Abstract
Emerging evidence suggests that cognitive impairments may result from various factors, such as neuroinflammation, oxidative stress, mitochondrial damage, impaired neurogenesis, synaptic plasticity, blood–brain barrier (BBB) disruption, amyloid β protein (Aβ) deposition, and gut dysbiosis. Meanwhile, dietary polyphenol intake in a recommended dosage has been suggested to reverse cognitive dysfunction via various pathways. However, excessive intake of polyphenols could trigger unwanted adverse effects. Thus, this review aims to outline possible causes of cognitive impairments and how polyphenols alleviate memory loss via various pathways based on in vivo experimental studies. Thus, to identify potentially relevant articles, the keywords (1) nutritional polyphenol intervention NOT medicine AND neuron growth OR (2) dietary polyphenol AND neurogenesis AND memory impairment OR (3) polyphenol AND neuron regeneration AND memory deterioration (Boolean operators) were used in the Nature, PubMed, Scopus, and Wiley online libraries. Based on the inclusion and exclusion criteria, 36 research papers were selected to be further reviewed. The outcome of all the studies included supports the statement of appropriate dosage by taking into consideration gender differences, underlying conditions, lifestyle, and causative factors for cognitive decline, which will significantly boost memory power. Therefore, this review recapitulates the possible causes of cognitive decline, the mechanism of polyphenols involving various signaling pathways in modulating the memory, gut dysbiosis, endogenous antioxidants, bioavailability, dosage, and safety efficacy of polyphenols. Hence, this review is expected to provide a basic understanding of therapeutic development for cognitive impairments in the future.
1. Introduction
Neurological disorders are becoming a global burden, and it is estimated that there might be an upsurge in the prevalence of neurological disorders, especially in low- and middle-income countries, over the next 10 years. Since the brain is the most vulnerable organ and the main target for neurological conditions, severe disability and poor quality of life are often seen in people with neurological conditions. One of the common manifestations of neurological conditions is cognitive decline [1]. Neurological disorders have been classified as one of the most common causes of morbidity and are ranked second in the global causes of morbidity [2]. Interventions to manage or treat neurological conditions can be very challenging due to the presence of the highly selective semi-permeable membrane known as the blood–brain barrier (BBB) and its unique capability to shield the brain from xenobiotics. Although conventional therapeutics are effective, their therapeutic efficacy is still below the optimum level. Therefore, there is currently a research focus on developing methods deliver chemical across the BBB [3]. Hence, natural products are gaining more popularity as preventive medicine and therapeutics in the treatment of neurological disorders. As a result, the use of natural products has gained the attention of researchers—especially natural extracts that have antioxidant properties [4]. Polyphenols are said to be effective in preventing neurodegeneration and neuroinflammation, thereby preventing cognitive decline even in very small dosages [5]. This may be due to the ability of certain polyphenols—such as epigallocatechin, daidzein, genistein, equol, and nobiletin—to be highly permeable to the BBB [6]. Thus, this review addresses the various factors that contribute to cognitive impairment; the possible mechanisms by which polyphenols alleviate memory loss, including reducing neuroinflammation and oxidative stress, improving mitochondrial function and synaptic plasticity, modulating gut microbiota; and reducing Aβ deposition.
This review highlights the importance of appropriate dosage of polyphenols, taking into account individual factors, such as gender, and underlying conditions to avoid adverse effects, such as gastrointestinal discomfort, liver toxicity, and interference with nutrient absorption. The study concludes that while the animal experiments provide a strong foundation for the therapeutic potential of dietary polyphenols in cognitive decline, more research is needed to determine the optimal dosage and long-term effects of polyphenol intake in humans. The study also emphasizes the importance of considering individual factors when recommending polyphenol intake for memory enhancement. Overall, this review provides an overview of the potential therapeutic benefits of dietary polyphenols for cognitive impairment and highlights the need for further research in this area. The study’s goals and objectives are to review the evidence for the potential of dietary polyphenols to protect against cognitive decline and to identify the mechanisms by which polyphenols alleviate memory loss via various pathways based on in vivo experimental studies. The study is relevant and novel in that it provides a thorough review of the current literature on this topic and highlights the importance of appropriate dosage and individualized recommendations for polyphenol intake to achieve maximum benefits while avoiding adverse effects.
2. Polyphenols
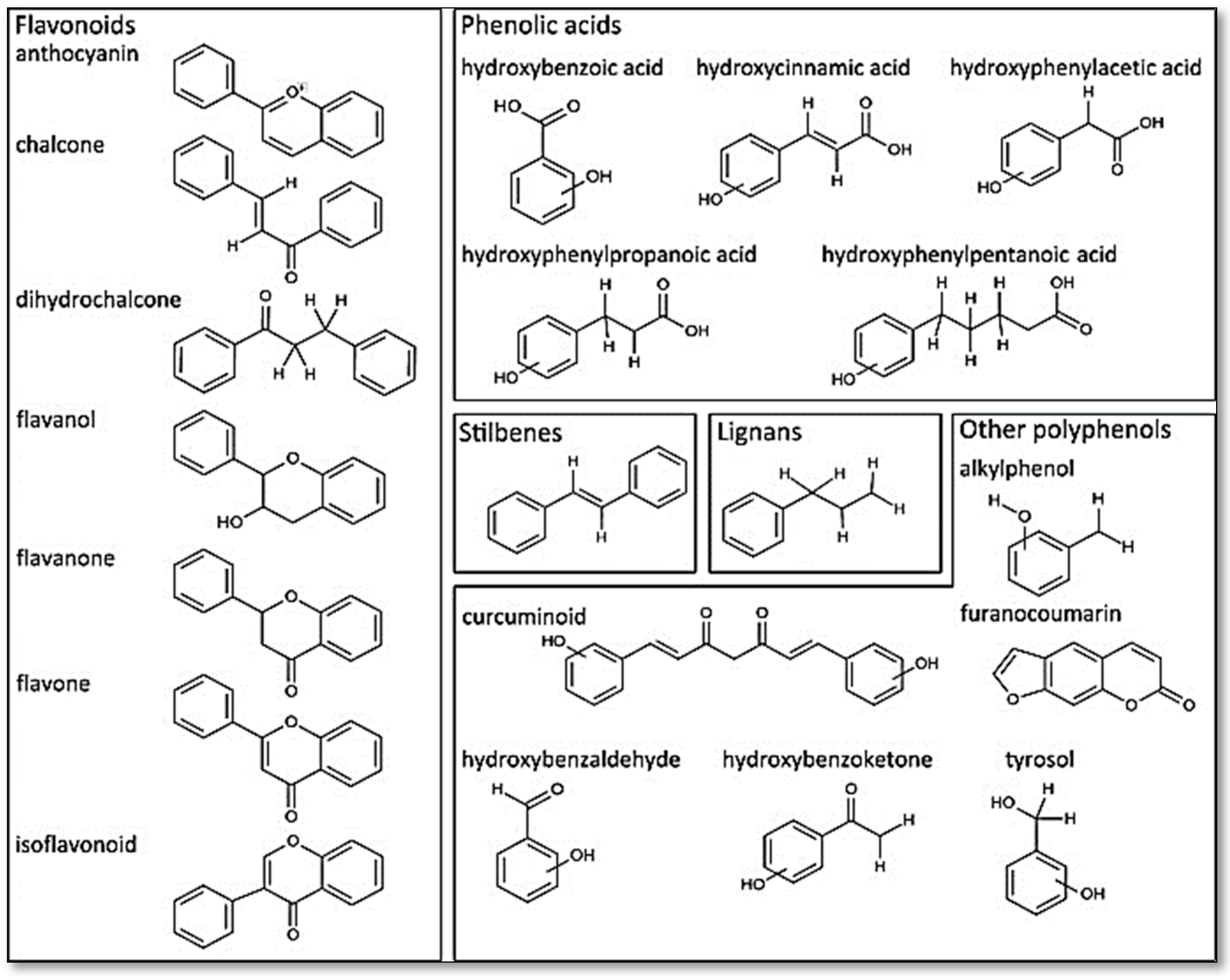
The polyphenols are a large and diverse group of naturally occurring compounds found in plants and are characterized by the presence of multiple phenol rings. They are widely distributed in the plant kingdom and are believed to play a key role in protecting plants against ultraviolet radiation, pathogens, and oxidative stress. To date, 8000 phenolic molecules have been identified [7]. Sources of polyphenols include fruit, vegetables, whole grains, nuts, seeds, and herbs. Polyphenols can be classified into two categories based on their chemical structure: flavonoids and non-flavonoids (phenolic acids, stilbenes, lignans, and others). They can be divided into many sub-classes depending on the number of phenol units within their molecular structures, their substituent groups, and/or the linkage type between phenol units [8]. The presence of phenolic compounds in plants can occur in both free and conjugated forms, with one or more sugar residues joined by β -glycosidic bonds to a hydroxyl group or an aromatic ring’s carbon atom [9]. Figure 1 provides some examples of distinct structures of polyphenols.
