Biochanin A Ameliorates Nephropathy in High-Fat Diet/Streptozotocin-Induced Diabetic Rats: Effects on NF-kB/NLRP3 Axis, Pyroptosis, and Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Cytotoxicity Studies
2.2.1. Induction of Hyperglycemia and BCA Treatment
2.2.2. Detection of Change in Mitochondrial Membrane Potential
2.2.3. Determination of ROS Generation in Cells
2.2.4. Cell Culture Treatment for Western Blotting
2.2.5. Immunofluorescence
2.3. Animals
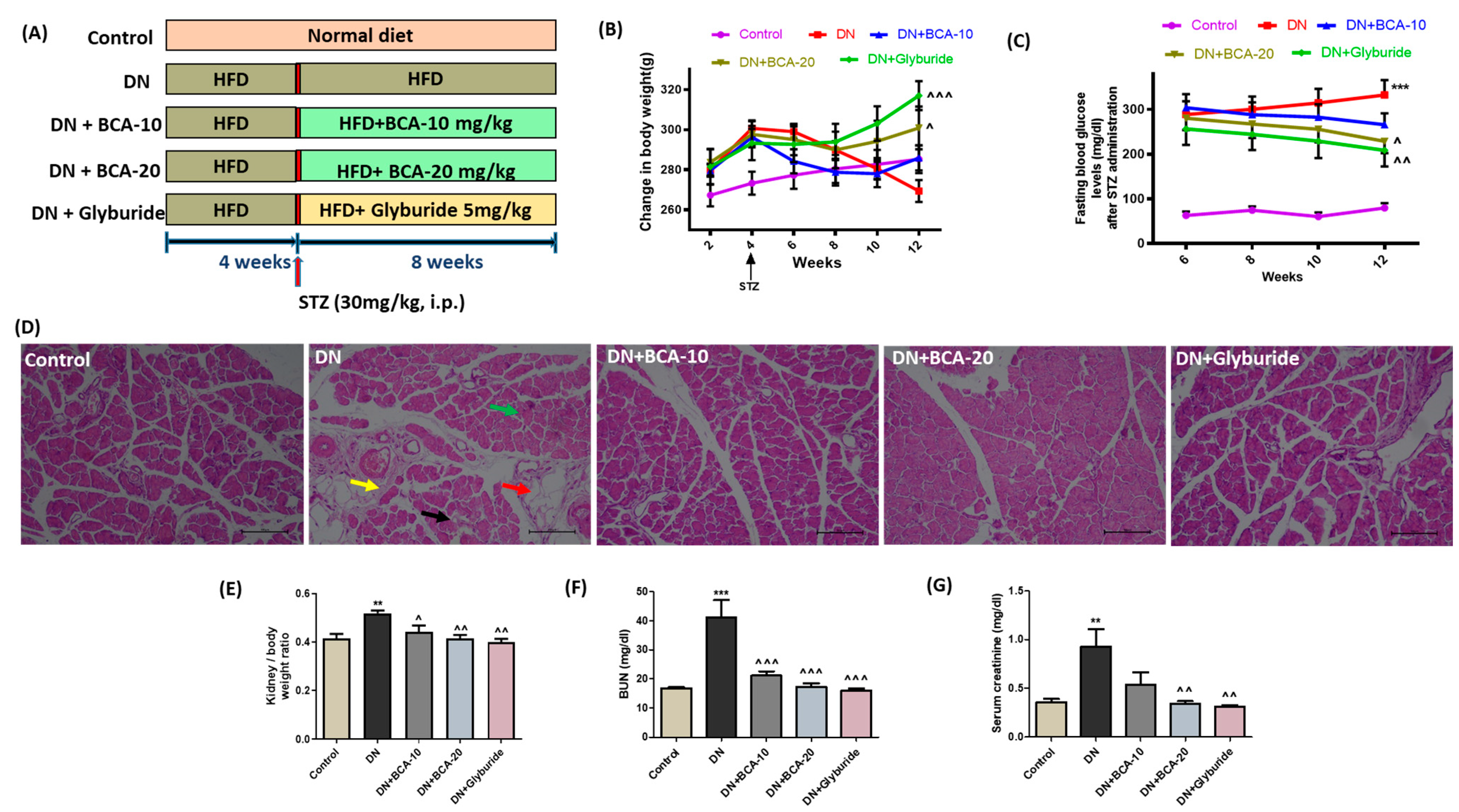
2.4. Induction of Diabetes in Rats and Experimental Design
2.5. Assessment of Blood Urea Nitrogen and Creatinine in the Serum
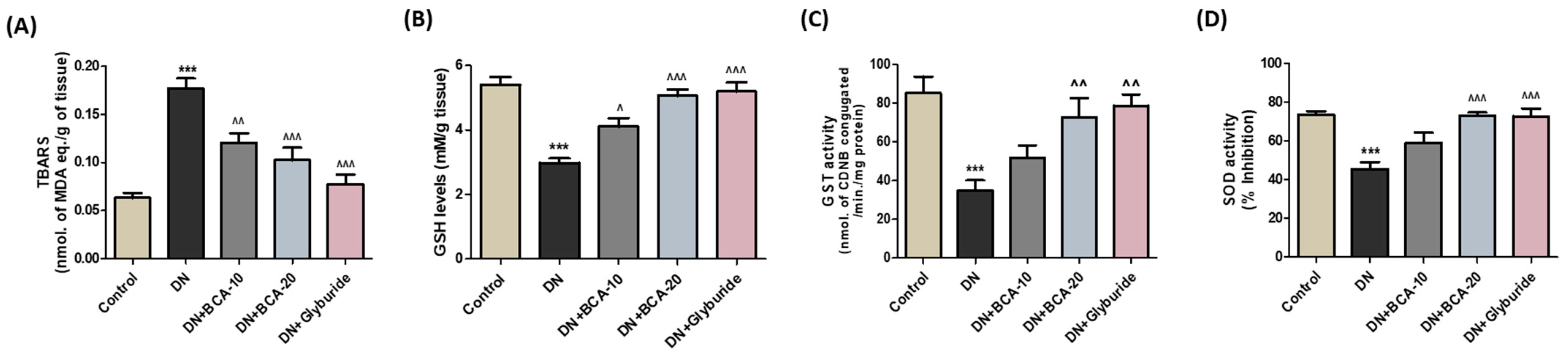
2.6. Assessment of Oxidative Stress Parameters in the Kidneys
2.7. Assessment of Pro-Inflammatory Cytokines by ELISA
2.8. Histopathology
2.9. Immunohistochemistry (IHC)
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
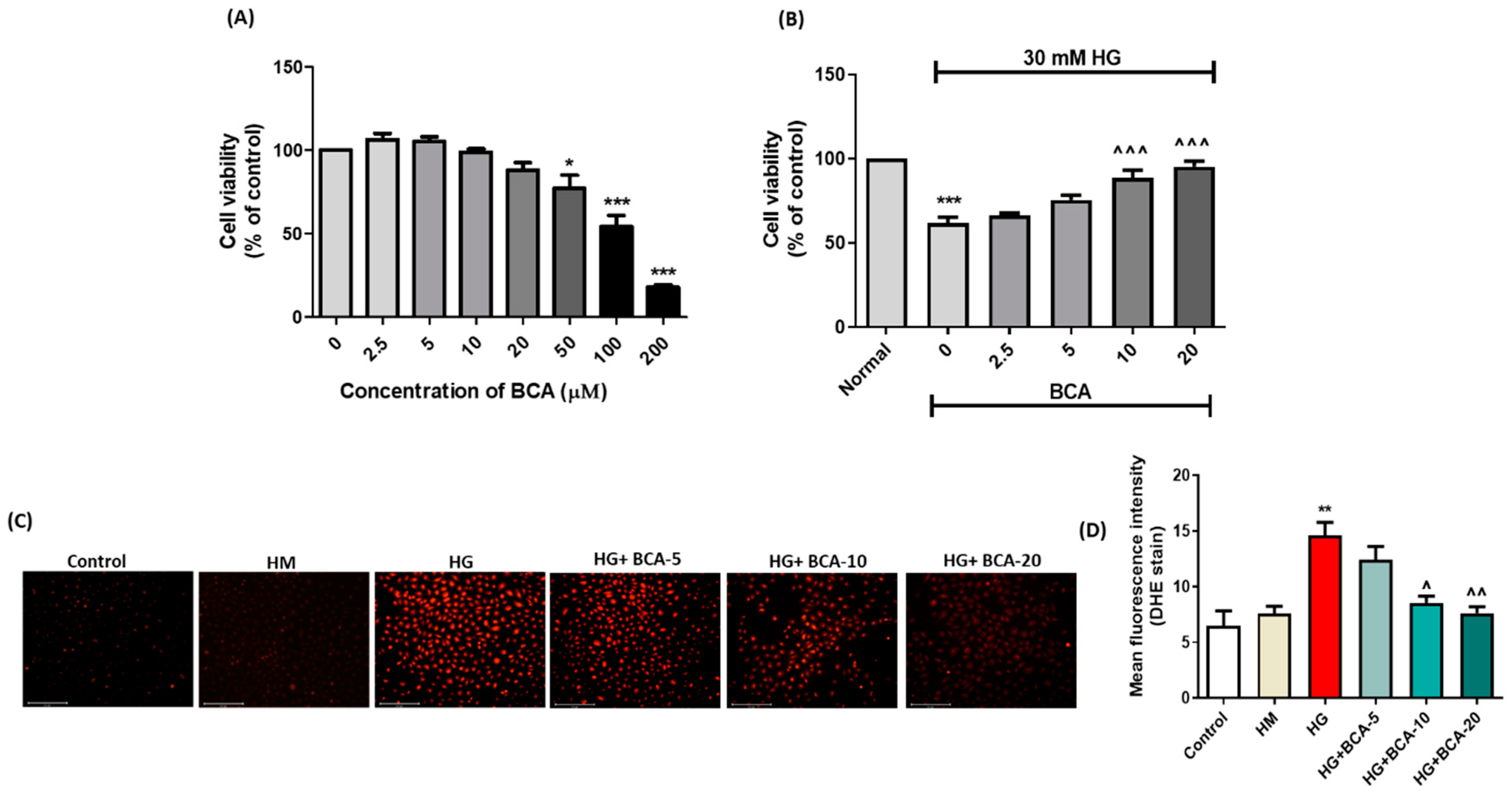
3.1. Effect of BCA on NRK-52E Cells Viability
3.2. Effect of BCA on ROS Production in the HG-Stimulated NRK-52E Cells
3.3. Effect of BCA on HG-Induced Changes in the Mitochondrial Membrane Potential and Apoptosis Proteins in NRK-52E Cells
3.4. Effect of BCA on HG-Induced NLRP3, Caspase-1, and Pyroptosis in NRK-52E Cells
3.5. Effect of BCA Treatment on Body Weight Changes, FBG, and Serum Kidney Injury Biomarkers in Diabetic Rats
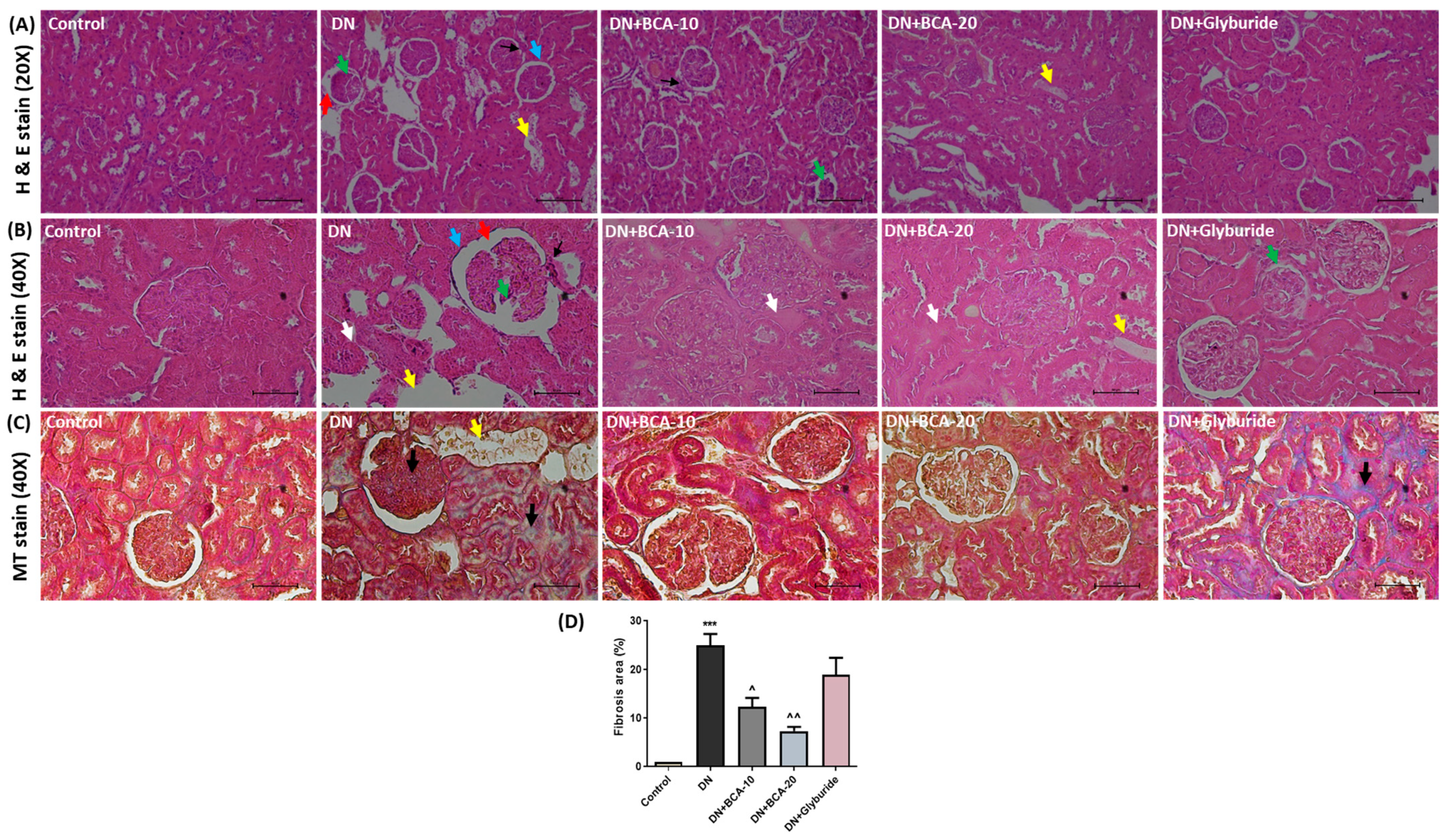
3.6. Effect of BCA on Kidney Histopathological Changes and Antioxidant Status
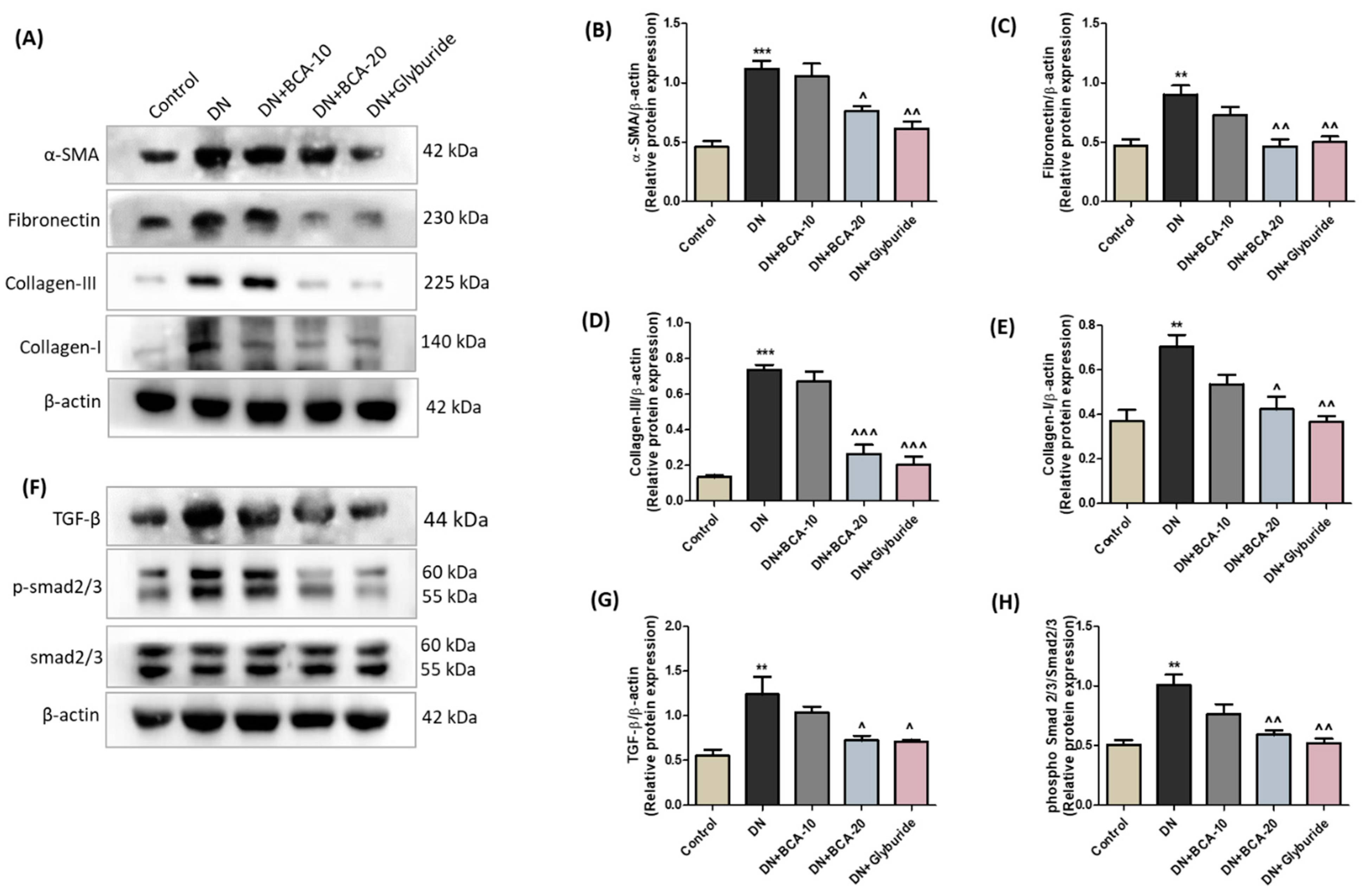
3.7. Effect of BCA on ECM Components and TGF-β/Smad Axis in Diabetic Kidneys
3.8. Effect of BCA on Renal NLRP3/Caspase-1 Signaling Axis in Diabetic Kidneys
