrarchus labrax) juveniles. Aquacult. Nutr. 2015, 21, 592–603. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein. J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.E.; García Rejón, L.; de la Higuera, M. Influence of handling and/or anaesthesia on stress response in rainbow trout. Effects on liver primary metabolism. Comp. Biochem. Physiol. A 1990, 95, 87–93. [Google Scholar] [CrossRef]
- Morales, A.E.; Pérez-Jiménez, A.; Hidalgo, M.C.; Abellán, E.; Cardenete, G. Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentex liver. Comp. Biochem. Physiol. C 2004, 139, 153–161. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assay of glutathione peroxidase. Methods Enzymol. 1984, 105, 115–121. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using gluthatione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Vandeputte, C.; Guizon, I.; Genestie-Denis, I.; Vannier, B.; Lorenzon, G. A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: Performance study of a new miniaturized protocol. Cell Biol. Toxicol. 1994, 10, 415–421. [Google Scholar] [CrossRef]
- Castro, C.; Diogenes, A.F.; Coutinho, F.; Panserat, S.; Corraze, G.; Pérez-Jiménez, A.; Peres, H.; Oliva-Teles, A. Liver and intestine oxidative status of gilthead sea bream fed vegetable oil and carbohydrate rich diets. Aquaculture 2016, 464, 665–672. [Google Scholar] [CrossRef]
- Belforti, M.; Gai, F.; Lussiana, C.; Renna, M.; Malfatto, V.; Rotolo, L.; De Marco, M.; Dabbou, S.; Schiavone, A.; Zoccarato, I.; et al. Tenebrio molitor meal in rainbow trout (Oncorhynchus mykiss) diets: Effects on animal performance, nutrient digestibility and chemical composition of fillets. Ital. J. Anim. Sci. 2015, 14, 4170. [Google Scholar] [CrossRef]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Anim. Feed Sci. Technol. 2016, 220, 34–45. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.; De Haro, C.; Sanz, A.; Trenzado, C.E.; Villareces, S.; Barroso, F.G. Nutritional evaluation of Tenebrio molitor meal as fishmeal substitute for tilapia (Oreochromis niloticus) diet. Aquac. Nutr. 2016, 22, 943–955. [Google Scholar] [CrossRef]