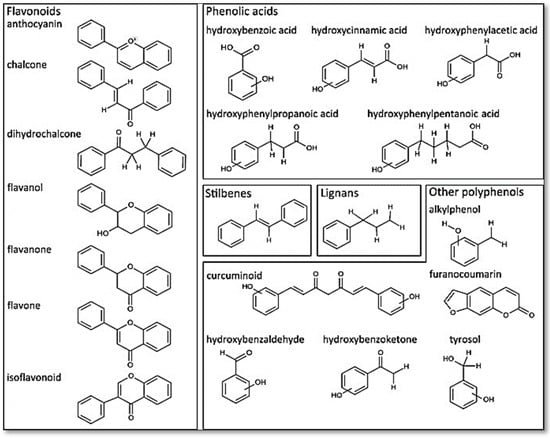
Figure 1.
Distinct substructure of polyphenols. Figure reused under the permission granted by http://creativecommons.org/licenses/by/4.0/ (accessed on 27 April 2023) [10].
2.1. Flavonoids
Flavonoids such as flavonols (found in onions, kale, and tea), flavanones (found in citrus fruits), isoflavaones (found in onions, kale, and tea), flavones, flavan-3-ols (found in tea, cocoa, and some fruits), and anthocyanins are among the most abundant polyphenols available in plants or food and are the most commonly studied. The great variability of the molecules (about 6000 distinct structures) comes from modifications of the core structures, such as by hydroxylation, methylation, glycosylation, and acylation, among others [11]. These modifications can occur at different positions of the rings, leading to a wide variety of flavonoid compounds with different biological activities and functions. Flavonoids can be found as glycosides or aglycones despite the fact that their basic structures are aglycones (the nonsugar part of the corresponding glycoside). All flavonoids share the same basic structure of diphenyl propanes (C6-C3-C6); they are composed of two aromatic rings (A and B) joined by a three-carbon bridge (C) and contain a carbonyl group (C=O) at position 1 in ring C, forming a central pyrane ring [12].
2.2. Flavanones
Flavonones are a type of compound derived from a chalcone-like compound and use flavan-4-ol as a substrate. The heterocyclic ring in flavanones or dihydroxyflavones (hesperetin, naringenin, eridictyol, sylibin, isosakuratenin), has a saturated three-carbon chain without a hydroxyl group at the C3 position, which makes it different from flavonols and flavones. In addition, due to their distinct substitution patterns, flavanones are characterized by a significant number of substituted derivatives, such as prenylated flavanones and benzylated flavanones [13]. Flavanones are recognized as important phytochemicals and are mainly found in high concentrations in citrus fruits, such as lemons (e.g., eriodictyol) and oranges (e.g., hesperidin), and most of these compounds are found in aglycone forms [14].
2.3. Flavones
Flavones differ from other flavonoids since they contain a double bond between the atoms C2 and C3 and a ketone group at the atom C4. Some of the flavones include luteolin, apigenin, chrysin, baicalein, tangeritin, diosmetin, orientin, and scoparin. They are synthesized from flavanones utilizing flavone synthase, which catalyzes the oxidation of C2 and C3 atoms and the formation of the double bond between them. A high degree of chemical diversity is produced by various modifications of the flavone backbone, which leads to a variety of biological actions. Flavones from plants are typically conjugated as 7-O-glycosides via glycosylation, which can enhance the solubility and biological activity of flavone [15,16]
2.4. Isoflavones
Isoflavones are a group of isoflavonoids primarily found in legumes, such as lentils, fava beans, and soybeans, which are the main source of isoflavones in the human diet. Isoflavones resemble estrogens structurally, especially 17-estradiol. Isoflavones resemble estradiol in that they have hydroxyl groups in the C7 and C4 positions and have ring B attached to ring C at the C3 position of the latter ring. Isoflavones are known for their ability to mimic the effects of estrogen in the body by binding to estrogen receptors, and as such, they are sometimes referred to as phytoestrogens. Some of the common isoflavones include genistein, daidzein, and glycitein [17,18].
2.5. Flavonols
Flavonols (dihydroflavonols) are the 3-hydroxy derivatives of flavanones that are synthesized by flavonol synthase. They consist double bond between C2 and C3 and oxygen (a ketone group) in C4 and particularly with flavones by the presence of a hydroxyl group in the C3 position [19]. The main sources of flavanols are cocoa, followed by dark chocolate, berries, strawberry, and nuts. Some foods or plants that are rich in flavonols may also contain significant amounts of catechins and proanthocyanidins [20]. However, the concentration of these compounds can vary greatly depending on the specific food or plant source. Some commonly studied compounds in this group are myricetin, kaempferol, and quercetin [21].
2.6. Phenolic Acid
Phenolic acids are a type of organic compound that is widely distributed in plants. They can be found in many vegetables and fruits (caffeic acid), berries and nuts (ellagic acid), and coffee and some fruit (chlorogenic acid). They are derived from benzoic acid or cinnamic acid and are characterized by the presence of one or more hydroxyl groups (−OH) attached to an aromatic ring. The structure of phenolic acids can vary depending on the number and position of the hydroxyl groups on the ring. Phenolic acids can be classified into two main classes based on their structure which are hydroxybenzoic acids (HBAs) and hydroxycinnamic acids (HCAs). HBAs have a benzoic acid backbone and one or more hydroxyl groups (−OH) attached to them, and examples of HBAs include gallic acid, protocatechuic acid, vanillic acid, and syringic acid. They are found either as free acids or in their conjugated forms (glycosides or esters). Meanwhile, a cinnamic acid backbone with one or more hydroxyl groups (-OH) attached to it is present in HCAs. Examples of hydroxycinnamic acids include caffeic acid, ferulic acid, and sinapic acid [22].
2.7. Lignans
Lignans consist of two propylbenzene units (C6-C3) linked together between the β-position in C8 of the propane side chains. The C9 and C9’ positions of lignans are substituted in different patterns, resulting in a wide range of different structural forms [23,24]. Lignans are found in legumes, seeds, and vegetable oils; they are mainly found in their free forms, while the glycosylated structure is not abundant [25]. The most commonly studied lignans are pinoresinol, secoisolariciresinol, medioresinol, lariciresinol, and syringaresinol [26].
2.8. Stilbenes
Stilbenes (1,2-diarylethenes) are a group of organic compounds that are composed of two benzene rings connected by a two-carbon ethylene bridge (−CH=CH−). The simplest stilbene is trans-stilbene, which has two phenyl groups attached to each end of the ethylene bridge. Stilbenes can exist as a glycosylated compound (major) or freeform (minor). This secondary metabolite is derived from the phenylpropanoid pathway commonly found in plants such as peanuts, grapes, and some berries. One of the most well-known stilbenes is resveratrol. Resveratrol (cis and trans), which is highly concentrated and naturally present in the fresh skin of red grapes, is identified as a main compound of stilbenes [25,27].
3. Cognitive Decline
Several factors contribute to cognitive decline. Some of the common causes include neuroinflammation, oxidative stress, synaptic plasticity, disruptions in the blood–brain barrier (BBB), mitochondrial damage, β-amyloid (Aβ) deposition, impaired neurogenesis, and altered gut–brain axis. Thus, this section briefly explains the possible causes that could lead to cognitive impairments.
3.1. Neuroinflammation
A key cause of cognitive decline and neurodegenerative disorders, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, and amyotrophic lateral sclerosis, is neuroinflammation. Almost all diseases of the central nervous system exhibit neuroinflammation, which is now widely acknowledged as a potential mediator of cognitive decline. Neurodegeneration and advanced age both increase the levels of systemic inflammation. For example, age-related changes in neuroinflammatory responses, such as glial activation and increased production of proinflammatory cytokines, can contribute to abnormal neuronal signaling and cognitive decline. However, it is also possible that abnormal neuronal signaling may trigger the release of proinflammatory cytokines and activate glial cells, leading to a neuroinflammatory response. Furthermore, other factors, such as activated microglia, astrocytes, inflammasomes, and bad cholesterol, can also contribute to the deterioration of the central nervous system microenvironment and exacerbate neuroinflammation and cognitive decline [28,29,30].