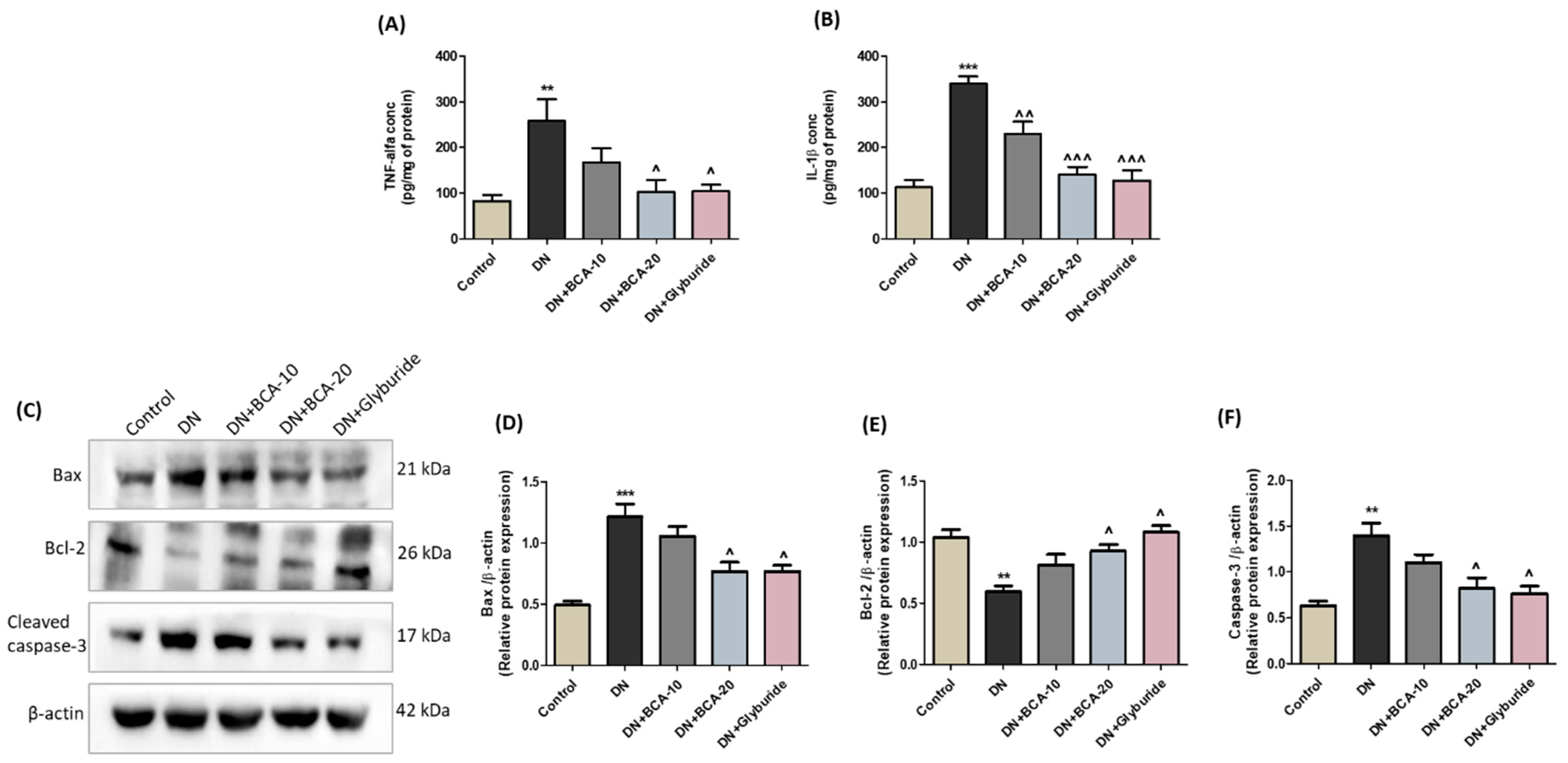
3.9. Effect of BCA on NF-kB (p65) and Pro-Inflammatory Cytokines in Diabetic Kidneys
3.10. Effect of BCA on Apoptosis-Associated Proteins in Diabetic Kidneys
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisa, N.H.; Khodir, A.E.; El-Sherbiny, M.; Elsherbiny, N.M.; Said, E. Phenethyl Isothiocyanate Attenuates Diabetic Nephropathy via Modulation of Glycative/Oxidative/Inflammatory Signaling in Diabetic Rats. Biomed. Pharmacother. 2021, 142, 111666. [Google Scholar] [CrossRef] [PubMed]
- Abdou, H.M.; Abd Elkader, H.T.A.E. The Potential Therapeutic Effects of Trifolium Alexandrinum Extract, Hesperetin and Quercetin against Diabetic Nephropathy via Attenuation of Oxidative Stress, Inflammation, GSK-3β and Apoptosis in Male Rats. Chem. Biol. Interact. 2022, 352, 109781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Xu, S.J.; Qian, J.C.; Yang, L.B.; Chen, P.Q.; Wang, Y.; Hu, X.; Zhang, Y.L.; Luo, W.; Liang, G. Pharmacological Inhibition of MyD88 Suppresses Inflammation in Tubular Epithelial Cells and Prevents Diabetic Nephropathy in Experimental Mice. Acta Pharmacol. Sin. 2022, 43, 354–366. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Ram, C.; Jha, A.K.; Ghosh, A.; Gairola, S.; Syed, A.M.; Murty, U.S.; Naidu, V.G.M.; Sahu, B.D. Targeting NLRP3 Inflammasome as a Promising Approach for Treatment of Diabetic Nephropathy: Preclinical Evidences with Therapeutic Approaches. Eur. J. Pharmacol. 2020, 885, 173503. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Chu, L.W.; Chen, J.Y.; Hsieh, S.L.; Chang, Y.C.; Dai, Z.K.; Wu, B.N. Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells 2020, 9, 1948. [Google Scholar] [CrossRef]