- Iaconisi, V.; Marono, S.; Parisi, G.; Gasco, L.; Genovese, L.; Maricchiolo, G.; Bovera, F.; Piccolo, G. Dietary inclusion of Tenebrio molitor larvae meal: Effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo). Aquaculture 2017, 476, 49–58. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Glutathione Peroxidases. In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 399–404. [Google Scholar] [CrossRef]
- Castro, C.; Coutinho, F.; Iglesias, P.; Oliva-Teles, A.; Couto, A. Chlorella sp. and Nannochloropsis sp. inclusion in plant-based diets modulate the intestine and liver antioxidant mechanisms of European sea bass juveniles. Front. Vet. Sci. 2020, 7, 607575. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, R.; Guerreiro, I.; Santos, R.A.; Coutinho, F.; Couto, A.; Serra, C.R.; Olsen, R.E.; Peres, H.; Oliva-Teles, A. Oxidative status and intestinal health of gilthead sea bream (Sparus aurata) juveniles fed diets with different ARA/EPA/DHA ratios. Sci. Rep. 2020, 10, 13824. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Elia, A.C.; Capucchio, M.T.; Caldaroni, B.; Magara, G.; Dörr, A.J.M.; Biasato, I.; Biasibetti, E.; Righetti, M.; Pastorino, P.; Prearo, M.; et al. Influence of Hermetia illucens meal dietary inclusion on the histological traits, gut mucin composition and the oxidative stress biomarkers in rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 496, 50–57. [Google Scholar] [CrossRef]
| Diets | ||||
|---|---|---|---|---|
| CTR | HM10 | HM20 | HM30 | |
| Ingredients (% dry weight basis) | ||||
| Fish meal 1 | 40.0 | 30.1 | 20.1 | 10.2 |
| Soluble fish protein concentrate 2 | 2.5 | 2.5 | 2.5 | 2.5 |
| Tenebrio molitor 3 | - | 10.0 | 20.0 | 30.0 |
| Wheat gluten 4 | 5.0 | 5.0 | 5.0 | 5.0 |
| Corn gluten 5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Soybean meal 6 | 14.0 | 14.0 | 14.0 | 14.0 |
| Wheat meal 7 | 15.0 | 14.3 | 13.6 | 12.8 |
| Fish oil | 12.3 | 12.1 | 12.0 | 11.8 |
| Vitamin premix 8 | 1.0 | 1.0 | 1.0 | 1.0 |
| Mineral premix 9 | 1.0 | 1.0 | 1.0 | 1.0 |
| Choline chloride (50%) | 0.5 | 0.5 | 0.5 | 0.5 |
| Binder 10 | 1.0 | 1.0 | 1.0 | 1.0 |
| Taurine 11 | 0.2 | 0.2 | 0.2 | 0.2 |
| Dibasic calcium phosphate | - | 0.8 | 1.6 | 2.5 |
| Proximate analyses (% dry weight basis) | ||||
| Dry matter | 93.7 | 91.8 | 94.3 | 90.8 |
| Crude protein | 50.3 | 50.1 | 49.7 | 49.7 |
| Crude fat | 19.0 | 18.7 | 19.2 | 19.9 |
| Ash | 9.2 | 8.8 | 8.5 | 8.5 |
| Energy (kJ g−1) | 23.2 | 23.6 | 23.1 | 23.9 |