3.2. Oxidative Stress
Studies on cells, biochemistry, and molecules have revealed close connections between oxidative stress and cognitive decline with age and age-related neurological disorders. Reduced repair and oxidative damage to nuclear and mitochondrial DNA are symptoms of brain aging. The antioxidant pathways are complex defense mechanisms that guard against oxidative damage to the human body. They include enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and many other nonenzymatic antioxidants that are either endogenous, such as glutathione (GSH), or dietary, such as vitamins A, C, and E and carotenoids. Thus, the degeneration of neurons, which is typically evident in brain illnesses, is caused by the accumulation of oxidative stress damage from oxidized proteins, glycated products, and lipid peroxidation. Cerebrovascular disorders are known to cause cognitive decline and are defined as vascular lesions [31,32].
3.3. Mitochondrial Damage
The mitochondria are the cell’s power plants, producing most of the adenosine triphosphate (ATP) through oxidative phosphorylation [33]. Among all cell types, neurons are among the most intensely energy- (ATP-) consuming cells due to their high metabolic demands. This is because the maintenance of ionic gradients across the neuronal cell membrane and the firing of action potentials require a substantial amount of ATP [34]. Additionally, another reason could be due to the need to preserve the ionic gradients necessary for continuing electrophysiological activity, neurotransmission, and temporary synaptic plasticity [35]. For example, low basal cytoplasmic concentrations and storage in calcium (Ca2+) release organelles are necessary for efficient intracellular Ca2+ signaling and are maintained by ATP-dependent mechanisms. Moreover, damaged mitochondria are important sites for producing free radicals and can cause apoptosis by releasing cytochrome c into the cytoplasm. Hence, even a slight decline in mitochondrial function may cause harm to neurons. The “mitochondrial theory of aging” proposes that mitochondria accumulate oxidative damage as reparative effectiveness declines, leading to inadequate cellular bioenergetics. In turn, metabolic disturbance results in altered synaptic plasticity, which impairs cognitive function, decreased neural resistance to other types of damage, and subsequent neurodegeneration. By leaking electrons throughout the electron transport cascade for oxidative phosphorylation, mitochondria produce free radicals that cause oxidative stress and impair cellular signaling and metabolic processes [36,37].
3.4. Synaptic Plasticity
Modifications to the morphology and function of synapses control synaptic plasticity, which is directly related to memory and learning processes. The two main manifestations of synaptic plasticity are long-term potentiation and long-term depression. The dendritic membrane contains projecting, tiny, highly dynamic structures called dendritic spines. These spines contain signal transduction molecules, scaffolding proteins, ion channels, cytoskeleton components, and postsynaptic density, which are mostly composed of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) and NMDAR [38]. Synaptic plasticity is mediated through structural alterations such as spine elongation and contraction, shape variations, spine distribution/density, and other functions. In the hippocampus and neocortex, abnormal synaptic structure/morphology and decreased spine density are early occurrences and significant changes that are associated with cognitive problems. Such changes in synaptic activity may enhance or impair rhythmic electrical activity, which in turn affects plasticity, demonstrating a two-way interaction [39].
3.5. Impaired Neurogenesis
The process through which new neurons are created in the brain is known as neurogenesis. Therefore, impaired neurogenesis could result in cognitive impairments. Studies show an activity-sensing property of hippocampal neural progenitor cells via Cav1.2/1.3 (L-type) Ca2+ channels and N-methyl-D-aspartate (NMDA) receptors, suggesting that excitation of the local neural network may regulate the neurogenic process. Neurogenesis modulates hippocampal network activity to enable memory storage at different levels [40]. In fact, during hippocampus neuronal development, such activity-dependent responses may aid in the creation of new memories and the erasure of old ones [41]. Progressive memory impairment is associated with degeneration of the hippocampus. The dentate gyrus of the hippocampus is a region critical for learning and memory functions. This will interpret the neurogenesis process and cause balance at brain hemostasis. When the neurogenesis process is not able to process, it may cause cognitive decline [42,43].
3.6. Blood–Brain Barrier Disruption
The BBB is essential for sustaining the neural tissue’s unique milieu. It facilitates communication while separating the brain parenchyma from the peripheral circulation system [44]. The neurovascular unit, which includes pericytes, smooth muscle cells, astrocytes, microglia, oligodendroglia, and neurons, forms the continuous non-fenestrated endothelial cells that make up the BBB at the cellular level [45]. The BBB is selectively permeable, meaning it allows essential nutrients to enter the brain while preventing harmful substances and toxins from crossing into the brain [44]. During the natural aging process, the BBB undergoes a range of structural and functional changes, causing the BBB to break down. Alterations in the composition and organization of the endothelial cell junctions, changes in the number and morphology of pericytes and astrocyte end-feet, and increases in oxidative stress and inflammation are some of the changes that can occur in the natural aging process in the BBB. Resultantly, the permeability of the BBB will increase, allowing potentially harmful substances to cross the BBB and induce damage. The BBB breaks down in the brain, which causes an increase in BBB permeability and a decrease in cerebral blood flow. As aging occurs, the capacity of neovascularization declines, as does the capillary density of the brain vasculature. According to research, the first occurrence of BBB breakdown in the aging hippocampus is what may cause cognitive impairments. The growing dysfunctional state of the brain endothelium is associated with abnormal BBB alterations [46,47].
Along with neurodegenerative diseases, such as Alzheimer’s, Parkinson, amyotrophic lateral sclerosis, multiple sclerosis, and Huntington’s disease [48], virus infection (ex. herpes simplex virus encephalitis) [49] and brain tumors [50] could also disrupt the normal structure of the BBB. In multiple sclerosis, the immune system attacks and damages the myelin sheath that surrounds nerve fibers in the brain and spinal cord, causing inflammation and disruption of the BBB, which allows the immune cells and antibodies to enter the brain [51]. Meanwhile, in the case of herpes simplex virus (HSV) encephalitis, the virus can directly infect and damage the BBB, leading to vascular brain edema, hemorrhage, and leukocyte infiltration, thereby permitting the entrance of viruses, immune cells, and cytokines into the brain parenchyma [49]. Similarly, in the vicinity of brain tumors, the integrity of BBB is disrupted due to the uncontrolled division of tumor cells and metastases and the presence of inflammation, as the tumor cells themselves produce inflammatory molecules [50].
3.7. β-Amyloid (Aβ) Deposition
A protein fragment called beta-amyloid (Aβ) is more frequently found in the brain as sticky, starch-like plaques [52]. The amyloid precursor proteins are cut into beta-amyloid protein fragments. According to a widely held belief, these protein pieces are broken down and discarded in a brain that is functioning ideally, but in some circumstances, this does not happen, and the protein fragments instead build up in the brain to form plaques. Instead of a failure to clear these products, the plaques are caused by an overproduction of amyloid precursor protein, which starts the cytotoxic effects. The result could be cognitive deterioration [52].
3.8. Gut–Brain Axis
The gut–brain axis, a bidirectional communication network that connects the enteric and the brain, is recognized to have a close connection with the central nervous system. This involves the ability of the gut microbiota to produce and release neurotransmitters and neuroactive metabolites that can affect brain functioning. For instance, a low level of Bifidobacterium infantis resulted in decreased production of serotonin and a high concentration of tryptophan in the plasma [53]. A marked decrease in serotonin in the neurons is positively associated with an increased level of cortical Aβ deposition and cognitive impairment [54]. Conversely, the gut microbial compositions of Lactobacillus plantarum, Lactobacillus brevis, Bifidobacterium adolescentis, Bifidobacterium angulatum, and Bifidobacterium dentium are known for their ability to produce gamma-aminobutyric acid (GABA) [55]. As an inhibitory neurotransmitter, GABA can control neuronal activity, reduce postsynaptic transmission hyperactivity, and regulate neural firing in the hippocampal [56]. Similarly, gut dysbiosis and disturbances to the gut–brain axis have a causal relationship with the development of neurological conditions, specifically cognitive performance [57].
4. Materials and Methods
4.1. Search Strategy
The search for potentially relevant articles for this review was done based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) described elsewhere [58]. Boolean operators were used to identify the keywords for article searches. The selected keywords included (1) nutritional polyphenol intervention NOT medicine AND neuron growth OR (2) dietary polyphenol AND neurogenesis AND memory impairment OR (3) polyphenol AND neuron regeneration AND memory deterioration. The literature search was conducted from January 2013 to January 2023, thereby limiting the article search to the past 10 years of publication.
4.2. Inclusion Criteria
Only animal studies (in vivo) were considered to be included in this review. All articles published in the English language, within 10 years of publication, and with full-text accessibility were selected to be further screened. The selected articles must contain all of the following information: (1) animal model used; (2) intervention details, such as dosage duration, type, and method; (3) behavioral test used; and (4) findings focusing on cognition.