- Brosius, F.C. New Insights into the Mechanisms of Fibrosis and Sclerosis in Diabetic Nephropathy. Rev. Endocr. Metab. Disord. 2008, 9, 245–254. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.L.; Liu, T.T.; Lan, H.Y. TGF-beta as a Master Regulator of Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 7881. [Google Scholar] [CrossRef]
- Li, J.; Yue, S.; Fang, J.; Zeng, J.; Chen, S.; Tian, J.; Nie, S.; Liu, X.; Ding, H. MicroRNA-10a/b Inhibit TGF-β/Smad-Induced Renal Fibrosis by Targeting TGF-β Receptor 1 in Diabetic Kidney Disease. Mol. Ther.—Nucleic Acids 2022, 28, 488–499. [Google Scholar] [CrossRef]
- Chen, X.; Sun, L.; Li, D.; Lai, X.; Wen, S.; Chen, R.; Zhang, Z.; Li, Q.; Sun, S. Green Tea Peptides Ameliorate Diabetic Nephropathy by Inhibiting the TGF-β/Smad Signaling Pathway in Mice. Food Funct. 2022, 13, 3258–3270. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Sapian, S.; Budin, S.B.; Taib, I.S.; Mariappan, V.; Zainalabidin, S.; Chin, K.Y. Role of Polyphenol in Regulating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis in Diabetic Nephropathy. Endocr. Metab. Immune Disord.—Drug Targets 2021, 22, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Raheja, S.; Girdhar, A.; Lather, V.; Pandita, D. Biochanin A: A Phytoestrogen with Therapeutic Potential. Trends Food Sci. Technol. 2018, 79, 55–66. [Google Scholar] [CrossRef]
- Felix, F.B.; Vago, J.P.; Beltrami, V.A.; Araújo, J.M.D.; Grespan, R.; Teixeira, M.M.; Pinho, V. Biochanin A as a Modulator of the Inflammatory Response: An Updated Overview and Therapeutic Potential. Pharmacol. Res. 2022, 180, 106246. [Google Scholar] [CrossRef]