| Chitin | 0.0 | 0.74 | 0.97 | 1.47 |
| Insect Meal | Diets | ||||
|---|---|---|---|---|---|
| Fatty Acids | Tenebrio Molitor | CTR | TM10 | TM20 | TM30 |
| 12:0 | 0.48 | 0.12 | 0.22 | 0.21 | 0.24 |
| 14:0 | 4.10 | 8.61 | 7.79 | 7.72 | 7.40 |
| 15:0 | 0.38 | 0.88 | 0.79 | 0.70 | 0.68 |
| 16:0 | 20.1 | 25.3 | 24.3 | 24.2 | 23.5 |
| 17:0 | 0.36 | 0.49 | 0.49 | 0.46 | 0.43 |
| 18:0 | 3.74 | 3.16 | 3.24 | 3.16 | 3.11 |
| ΣSFA | 29.2 | 38.6 | 36.8 | 36.4 | 35.4 |
| 14:1 | 0.18 | 0.33 | 0.29 | 0.27 | 0.26 |
| 16:1 | 3.37 | 8.34 | 7.62 | 7.38 | 6.82 |
| 17:1 | 0.00 | 0.15 | 0.15 | 0.06 | 0.05 |
| 18:1 | 35.2 | 22.7 | 24.3 | 26.1 | 27.3 |
| 20:1 | 0.00 | 2.27 | 2.31 | 1.64 | 1.67 |
| 22:1 | 0.00 | 1.17 | 1.27 | 1.07 | 0.87 |
| ΣMUFA | 38.7 | 35.0 | 35.9 | 36.5 | 36.9 |
| 18:2 n−6 | 28.7 | 8.77 | 11.2 | 13.6 | 15.5 |
| 20:2 n−6 | 0.00 | 0.21 | 0.21 | 0.15 | 0.10 |
| 20:4 n−6 | 0.00 | 0.45 | 0.42 | 0.29 | 0.14 |
| Σn−6 PUFA | 28.7 | 9.43 | 11.9 | 14.0 | 15.8 |
| 18:3 n−3 | 1.17 | 2.26 | 2.20 | 2.16 | 1.99 |
| 18:4 n−3 | 0.00 | 2.23 | 1.99 | 1.73 | 1.52 |
| 20:4 n−3 | 0.00 | 0.30 | 0.21 | 0.00 | 0.15 |
| 20:5 n−3 | 0.00 | 4.51 | 4.16 | 3.65 | 3.16 |
| 22:6 n−3 | 0.00 | 3.57 | 3.44 | 2.77 | 2.30 |
| Σn−3 PUFA | 1.17 | 12.9 | 12.0 | 10.31 | 9.11 |
| Σn−3 LC-PUFA | 0.00 | 8.39 | 7.81 | 6.42 | 5.61 |
| Ratios | |||||
| SFA:PUFA | 0.96 | 1.58 | 1.43 | 1.39 | 1.33 |
| n3:n6 | 0.04 | 1.37 | 1.01 | 0.74 | 0.58 |
| Unsaturation Index a | 101.0 | 122.5 | 123.0 | 118.8 | 116.2 |
| Diets | One-Way ANOVA | Polynomial Contrasts | ||||||
|---|---|---|---|---|---|---|---|---|
| Fatty Acids | Initial | CTR | TM10 | TM20 | TM30 | p-Value | Linear | Quadratic |
| 14:0 | 3.8 | 6.20 ± 0.25 b | 5.93 ± 0.63 ab | 5.39 ± 0.24 ab | 4.98 ± 0.19 a | 0.016 | 0.002 | 0.749 |
| 15:0 | 0.5 | 0.78 ± 0.032 | 0.57 ± 0.339 | 0.68 ± 0.042 | 0.61 ± 0.012 | 0.483 | 0.364 | 0.501 |
| 16:0 | 24.3 | 25.3 ± 1.09 | 24.6 ± 1.94 | 23.0 ± 0.47 | 22.5 ± 0.46 | 0.058 | 0.011 | 0.838 |
| 17:0 | 0.4 | 0.49 ± 0.04 b | 0.41 ± 0.024 ab | 0.39 ± 0.025 a | 0.38 ± 0.013 a | 0.007 | 0.002 | 0.086 |
| 18:0 | 4.8 | 3.43 ± 0.11 b | 3.01 ± 0.14 a | 3.12 ± 0.10 a | 3.18 ± 0.09 ab | 0.010 | 0.056 | 0.006 |
| ΣSFA | 33.9 | 36.2 ± 1.38 b | 34.5 ± 2.77 ab | 32.6 ± 0.61 ab | 31.7 ± 0.65 a | 0.035 | 0.006 | 0.673 |