4.3. Exclusion Criteria
All secondary articles, thesis dissertations, proceedings, patents, and case reports were excluded from being further reviewed. Those articles that were written in any other language other than English and articles that contained inadequate details as described in the inclusion criteria were automatically rejected from being further reviewed. Any articles that focused on in vitro, ex vivo, or human trials (prospective or retrospective) were also excluded.
4.4. Data Extraction
Initial data extraction was performed by 4 reviewers independently (R.N., J.K., S.S.B., S.H.T., and H.B) by screening the title, abstract, and full text. All articles that meet the inclusion criteria were selected to be included in the review. Upon agreement, a standardized data extraction sheet was created in which all of the reviewers extract data based on the selected article independently. The data extraction table is shown in Table 1. Any disagreements during data extraction were resolved by discussing with the fifth reviewer (H.S).

Table 1.
Natural polyphenols for neuroprotection in cognitive impairments.
5. Results
5.1. Literature Search and Article Selection
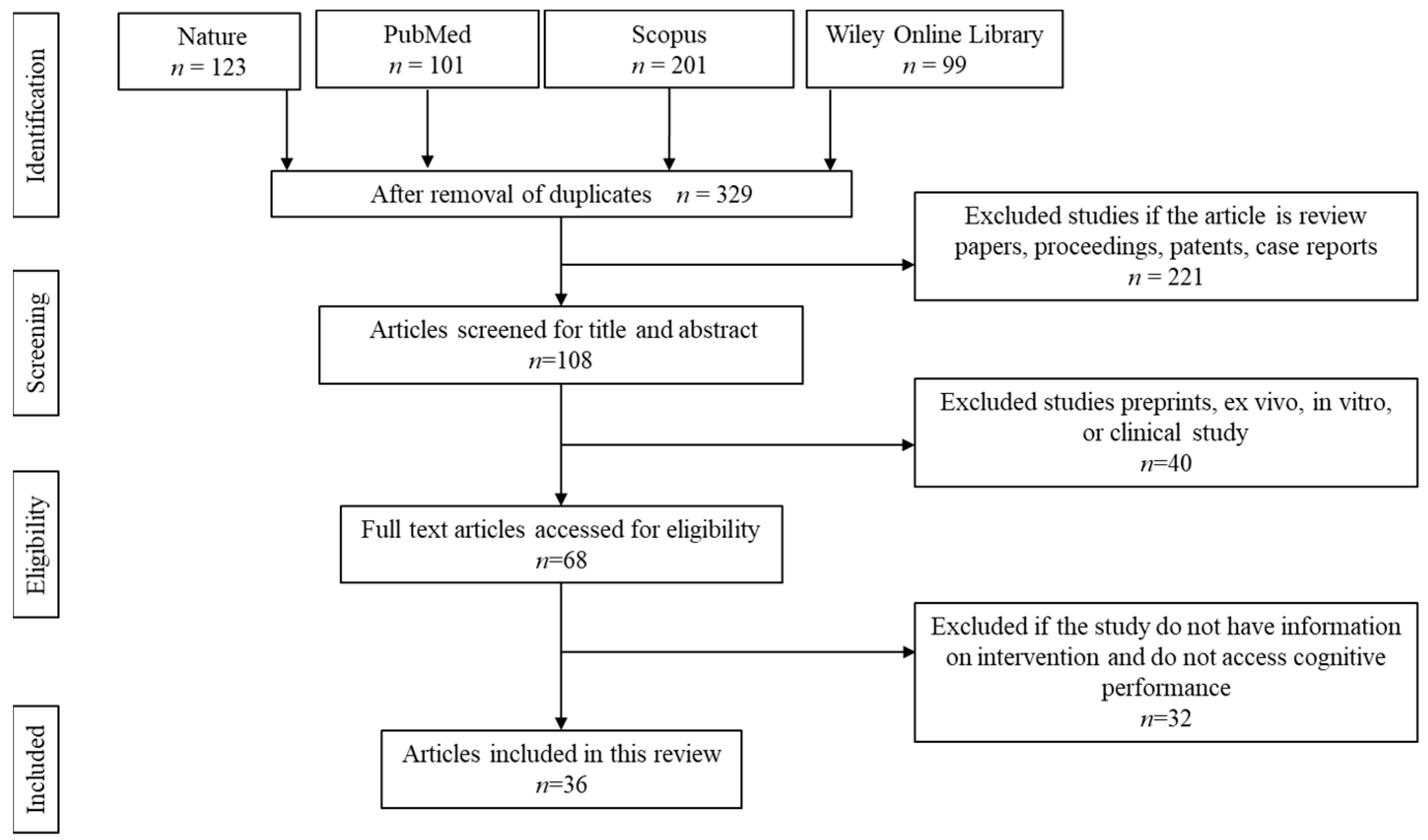
The article selection and screening processes were conducted as shown in Figure 2. The initial screening resulted in a total of 524 articles. About 195 articles were removed owing to duplication. Of the remaining 329 articles, 221 articles were removed due to being secondary literature, case reports, proceedings, and patents. Another 40 articles were removed because the articles focused on in vitro, ex vivo, retrospective, or prospective studies. The remaining 68 articles were screened and accessed for full-text eligibility. From there, 32 articles were removed because the articles did not specify the details on the dose, duration, and type of intervention or if the study did not assess cognitive performance.
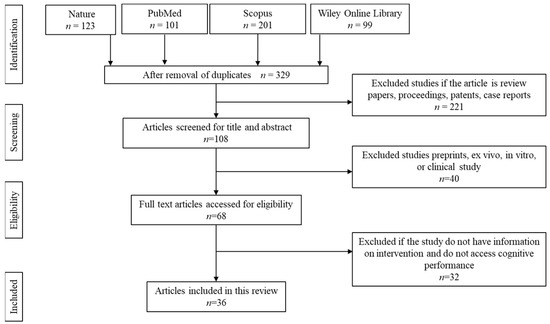
Figure 2.
Article screening and selection.
5.2. Mechanism of Polyphenols Alleviating Cognitive Decline
In vivo studies conducted for the past 10 years show that numerous incidents can cause cognitive decline as summarized in Table 1. Some of the pathological changes that induce cognitive impairments are neuroinflammation [59,71], oxidative stress [60,63,64,66,77], mitochondrial damage, impaired neurogenesis, disrupted blood–brain barrier (BBB) [92], and Aβ deposition [61,62,67,89,91]. In this context, polyphenols have proven to ameliorate cognitive decline and reverse pathological changes that occur in neurodegenerative diseases. For instance, dietary intake of polyphenol-rich fruits, such as apples (Ralls) [62], Prunus Salicina [67], Vitis vinifera [69], and Boswellia serrata gum [77] can reverse neuropathological changes associated with cognitive impairments in Alzheimer’s disease. Polyphenol intake is positively associated with cognitive performance improvements regardless of underlying conditions. One of the main reasons for this could be the ability of the polyphenols to cross the BBB and exhibit their effects. Since the BBB is selectively permeable, the relatively small size of the polyphenols enables them to cross the BBB and reach the targeted part of the brain (neurons) to exhibit their neuroprotective effects [96]. Polyphenols, being strong natural antioxidants, can neutralize excessive free radicals in the brain, thereby suppressing the activity of ROS. Thus, this section explains the mechanism of polyphenols in different pathways involved in cognition.
Polyphenols are proven to suppress neuroinflammation by suppressing proinflammatory factors, such as IL-1β, IL-6, and TNF-α, in the cerebral cortex and hippocampus. Microglial activation in neurotoxic conditions (M1) may stimulate the excessive release of proinflammatory cytokines, nitric oxides, and ROS, which may further activate the NLRP3 inflammasome signaling pathway [97]. The aggregation of Aβ is one of the causes of NLRP3 pathway activation. In such condition, upon activation NLRP3 will bind to the pyrin domain (death fold protein) and stimulates protease pro-caspase-1 to configure NLRP3 inflammasome, thereby pro-caspase-1 will become activated which is now known as caspase-1. Thus, caspase-1 is now free to bind with the inactive forms of proinflammatory cytokines and may induce the release of uncontrolled proinflammatory cytokines [98]. The strong proinflammatory signals may further induce pyroptosis in the neurons [99]. Hippocampal pyroptosis is one of the primary causes of cognitive decline in neurodegenerative diseases [100]. Conversely, Aβ may cleave to toll-like receptor 4 (TLR4) and induce microglial activation and excessive generation of cytokines. This may inhibit the microglial phagocytic process, causing the toxic form of Aβ (Aβ1–42) to increase, eventually leading to neuroinflammation [101]. Meanwhile, polyphenol compounds such as gallic acid and Salvianolic acid B can block the action of the NLRP3 inflammasome signaling pathway directly [102]. Several other polyphenols, such as curcumin, can inhibit the NLRP3 activity by blocking the action of the TLR-MyD88-NF-κB pathway, thereby preventing neuronal apoptosis [103,104]. In this, 100 mg/kg of curcumin is enough to inhibit TLR4-mediated proinflammatory cytokine release and repress microglial activation [104]. Another study shows that polyphenol (Catechin) intake could downregulate p38MAPK and NF-κBp65 signaling via the inhibition of TLR2, which may decrease the proinflammatory mediators and its signal transduction pathway [105].