- Ram, C.; Gairola, S.; Syed, A.M.; Kulhari, U.; Kundu, S.; Mugale, M.N.; Murty, U.S.; Sahu, B.D. Biochanin A Alleviates Unilateral Ureteral Obstruction-Induced Renal Interstitial Fibrosis and Inflammation by Inhibiting the TGF-Β1/Smad2/3 and NF-KB/NLRP3 Signaling Axis in Mice. Life Sci. 2022, 298, 120527. [Google Scholar] [CrossRef]
- Amri, J.; Alaee, M.; Babaei, R.; Salemi, Z.; Meshkani, R.; Ghazavi, A.; Akbari, A.; Salehi, M. Biochanin-A Has Antidiabetic, Antihyperlipidemic, Antioxidant, and Protective Effects on Diabetic Nephropathy via Suppression of TGF-Β1 and PAR-2 Genes Expression in Kidney Tissues of STZ-Induced Diabetic Rats. Biotechnol. Appl. Biochem. 2022, 69, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Liu, T.; Guo, T.; Tao, W.; Chen, X.; Chen, W.; Chen, L.; Xiao, Y. ATF4 Promotes Renal Tubulointerstitial Fibrosis by Suppressing Autophagy in Diabetic Nephropathy. Life Sci. 2021, 264, 118686. [Google Scholar] [CrossRef]
- Chen, M.F.; Liou, S.S.; Kao, S.T.; Liu, I.M. Erianin Protects against High Glucose-Induced Oxidative Injury in Renal Tubular Epithelial Cells. Food Chem. Toxicol. 2019, 126, 97–105. [Google Scholar] [CrossRef]
- El Gamal, H.; Eid, A.H.; Munusamy, S. Renoprotective Effects of Aldose Reductase Inhibitor Epalrestat against High Glucose-Induced Cellular Injury. Biomed Res. Int. 2017, 2017, 5903105. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Zhao, X.; Xing, C.; Sun, B. β-Aminoisobutyric Acid Ameliorates the Renal Fibrosis in Mouse Obstructed Kidneys via Inhibition of Renal Fibroblast Activation and Fibrosis. J. Pharmacol. Sci. 2017, 133, 203–213. [Google Scholar] [CrossRef]