| 14:1 | 0.1 | 0.25 ± 0.022 b | 0.23 ± 0.033 ab | 0.20 ± 0.025 ab | 0.17 ± 0.018 a | 0.016 | 0.002 | 0.687 |
| 16:1 | 7.5 | 8.07 ± 0.44 | 8.38 ± 0.31 | 8.06 ± 0.09 | 7.73 ± 0.32 | 0.183 | 0.147 | 0.118 |
| 17:1 | 0.0 | 0.16 ± 0.018 b | 0.13 ± 0.035 ab | 0.09 ± 0.007 a | 0.12 ± 0.003 ab | 0.011 | 0.008 | 0.020 |
| 18:1 | 28.2 | 24.1 ± 0.65 a | 25.3 ± 0.31 b | 27.0 ± 0.34 c | 29.1 ± 0.24 d | 0.000 | 0.000 | 0.114 |
| 20:1 | 1.2 | 2.34 ± 0.17 b | 2.06 ± 0.31 ab | 1.94 ± 0.13 ab | 1.72 ± 0.04 a | 0.024 | 0.004 | 0.802 |
| 22:1 | 0.5 | 0.66 ± 0.481 | 0.89 ± 0.226 | 0.88 ± 0.078 | 0.67 ± 0.085 | 0.600 | 0.993 | 0.198 |
| ΣMUFA | 37.5 | 35.5 ± 1.07 a | 37 ± 0.54 ab | 38.2 ± 0.21 bc | 39.5 ± 0.21 c | 0.000 | 0.000 | 0.868 |
| 18:2n−6 | 14.9 | 10.5 ± 0.28 a | 12.5 ± 0.13 b | 14.9 ± 0.42 c | 16.8 ± 0.3 d | 0.000 | 0.000 | 0.779 |
| 20:2n−6 | 0.0 | 0.23 ± 0.021 ab | 0.24 ± 0.018 b | 0.19 ± 0.022 a | 0.19 ± 0.02 ab | 0.023 | 0.010 | 0.954 |
| 20:4n−6 | 0.8 | 0.67 ± 0.025 | 0.55 ± 0.164 | 0.50 ± 0.088 | 0.45 ± 0.032 | 0.102 | 0.021 | 0.571 |
| Σn−6 PUFA | 15.8 | 11.4 ± 0.28 a | 13.3 ± 0.31 b | 15.6 ± 0.42 c | 17.5 ± 0.29 d | 0.000 | 0.000 | 0.925 |
| 18:3n−3 | 1.8 | 2.03 ± 0.082 c | 1.88 ± 0.026 b | 1.86 ± 0.035 ab | 1.73 ± 0.043 a | 0.001 | 0.000 | 0.693 |
| 18:4n−3 | 0.7 | 1.56 ± 0.076 d | 1.35 ± 0.042 c | 1.17 ± 0.025 b | 0.98 ± 0.045 a | 0.000 | 0.000 | 0.736 |
| 20:4n−3 | 0.2 | 0.33 ± 0.058 b | 0.26 ± 0.036 ab | 0.21 ± 0.035 a | 0.19 ± 0.012 a | 0.011 | 0.002 | 0.281 |
| 20:5n−3 | 2.7 | 3.94 ± 0.38 c | 3.34 ± 0.29 bc | 3.04 ± 0.26 ab | 2.50 ± 0.2 a | 0.002 | 0.000 | 0.851 |
| 22:6n−3 | 3.1 | 4.98 ± 0.9 b | 4.09 ± 0.71 ab | 3.72 ± 0.16 ab | 2.74 ± 0.28 a | 0.011 | 0.002 | 0.894 |
| Σn−3 PUFA | 8.5 | 12.8 ± 1.37 c | 10.9 ± 0.95 bc | 10 ± 0.44 ab | 8.1 ± 0.41 a | 0.001 | 0.000 | 0.950 |
| Σn−3 LC-PUFA | 6.0 | 9.2 ± 1.33 b | 7.7 ± 0.10 ab | 7.0 ± 0.42 ab | 5.4 ± 0.49 a | 0.005 | 0.001 | 0.983 |
| Ratios | ||||||||
| SFA:PUFA | 1.31 | 1.37 ± 0.11 | 1.31 ± 0.16 | 1.18 ± 0.04 | 1.16 ± 0.02 | 0.091 | 0.018 | 0.743 |
| n3:n6 | 0.54 | 1.13 ± 0.136 c | 0.82 ± 0.054 b | 0.64 ± 0.034 ab | 0.47 ± 0.036 a | 0.000 | 0.000 | 0.172 |
| Unsaturation Index 1 | 116.4 | 128.5 ± 6.33 | 123.7 ± 6.69 | 124.1 ± 2.32 | 118.8 ± 1.89 | 0.188 | 0.051 | 0.925 |
| Diets | One-Way ANOVA | Polynomial Contrasts | |||||