Besides, the ability to neutralize free radicals is another way polyphenols exhibit neuroprotective effects. High levels of free radicals can be inferred from suppressed levels of endogenous antioxidants, including SOD, CAT, GPx, and GSH. In this, polyphenolic compound, such as Chlorogenic acid [88] and 5-Caffeoylquinic acid [89], is known to be an excellent free radical scavenger. This is achieved through the activation of Akt phosphorylation. Phosphorylated Akt in turn may inhibit the activity of GSK-3β, causing the level of cyclin D1 to increase, thereby modulating the cell cycle [106] and promoting neurogenesis by initiating neuronal differentiation [107]. Aside from this, caffeoylquinic acids protect hippocampal neurons from hydrogen-peroxide-induced oxidative stress by upregulation of nicotinamide adenine dinucleotide phosphate (NADPH) [108]. An alternative method of using 5-Caffeoylquinic to neutralize free radicals is decreasing the level of lipid peroxidation in the hippocampus, thereby restoring cognitive performance [109]. A high level of ROS may mediate lipid peroxidation, which may cause neuronal damage. In the case of neurological disorders such as Alzheimer’s, lipid peroxidation may induce accumulation of Aβ, while in Parkinson’s, it may induce the localization of 4-hydroxy-2-nonenal within the Lewy bodies [110]. In such a scenario, cognitive dysfunction is a common pathological symptom that arises from the high level of lipid peroxidation.
In addition, polyphenols are believed to restore cognition via the modulation of mitochondrial metabolism in the hippocampus region of the brain [111]. Mitochondrial dysfunction can induce the release of NADPH oxidase from the Kreb cycle, thereby accelerating the mitochondrial electron transfer chain. Resultantly, this may raise ROS generation and oxidative stress in the hippocampus [112]. Dysfunctional mitochondria and biogenesis may evince a reduced level of SIRT-1 [113]. SIRT-1 plays a vital role in neuronal plasticity and shield against neuronal degeneration associated with cognitive decline [114]. Under redox conditions, SIRT-1 initiates differentiation in the neural progenitor cells in the astrocytes, thus triggering the accumulation of damaged mitochondrial DNA [115]. This happens as a result of disruption in the integrity of mitochondrial DNA due to a high level of oxidative stress [116]. Thus, the depletion of mitochondrial DNA may activate astrocytes, leading to spongiotic degeneration in the brain parenchyma, as well as severe neurodegeneration, which in due time will manifest as memory loss [117]. However, consumption of polyphenols such as resveratrol [82] and a polyphenol-rich diet comprising various fruits, vegetables, and nuts [90] have proven to improve the level of SIRT-1 drastically in the prefrontal cortex and hippocampus. It has been claimed that a polyphenols-enriched diet can stimulate SRT1460 and SRT218, which in turn may increase the level of SIRT-1 up to five-fold [118]. The increase of SIRT-1 suppresses oxidative stress in the mitochondria via deacetylation of Forkhead box O3 (FOXO3), which may enhance the formation of endogenous antioxidants such as manganese SOD and CAT [119].
In reality, polyphenol metabolites are proven to enter the BBB at a measurable amount across the endothelium [96]. Thus, they would be naturally occurring compounds with an ability to prevent constant injury to the neurons in the brain by providing a shield against the brain neurons [96]. Disruption in the cerebrovascular integrity is one of the most common pathological conditions that lead to cognitive dysfunction in neurological disorders [46,120,121,122]. However, dietary polyphenols can reverse these conditions. Recent discoveries have shown that polyphenols can reduce BBB permeability via various pathways. For instance, quercetin increases the level of SOD [123] and Nrf2 to maintain the permeability and integrity of the BBB by suppressing ROS [124]. Quercetin upregulates the level of claudin-5 and ZO-1 and downregulates the expression of MMP through Wnt/β-catenin downstream mediators to mitigate dysfunctional BBB [125]. As a result, the proinflammatory cytokines, such as NF-κB, TNF-α, and IL-6, will be reduced in the hippocampal region of the brain. At the same time, quercetin impedes the action of E-selectin in the cerebral endothelial cells, therewith preventing monocyte adhesion [126]. Since microglial activation may arise from the transmigration and adhesion of monocytes and enhance cognitive decline [127]; the role of quercetin here could prevent microgliosis, thereby preventing memory loss. Parallelly, polyphenols such as luteolin attenuate cognitive decline [60] through the inhibition of NF-κBp65 translocation, the p38MAPK pathway, and phosphorylated inhibitory κB kinase in the BBB, thus providing a shield against the barrier function of the BBB [128]. Similarly, resveratrol in animal models exhibits its strong antioxidant capacity by suppressing the excessive level of ROS in the BBB and astrocytes and the expression of (NADPH oxidase) 2 and 4 enzymes in the BBB endothelial cells [129].
In addition, administration of certain polyphenols can affect synaptic morphology [130]. For instance, oral administration of 20 mg/kg of resveratrol led to an increase in the length and number of dendritic spines in pyramidal neurons. Since dendrites play a vital role for synaptic function and plasticity, these synaptic morphological changes may have beneficial effects on cognitive function by promoting synaptic plasticity [131]. An intraperitoneal injection of 25 mg/kg of epigallocatechin gallate (EGCG) was found to be effective in restoring dendritic arborization and improving spine maturation in the brain by restoring spine density and maturation [132]. Another mechanism how polyphenols mitigate cognitive impairments is by abating neurotoxins such as excessive level of glutamate [133], and nitric oxide [134] in the brain. Hyperexcitability of glutamate signaling may result in neurodegenerative-associated cognitive decline by inducing neuronal apoptosis [135]. In this, green tea polyphenols enhance the expression of Bcl-2, thereby inhibiting the pore development in the mitochondria by hindering the translocation of Bax from the cytosol into the membrane of mitochondria. Resultantly, activation of caspase 3 and cytochrome C release will be stopped, thereby preventing neuronal apoptosis [133]. Another polyphenol can modulate nitric oxide formation and iNOS expression. Such action in microglia may prevent excessive production of cytokines, in that way preventing injury to the neurons [136]. A similar result was reported during intervening polyphenol-rich blueberry on kainic-acid-infused rat hippocampus. It has been outlined that polyphenol-rich blueberries enhance cognitive behavior by reducing the expression of insulin-like growth factor 1, IL-1β, TNF-α, and NF-κB [137].
5.3. The Interplay between Polyphenols, the Gut–Brain Axis, and Cognition
Polyphenols play a vital role in restoring cognition via the signaling pathway involving the gut-brain axis. This is because polyphenols must undergo biotransformation to obtain their metabolites, and this process is accommodated by the gut microbiome. Hence, the gut–brain axis acts as a neuroendocrine system that helps the polyphenols’ metabolites to cross the BBB and exhibit their effects. This may be achieved by various networks involving the enteric nervous system or neuroimmune system [57]. Disruption in the gut–brain axis homeostasis is one cause of cognitive decline [138]. A recent study shows that gut in dysbiosis, a particularly significant reduction in Eubacterium rectale—an anti-inflammatory bacterium—with an increased level of Escherichia coli and Shigella—proinflammatory bacteria—is one of the primary reasons for peripheral inflammation and cognitive decline. This hypothesis was further proven by the presence of abnormal deposition of amyloid in the brain (amyloidosis) [139]. Another study indicates that chronic infection of Helicobacter pylori is significantly linked to increased gastric atrophy and cognitive dysfunction. The study proves that Helicobacter pylori-infected Alzheimer’s is more susceptible to developing neuroinflammation and cerebrovascular lesions, leading to cognitive impairments [140]. Similarly, excessive level of Escherichia coli in the gut microbiota stimulates microgliosis and activates TNF-α, IL-12, and IL-6, eventually resulting in cognitive impairments [141]. In due course, the short-chain fatty acids from microbes mediate the interaction between the bacteria and the microglia cells. When this happens, the bacterial metabolites tend to deviate from colonic mucosa blood circulation, crossing the BBB and inducing neuroinflammation in the brain [142].