- Gairola, S.; Ram, C.; Syed, A.M.; Doye, P.; Kulhari, U.; Mugale, M.N.; Murty, U.S.; Sahu, B.D. Nootkatone Confers Antifibrotic Effect by Regulating the TGF-β/Smad Signaling Pathway in Mouse Model of Unilateral Ureteral Obstruction. Eur. J. Pharmacol. 2021, 910, 174479. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.F.; de Oliveira Silva, V.; de Moura, N.O.; de Carvalho Foureaux, R.; Orlando, D.R.; de Moura, R.F.; Pereira, L.J. Physical Exercise Improves Glycemic and Inflammatory Profile and Attenuates Progression of Periodontitis in Diabetic Rats (HFD/STZ). Nutrients 2018, 10, 1702. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, X.; Long, S.R.; Wang, T.; Ge, H.; Wang, Y.; Yu, S.; Xue, Y.; Zhang, Y.; Li, X.; et al. Antidiabetic Effects of Pterostilbene through PI3K/Akt Signal Pathway in High Fat Diet and STZ-Induced Diabetic Rats. Eur. J. Pharmacol. 2019, 859, 172526. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Seritean Isac, P.N.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative Stress, and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives. Biomolecules 2022, 12, 1227. [Google Scholar] [CrossRef]
- Maruno, S.; Tanaka, T.; Nangaku, M. Exploring Molecular Targets in Diabetic Kidney Disease. Kidney Res. Clin. Pract. 2022, 41 (Suppl. S2), S33–S45. [Google Scholar] [CrossRef]
- Wang, F.; Li, R.; Zhao, L.; Ma, S.; Qin, G. Resveratrol Ameliorates Renal Damage by Inhibiting Oxidative Stress-Mediated Apoptosis of Podocytes in Diabetic Nephropathy. Eur. J. Pharmacol. 2020, 885, 173387. [Google Scholar] [CrossRef]