|---|---|---|---|---|---|---|---|
| CTR | TM10 | TM20 | TM30 | p-Value | Linear | Quadratic | |
| Lipids retention | 47.1 ± 2.3 c | 41.0 ± 3.3 bc | 38.6 ± 3.3 b | 29.3 ± 2.7 a | 0.000 | 0.000 | 0.368 |
| 16:0 | 47.3 ± 3.3 c | 41.4 ± 1.4 bc | 36.5 ± 2.3 ab | 30.0 ± 4.4 a | 0.001 | 0.000 | 0.890 |
| ΣSFA | 44.5 ± 2.8 c | 38.4 ± 1.8 bc | 34.3 ± 2.2 ab | 28.1 ± 3.9 a | 0.001 | 0.000 | 0.962 |
| 18:1n−9 | 49.3 ± 3.8 b | 42.3 ± 4 ab | 39.7 ± 3.9 ab | 34.2 ± 5.7 a | 0.019 | 0.003 | 0.780 |
| ΣMUFA | 47.7 ± 3.8 b | 42.3 ± 4 ab | 40.4 ± 3.6 ab | 34.4 ± 5.4 a | 0.032 | 0.005 | 0.910 |
| 18:2n−6 | 54.6 ± 4.4 b | 44.8 ± 4.2 ab | 42.5 ± 4.8 ab | 35.4 ± 6.2 a | 0.010 | 0.002 | 0.654 |
| Σn−6 PUFA | 55.3 ± 4.4 b | 45.2 ± 4.9 ab | 43.0 ± 4.9 ab | 36.1 ± 6.3 a | 0.012 | 0.002 | 0.606 |
| 18:3n−3 | 42.7 ± 3.5 b | 35.3 ± 3 ab | 33.4 ± 3.4 ab | 27.6 ± 4.6 a | 0.007 | 0.001 | 0.705 |
| 20:5n−3 | 42.1 ± 3.9 b | 33.7 ± 5.6 ab | 32.7 ± 4.7 ab | 24.6 ± 2 a | 0.007 | 0.001 | 0.952 |
| 22:6n−3 | 67.3 ± 11.3 b | 50.3 ± 12.6 ab | 53.0 ± 3.7 ab | 36.6 ± 2.9 a | 0.018 | 0.004 | 0.949 |
| Σn−3 PUFA | 48.1 ± 4.9 b | 38.3 ± 6.3 ab | 38.2 ± 3.7 ab | 28.1 ± 2.6 a | 0.005 | 0.001 | 0.953 |
| Diets | One-Way ANOVA | Polynomial Contrasts | |||||
|---|---|---|---|---|---|---|---|
| CTR | TM10 | TM20 | TM30 | p-Value | Linear | Quadratic | |
| SOD | 91 ± 24 ab | 74 ± 18 a | 102 ± 14 bc | 119 ± 16 c | 0.000 | 0.000 | 0.014 |
| CAT | 320 ± 95 b | 325 ± 62 b | 278 ± 20 ab | 216 ± 42 a | 0.003 | 0.001 | 0.122 |
| G6 PDH | 72 ± 6 a | 82 ± 13 ab | 81 ± 10 ab | 94 ± 17 b | 0.010 | 0.002 | 0.668 |
| GR | 5.2 ± 0.51 a | 5.8 ± 1.16 ab | 6.0 ± 1.14 ab | 6.9 ± 1.34 b | 0.022 | 0.003 | 0.704 |
| GPX | 174 ± 35 c | 189 ± 29 c | 126 ± 23 b | 81 ± 24 a | 0.000 | 0.000 | 0.003 |
| tGSH | 1042 ± 91 b | 981 ± 130 b | 775 ± 119 a | 1007 ± 148 b | 0.000 | 0.130 | 0.002 |
| GSH | 1038 ± 91 b | 977 ± 129 b | 773 ± 118 a | 1002 ± 148 b | 0.000 | 0.126 | 0.002 |
| GSSG | 3.4 ± 1.48 | 4.4 ± 1.88 | 2.7 ± 1.31 | 4.5 ± 2.04 | 0.199 | 0.448 | 0.292 |
| OSI 1 | 0.66 ± 0.30 | 0.8 ± 0.36 | 0.7 ± 0.30 | 0.9 ± 0.39 | 0.512 | 0.252 | 0.813 |
| MDA | 16 ± 5 | 20 ± 10 | 14 ± 8 | 26 ± 19 | 0.150 | 0.194 | 0.289 |
| Diets | One-Way ANOVA | Polynomial Contrasts | |||||
|---|---|---|---|---|---|---|---|
| CTR | TM10 | TM20 | TM30 | p-Value | Linear | Quadratic | |