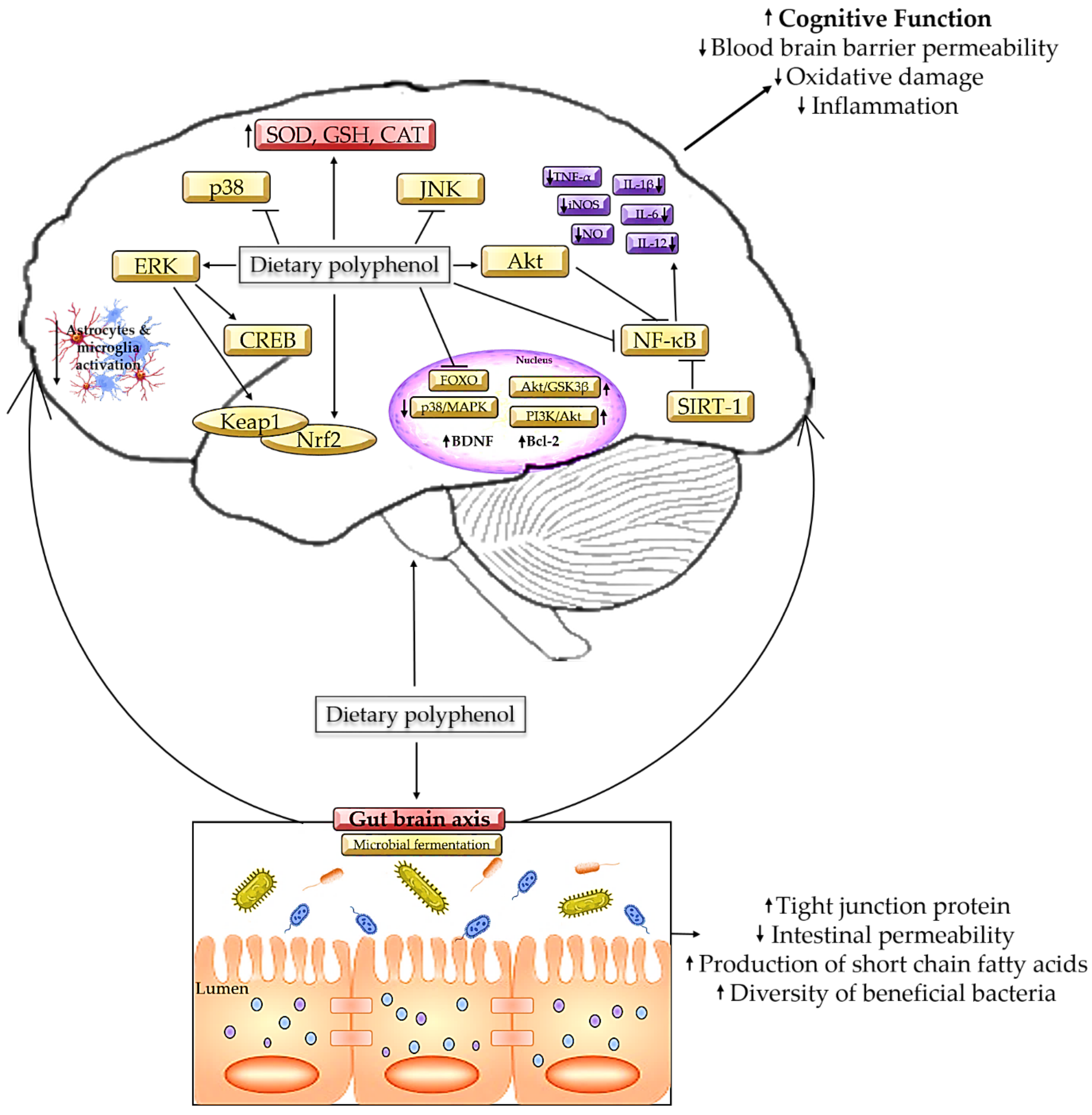
On the contrary, polyphenols could alleviate the damage caused by gut dysbiosis by modifying the colonic microbial composition by influencing bacterial growth and metabolism [8]. Clostridium perfringens produces epsilon toxin, which may cleave endothelial cells of the brain, enter the BBB, and induce cellular lesion and glutamate release [143], while Bacteroides spp. induce hyperintensity of white matter and hippocampal atrophy and cortical atrophy [144]. In both of these cases, the changes will result in cognitive impairment at some point. However, the high concentration of gallic acid and catechins in tea extract is proven to inhibit the growth of pathogenic bacteria such as Clostridium perfringens, Clostridium difficile, and Bacteroides spp. by stimulating the formation of phenylpropionic acid [145]. Comparably, a dietary intake of curcumin, 700 mg/3 times per day for 4 weeks, successfully eradicates Helicobacter pylori colonization [146]. Meanwhile, tea polyphenol epigallocatechin gallates act as pro-oxidants by increasing the level of intracellular oxidative stress to suppress the growth of Escherichia coli [147], while gallic acid is proven to hinder Shigella spp. by repressing the expression of the mdoH and OpgH genes [148]. Figure 3 summarizes the mechanism of polyphenols in alleviating cognitive decline.
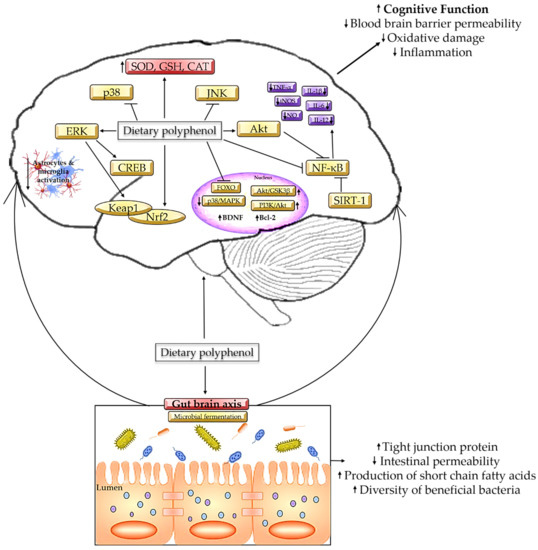
Figure 3.
Mechanism on polyphenols in alleviating cognitive decline.
5.4. Polyphenols and Endogenous Antioxidants
The first line of defense against an excessive amount of ROS is initiated by endogenous antioxidants. In these circumstances, SOD, CAT, and GPx are some of the enzymes that provide first-line defense against H2O2 and superoxide, while aldo-keto reductase, aldheyde dehydrogenase, and glutathione S-transferase (GST) act as a second-line defense against ROS [149]. Upon neutralization by polyphenols, the detoxified products will be cleared from the cells and can be further broken down via the mercapturic acid pathway [150]. Alternatively, a minimum amount of 300 g per day of dietary polyphenol intake maximizes the capacity of endogenous antioxidants via the Nrf2/antioxidant response element (ARE) signaling mechanism and its downstream modulators. Since Nrf2 is the primary regulator for genes responsible for GSH, redox reaction, and antioxidant response; Nrf2 is involved in the modulation of dietary polyphenols and protection against ROS-induced oxidative stress [151]. Upon sensing a rise in oxidative stress, Kelch-like ECH-associated protein 1 (Keap1)—the regulator of Nrf2—restricts the degradation of protein ubiquitination [152]. Subsequently, the accumulation of Nrf2 is increased by transforming phenolics into electrophilic quinones and hydroquinones [153] in the nucleus, thereby increasing the synthesis of endogenous antioxidants of SOD, CAT, and peroxiredoxin [152]. Conversely, a gender-specific study revealed that females tend to have high levels of endogenous antioxidants compared to males. One of the reasons for this could be due to the high level of estrogen in females that modulates the regulatory function of mitochondria [154]. Thus, men need a slightly higher intake of dietary polyphenols compared to women [155] to maximize the protective effects of existing endogenous antioxidants.