- Wei, P.Z.; Szeto, C.C. Mitochondrial Dysfunction in Diabetic Kidney Disease. Clin. Chim. Acta 2019, 496, 108–116. [Google Scholar] [CrossRef]
- Song, S.; Shi, C.; Bian, Y.; Yang, Z.; Mu, L.; Wu, H.; Duan, H.; Shi, Y. Sestrin2 Remedies Podocyte Injury via Orchestrating TSP-1/TGF-Β1/Smad3 Axis in Diabetic Kidney Disease. Cell Death Dis. 2022, 13, 663. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Zhao, J.; Guo, X.; Luo, Y.; Hu, W.; Zhao, T. Tumor Necrosis Factor Receptor-Associated Protein 1 Protects against Mitochondrial Injury by Preventing High Glucose-Induced MPTP Opening in Diabetes. Oxid. Med. Cell. Longev. 2020, 2020, 6431517. [Google Scholar] [CrossRef]
- Patial, V.; Katoch, S.; Chhimwal, J.; Singh, P.P.; Suresh, P.S.; Padwad, Y. Tinospora Cordifolia Activates PPARγ Pathway and Mitigates Glomerular and Tubular Cell Injury in Diabetic Kidney Disease. Phytomedicine 2021, 91, 153663. [Google Scholar] [CrossRef]
- Xiao, Y.; Deng, J.; Li, C.; Gong, X.; Gui, Z.; Huang, J.; Zhang, Y.; Liu, Y.; Ye, X.; Li, X. Epiberberine Ameliorated Diabetic Nephropathy by Inactivating the Angiotensinogen (Agt) to Repress TGFβ/Smad2 Pathway. Phytomedicine 2021, 83, 153488. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C.; De Fuentes, M.M.; García-Pérez, J. Inflammatory Molecules and Pathways in the Pathogenesis of Diabetic Nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhou, T.J.; Ren, G.L.; Cai, L.; Meng, X.M. Novel Insights into NOD-like Receptors in Renal Diseases. Acta Pharmacol. Sin. 2022, 43, 2789–2806. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhu, F.; Xu, Z.; Xiong, J. Role of Inflammasomes in Kidney Diseases via Both Canonical and Non-Canonical Pathways. Front. Cell Dev. Biol. 2020, 8, 106. [Google Scholar]