| SOD | 333 ± 101 | 387 ± 130 | 370 ± 105 | 259 ± 37 | 0.065 | 0.140 | 0.023 |
| CAT | 46.8 ± 28.2 a | 71.5 ± 29.2 a | 67.8 ± 16.6 a | 101.4 ± 12.6 b | 0.000 | 0.000 | 0.567 |
| G6PDH | 3.28 ± 1.08 | 5.73 ± 2.56 | 4.92 ± 2.31 | 4.20 ± 2.33 | 0.116 | 0.547 | 0.034 |
| GR | 7.38 ± 1.92 a | 10.23 ± 2.72 b | 8.97 ± 1.31 ab | 7.19 ± 2.07 a | 0.011 | 0.558 | 0.002 |
| GPX | 43 ± 7.6 b | 42.2 ± 10.1 b | 40.3 ± 10.0 b | 25.4 ± 10.1 a | 0.002 | 0.001 | 0.042 |
| tGSH | 845 ± 189 | 768 ± 49 | 919 ± 301 | 877 ± 246 | 0.599 | 0.467 | 0.822 |
| GSH | 830 ± 189 | 747 ± 50 | 898 ± 299 | 858 ± 246 | 0.605 | 0.495 | 0.785 |
| GSSG | 14.7 ± 3.17 a | 16.8 ± 3.59 ab | 21.6 ± 6.83 b | 19.4 ± 4.25 ab | 0.026 | 0.013 | 0.205 |
| OSI 1 | 3.67 ± 1.11 | 4.15 ± 1.11 | 4.45 ± 1.33 | 4.77 ± 1.73 | 0.379 | 0.088 | 0.866 |
| MDA | 102 ± 31 | 76 ± 30 | 69 ± 14 | 102 ± 37 | 0.041 | 0.921 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerreiro, I.; Castro, C.; Serra, C.R.; Coutinho, F.; Couto, A.; Peres, H.; Pousão-Ferreira, P.; Corraze, G.; Oliva-Teles, A.; Enes, P. Feeding Yellow Worms to Meagre: Effects on Whole-Body Fatty Acid Profile and Hepatic and Intestine Oxidative Status. Antioxidants 2023, 12, 1031. https://doi.org/10.3390/antiox12051031
Guerreiro I, Castro C, Serra CR, Coutinho F, Couto A, Peres H, Pousão-Ferreira P, Corraze G, Oliva-Teles A, Enes P. Feeding Yellow Worms to Meagre: Effects on Whole-Body Fatty Acid Profile and Hepatic and Intestine Oxidative Status. Antioxidants. 2023; 12(5):1031. https://doi.org/10.3390/antiox12051031
Chicago/Turabian StyleGuerreiro, Inês, Carolina Castro, Cláudia R. Serra, Filipe Coutinho, Ana Couto, Helena Peres, Pedro Pousão-Ferreira, Geneviève Corraze, Aires Oliva-Teles, and Paula Enes. 2023. "Feeding Yellow Worms to Meagre: Effects on Whole-Body Fatty Acid Profile and Hepatic and Intestine Oxidative Status" Antioxidants 12, no. 5: 1031. https://doi.org/10.3390/antiox12051031
APA StyleGuerreiro, I., Castro, C., Serra, C. R., Coutinho, F., Couto, A., Peres, H., Pousão-Ferreira, P., Corraze, G., Oliva-Teles, A., & Enes, P. (2023). Feeding Yellow Worms to Meagre: Effects on Whole-Body Fatty Acid Profile and Hepatic and Intestine Oxidative Status. Antioxidants, 12(5), 1031. https://doi.org/10.3390/antiox12051031