6. Bioavailability of Dietary Polyphenols
Despite many encouraging findings on the potential effectiveness of dietary polyphenols towards health benefits, the bioavailability of dietary polyphenols remains a main dilemma and has yet to be resolved. Bioavailability can be defined as the proportion of the nutrient that is digested, absorbed, and made usable at the location of the action. The majority of dietary polyphenolic compounds exhibit low bioavailability and are primarily excreted in feces after entering the gut, which results in their poor bioaccessibility [156,157]. As reported by Shivashankara and Acharya (2010), the bioavailability of dietary polyphenols can be ranked in the following order: isoflavones > flavanols > flavanones > flavonols > anthocyanins [158]. High dietary polyphenol intake does not always correspond with high polyphenol bioavailability [159]. The bioavailability of dietary polyphenols can vary depending on several factors, including the type of dietary polyphenol, the food matrix it is found in, food processing, digestive enzymes, the microenvironment of the intestine, metabolism rate, and gut microbiota composition. The main factor that has been identified as causing poor bioavailability of dietary polyphenols is a first-pass metabolism that involves phase II conjugation, such as like methylation, glucuronidation, or sulfonation [160]. This metabolic reaction will convert dietary polyphenol into hydrophilic conjugates known as glucuronides and sulfates, which are then easily eliminated via urine [160,161,162].
Biotransformations promoted by the gut microbiota can have a significant impact on the bioavailability of polyphenols. The gut microbiota can transform a polyphenol through enzymatic reactions, such as fermentation, oxidation, reduction, and hydrolysis. These biotransformations can alter the chemical structure of these compounds, affecting their solubility, stability, and absorption. One of the factors that can influence the level of biotransformations of polyphenols in the gut is the individual diversity of intestinal microbiota. For instance, deglycosylation can be performed by a wide array of gut microbial species and genera; others require particular enzymes that are only expressed by a given species or strain. Deglycosylation will produce an aglycone form of the polyphenol which reduces both the bioaccessibility and bioavailability of phytochemicals [21,163,164].
7. Dosage and Safety Concerns of Dietary Polyphenols
The effective dosage of dietary polyphenols can vary depending on the specific compound. Some studies have suggested that lower doses of certain polyphenols, such as resveratrol, may be more effective than higher doses at providing neuroprotection against cognitive decline [165,166]. When evaluating the therapeutic potential of dietary polyphenols, CNS (central nervous system) penetrability is an important consideration. The ability of a compound to penetrate the CNS can be influenced by factors such as molecular weight, lipophilicity, and the presence of polar groups in the compound. Some polyphenols, such as curcumin, have poor CNS penetrability due to their low lipophilicity and high molecular weight [167]. In contrast, others—such as EGCG—have been shown to readily cross the BBB even in very low doses [168]. The amount of dietary polyphenol intake influences the beneficial effect it could exhibit. A study published by Perry et al. (2018) indicates that daily consumption of 500 mg of polyphenol could help to reduce oxidative stress, while 1000 mg/day is enough to suppress inflammation. Similarly, up to 1500 mg of dietary polyphenol intake could enhance mitochondrial synthesis [169]. Based on epidemiological data collected, it has been suggested that a mean consumption of 900 mg/day is the daily recommended dosage [170]. However, the minimal dosage requirements could differ for each individual based on underlying disease condition, daily lifestyle, geographical location, or source of polyphenol. For example, for obese subjects, a daily dosage of not more than 1200 mg is recommended daily [171], while the safe dose for pregnant women would be 500 mg/day [172]. Conversely, the dosage effects of polyphenols may depend on the age and strain of the animals. For example, one study found that chronic treatment with resveratrol improved cognitive function in aged rats [173] but not in young mice [174]. Similarly, a study on the effects of blueberry extract on cognitive function found that supplementation improved memory in aged mice [175] but had no effect on young mice. In terms of strain differences, a study comparing the effects of green tea extract on cognitive function in two different strains of mice found that the extract improved memory in SAMP8 but not in the SAMR1 [176]. It is important to know the recommended dosage, as excessive consumption of polyphenols could induce adverse effects.
A toxicity evaluation shows that continuous intake of quercetin for 2 years at 40,000 ppm daily has induced chronic nephropathy and carcinogenic symptoms in the kidney in Fischer 344 male rats. Thus, from the documented symptoms, quercetin has been termed a genotoxic chemical [177]. Similarly, a daily intake of 40 mg of quercetin for 50 weeks decreased the life span of male LACA mice, while the same dose in female LACA mice increased the life expectancy [178]. Therewithal, a diet supplement containing 2% of caffeic acid in Fischer 344 rats and C57BL/6N x C3H/HeN F1 mice resulted in aggressive carcinogenic development in the abdominal cavity and renal tubule [179]. One of the possible underlying causes for this could be due to the ability of quercetin to inhibit catechol O-methyltransferase (COMT) [180] which in turn may trigger redox reaction in catecholestrogens, eventually accelerating the progression of estradiol-induced carcinogenesis [181]. Similarly, 1% of green tea catechins diet supplements for 33 weeks resulted in cancer cell proliferation in Fischer 344 male rats [182]. Another study theorizes that polyphenol-rich tea consumption positively correlates with iron deficiency in women [183], while proanthocyanidins escalate the level of endogenous digestive proteins, such as protease, lipase, and biliary acid [184]. Additionally, grape seed polyphenolic extract exhibited pro-oxidants and deleterious effects in cells [185] and interfered with the pharmacokinetics and bioavailability of certain drugs (benzodiazepines and terfenadine) [186].
In toto, while some polyphenols have been shown to have potential health benefits, such as antioxidant and anti-inflammatory effects in restoring cognition as discussed in this study, others may have harmful effects (carcinogenic or genotoxic) at high doses [181]. One challenge in evaluating the potential toxicity of polyphenols is the variability in the types and amounts found in different foods. Additionally, some polyphenols may be transformed by gut bacteria into metabolites that have different effects on the body than the original compound [187]. Although there is some evidence to suggest that high doses of certain polyphenols, such as catechins found in green tea, may have toxic effects on the liver in very rare cases [188], these effects have typically been observed in studies using very high doses that are unlikely to be encountered in normal dietary intake. Moderate long-term use of certain polyphenols, such as resveratrol found in grapes and red wine, has been associated with potential health benefits, such as improved memory function as described in Table 1. However, the evidence for these benefits is still limited, and more research is needed to determine optimal doses and potential risks.
8. Conclusions
In conclusion, there has been growing interest in the potential of dietary polyphenols to protect against cognitive decline. The reviewed animal studies have provided strong evidence that polyphenol intake can reverse cognitive dysfunction via various pathways, including reducing neuroinflammation, oxidative stress, and Aβ deposition, improving mitochondrial function and synaptic plasticity and modulating gut microbiota. However, it is important to note that excessive intake of polyphenols can also have adverse effects, such as gastrointestinal discomfort, liver toxicity, and interference with nutrient absorption. Therefore, appropriate dosage taking into account individual factors, such as gender, underlying conditions, and lifestyle, is crucial. Hence, while the animal experiments provide a strong foundation for the therapeutic potential of dietary polyphenols in cognitive decline, more research is needed to determine the optimal dosage and long-term effects of polyphenol intake in humans. It is also important to consider individual factors when recommending polyphenol intake for memory enhancement. Nevertheless, the findings suggest that dietary polyphenols hold promise as a safe and effective therapeutic strategy for cognitive impairment, especially when integrated with other lifestyle changes.
9. Limitation
One limitation of this study could be the sex of the animal models used in the study. Since most of the studies use male animal models, the outcomes may not accurately reflect the effects of dietary polyphenols on female subjects. This may limit the generalizability of the findings to female subjects, as there might be evidence to suggest that the neuroprotective effects of polyphenols may differ between males and females due to differences in sex hormones and other biological factors. Thus, future studies should aim to include both male and female animal models to better understand the sex-specific effects of polyphenols on brain health. This is to avoid potential biases and highlight the importance of considering sex differences in future studies. Additionally, although the selected papers may suggest that polyphenols compounds have a protective effect against neuronal death, the manuscripts may not sufficiently address the concentrations at which these effects occur. This could be seen as a limitation of the study, as it may not provide a comprehensive understanding of how the compounds function in vivo. Another limitation is that animal models may not always translate directly to human subjects. The effects of dietary polyphenols on cognitive decline and neuroprotection in humans may be different than those observed in animal models. Additionally, the specific type and dose of polyphenols used in animal models may not accurately reflect the polyphenol intake of humans. Human diets are more complex and may contain a wider variety of polyphenols than those used in animal studies. Finally, it is important to note that the effects of polyphenols on cognitive decline and neuroprotection are likely to be influenced by a variety of factors beyond diet, including genetics, lifestyle, and environmental factors.