- Yi, H.; Peng, R.; Zhang, L.Y.; Sun, Y.; Peng, H.M.; Liu, H.D.; Yu, L.J.; Li, A.L.; Zhang, Y.J.; Jiang, W.H.; et al. LincRNA-Gm4419 Knockdown Ameliorates NF-ΚB/NLRP3 Inflammasome-Mediated Inflammation in Diabetic Nephropathy. Cell Death Dis. 2017, 8, e2583. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Xu, Y. Pyroptosis in Kidney Disease. J. Mol. Biol. 2022, 434, 167290. [Google Scholar] [CrossRef]
- Ram, C.; Gairola, S.; Syed, A.M.; Verma, S.; Mugale, M.N.; Sahu, B.D. Carvacrol Preserves Antioxidant Status and Attenuates Kidney Fibrosis via Modulation of TGF-Β1/Smad Signaling and Inflammation. Food Funct. 2022, 13, 10587–10600. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Muhsin, S.A.; Choi, M.E. TGF-β Signaling in the Kidney: Profibrotic and Protective Effects. Am. J. Physiol.—Ren. Physiol. 2016, 310, F596–F606. [Google Scholar] [CrossRef]
- Dong, L.; Yu, L.; Zhong, J. Histone Lysine-Specific Demethylase 1 Induced Renal Fibrosis via Decreasing Sirtuin 3 Expression and Activating TGF-Β1/Smad3 Pathway in Diabetic Nephropathy. Diabetol. Metab. Syndr. 2022, 14, 2. [Google Scholar] [CrossRef]
- Zhang, X.X.; Liu, Y.; Xu, S.S.; Yang, R.; Jiang, C.H.; Zhu, L.P.; Xu, Y.Y.; Pan, K.; Zhang, J.; Yin, Z.Q. Asiatic Acid from Cyclocarya Paliurus Regulates the Autophagy-Lysosome System via Directly Inhibiting TGF-β Type I Receptor and Ameliorates Diabetic Nephropathy Fibrosis. Food Funct. 2022, 13, 5536–5546. [Google Scholar] [CrossRef]
- Moon, Y.J.; Sagawa, K.; Frederick, K.; Zhang, S.; Morris, M.E. Pharmacokinetics and Bioavailability of the Isoflavone Biochanin A in Rats. AAPS J. 2006, 8, E433–E442. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Wahajuddin; Jain, G.K. Intravenous pharmacokinetics and oral bioavailability of biochanin A in female rats. Med. Chem. Res. 2011, 20, 1627–1631. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ram, C.; Gairola, S.; Verma, S.; Mugale, M.N.; Bonam, S.R.; Murty, U.S.; Sahu, B.D. Biochanin A Ameliorates Nephropathy in High-Fat Diet/Streptozotocin-Induced Diabetic Rats: Effects on NF-kB/NLRP3 Axis, Pyroptosis, and Fibrosis. Antioxidants 2023, 12, 1052. https://doi.org/10.3390/antiox12051052
Ram C, Gairola S, Verma S, Mugale MN, Bonam SR, Murty US, Sahu BD. Biochanin A Ameliorates Nephropathy in High-Fat Diet/Streptozotocin-Induced Diabetic Rats: Effects on NF-kB/NLRP3 Axis, Pyroptosis, and Fibrosis. Antioxidants. 2023; 12(5):1052. https://doi.org/10.3390/antiox12051052
Chicago/Turabian StyleRam, Chetan, Shobhit Gairola, Shobhit Verma, Madhav Nilakanth Mugale, Srinivasa Reddy Bonam, Upadhyayula Suryanarayana Murty, and Bidya Dhar Sahu. 2023. "Biochanin A Ameliorates Nephropathy in High-Fat Diet/Streptozotocin-Induced Diabetic Rats: Effects on NF-kB/NLRP3 Axis, Pyroptosis, and Fibrosis" Antioxidants 12, no. 5: 1052. https://doi.org/10.3390/antiox12051052
APA StyleRam, C., Gairola, S., Verma, S., Mugale, M. N., Bonam, S. R., Murty, U. S., & Sahu, B. D. (2023). Biochanin A Ameliorates Nephropathy in High-Fat Diet/Streptozotocin-Induced Diabetic Rats: Effects on NF-kB/NLRP3 Axis, Pyroptosis, and Fibrosis. Antioxidants, 12(5), 1052. https://doi.org/10.3390/antiox12051052