Author Contributions
Conceptualization, R.N., M.D.Y., H.B. and H.E.; methodology, M.D.Y.; software, S.H.T.; validation, S.H.T., S.S.B. and J.K.; formal analysis, R.N.; investigation, R.N.; resources, H.B.; data curation, R.N.; writing—original draft preparation, R.N., M.D.Y., H.B. and H.E.; writing—review and editing, R.N., S.S.B., H.S. and H.E.; visualization, S.H.T.; supervision, J.K. and H.B.; project administration, S.S.B.; funding acquisition, H.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Higher Education, Malaysia, through the Fundamental Research Grant Scheme, with the reference number Universiti Putra Malaysia 04-0L-20-2274FR with the project code FRGS/1/2020/SKK0/UPM/02/4.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thakur, K.T.; Albanese, E.; Giannakopoulos, P.; Jette, N.; Linde, M.; Prince, M.J.; Steiner, T.J.; Dua, T. Neurological Disorders. In Mental, Neurological, and Substance Use Disorders: Disease Control Priorities; Patel, V., Chisholm, D., Dua, T., Laxminarayan, R., Medina-Mora, M.E., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2016; Volume 4, pp. 87–107. ISBN 978-1-4648-0428-1. [Google Scholar]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 480. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Sriramoju, B.; Kanwar, R.K. Neurological disorders and therapeutics targeted to surmount the blood-brain barrier. Int. J. Nanomed. 2012, 7, 3259–3278. [Google Scholar] [CrossRef] [PubMed]
- Pohl, F.; Kong, P.; Lin, T. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [PubMed]
- De Vries, K.; Medawar, E.; Korosi, A.; Witte, A.V. The Effect of Polyphenols on Working and Episodic Memory in Non-pathological and Pathological Aging: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 720756. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of Blood–Brain Barrier Permeability of Polyphenols, Anthocyanins, and Their Metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Tomás-Barberán, F.A.; García-Villalba, R. Structural Diversity of Polyphenols and Distribution in Foods. In Dietary Polyphenols; Tomás-Barberán, F.A., González-Sarrías, A., García-Villalba, R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2020; pp. 1–29. ISBN 9781119563754. [Google Scholar]
- Lacroix, S.; Klicic Badoux, J.; Scott-Boyer, M.P.; Parolo, S.; Matone, A.; Priami, C.; Morine, M.J.; Kaput, J.; Moco, S. A computationally driven analysis of the polyphenol-protein interactome. Sci. Rep. 2018, 8, 2232. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- De Giada, M.L.R. Food phenolic compounds: Main classes, sources and their antioxidant power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-Gonzalez, J.A., Ed.; Intech: London, UK, 2013; pp. 87–112. ISBN 978-953-51-5374-0. [Google Scholar]
- De Oliveira, L.D.L.; De Carvalho, M.V.; Melo, L. Health promoting and sensory properties of phenolic compounds in food. Food Sci. Technol. 2014, 61, 764–779. [Google Scholar] [CrossRef]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From Biosynthesis to Health Benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- La Szeja, W.; Grynkiewicz, G.; Rusin, A. Isoflavones, their Glycosides and Glycoconjugates. Synthesis and Biological Activity. Curr. Org. Chem. 2017, 21, 235. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, F.; Brombo, G.; Zuliani, G. Nootropics, functional foods, and dietary patterns for prevention of cognitive decline. In Nutrition and Functional Foods for Healthy Aging; Watson, R.R., Ed.; Elsevier Inc.: Ferrara, Italy, 2017; pp. 211–232. ISBN 9780128092996. [Google Scholar]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Suzuki, S.; Umezawa, T. Biosynthesis of lignans and norlignans. J. Wood Sci. 2007, 53, 273–284. [Google Scholar] [CrossRef]
- Willför, S.M.; Smeds, A.I.; Holmbom, B.R. Chromatographic analysis of lignans. J. Chromatogr. A 2005, 1112, 64–77. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, O.; Fernando, W.B.; Lumanlan, J.; Ademuyiwa, O.; Jayasena, V. Role of phenolic acid, tannins, stilbenes, lignans and flavonoids in human health—A review. Int. J. Food Sci. Technol. 2022, 57, 6326–6335. [Google Scholar] [CrossRef]
- Alam, A.; Hana, Z.; Jin, Z.; Suen, K.C.; Ma, D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine 2018, 37, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Editorial: Neuroinflammation and Cognition. Front. Aging Neurosci. 2018, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Lecca, D.; Jung, Y.J.; Scerba, M.T.; Hwang, I.; Kim, Y.K.; Kim, S.; Modrow, S.; Tweedie, D.; Hsueh, S.C.; Liu, D.; et al. Role of chronic neuroinflammation in neuroplasticity and cognitive function: A hypothesis. Alzheimer’s Dement. 2022, 18, 2327–2340. [Google Scholar] [CrossRef]
- Kandlur, A.; Satyamoorthy, K.; Gangadharan, G. Oxidative Stress in Cognitive and Epigenetic Aging: A Retrospective Glance. Front. Mol. Neurosci. 2020, 13, 41. [Google Scholar] [CrossRef]
- Revel, F.; Gilbert, T.; Roche, S.; Drai, J.; Blond, E.; Ecochard, R.; Bonnefoy, M. Influence of Oxidative Stress Biomarkers on Cognitive Decline. J. Alzheimer’s Dis. 2015, 45, 553–560. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, R.; Qiao, L.; Du, Y. The molecular dynamics of neural metabolism during the action potential. Sci. China Technol. Sci. 2014, 57, 857–863. [Google Scholar] [CrossRef]
- Kann, O.; Kovács, R. Mitochondria and neuronal activity. Am. J. Physiol. Cell Physiol. 2006, 292, C641–C656. [Google Scholar] [CrossRef] [PubMed]
- Khacho, M.; Clark, A.; Svoboda, D.S.; MacLaurin, J.G.; Lagace, D.C.; Park, D.S.; Slack, R.S. Mitochondrial dysfunction underlies cognitive defects as a result of neural stem cell depletion and impaired neurogenesis. Hum. Mol. Genet. 2017, 26, 3327–3341. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Kim, S.; Nam, Y.; Jung, U.J.; Kim, S.R. Mitochondrial dysfunction as a driver of cognitive impairment in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 4850. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.M.; Parasuraman, R. Neuronal and cognitive plasticity: A neurocognitive framework for ameliorating cognitive aging. Front. Aging Neurosci. 2010, 2, 150. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.M.; Froemke, R.C.; Burden, S.J. Synaptic plasticity and cognitive function are disrupted in the absence of Lrp4. Elife 2014, 3, 4287. [Google Scholar] [CrossRef]
- Moosmang, S.; Haider, N.; Klugbauer, N.; Adelsberger, H.; Langwieser, N.; Müller, J.; Stiess, M.; Marais, E.; Schulla, V.; Lacinova, L.; et al. Role of Hippocampal Cav1.2 Ca2+ Channels in NMDA Receptor-Independent Synaptic Plasticity and Spatial Memory. J. Neurosci. 2005, 25, 9883–9892. [Google Scholar] [CrossRef]
- Yau, S.Y.; Li, A.; So, K.F. Involvement of Adult Hippocampal Neurogenesis in Learning and Forgetting. Neural Plast. 2015, 2015, 717958. [Google Scholar] [CrossRef]
- Hollands, C.; Tobin, M.K.; Hsu, M.; Musaraca, K.; Yu, T.S.; Mishra, R.; Kernie, S.G.; Lazarov, O. Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer’s disease by compromising hippocampal inhibition. Mol. Neurodegener. 2017, 12, 64. [Google Scholar] [CrossRef]
- Rodríguez, J.J.; Jones, V.C.; Tabuchi, M.; Allan, S.M.; Knight, E.M.; LaFerla, F.M.; Oddo, S.; Verkhratsky, A. Impaired Adult Neurogenesis in the Dentate Gyrus of a Triple Transgenic Mouse Model of Alzheimer’s Disease. PLoS ONE 2008, 3, e2935. [Google Scholar] [CrossRef]
- Ronaldson, P.T.; Davis, T.P. Regulation of blood–brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow Metab. 2020, 40, S6–S24. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Barisano, G.; Montagne, A.; Kisler, K.; Schneider, J.A.; Wardlaw, J.M.; Zlokovic, B.V. Blood-brain barrier link to human cognitive impairment and Alzheimer’s. Nat. Cardiovasc. Res. 2022, 1, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, V.; Fann, D.Y.; Dinh, Q.N.; Kim, H.A.; De Silva, T.M.; Lai, M.K.P.; Chen, C.L.H.; Drummond, G.R.; Sobey, C.G.; Arumugam, T.V. Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics 2022, 12, 1639–1658. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qiu, K.; He, Q.; Lei, Q.; Lu, W. Mechanisms of Blood-Brain Barrier Disruption in Herpes Simplex Encephalitis. J. Neuroimmune Pharmacol. 2018, 14, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, S.; Morales, J.E.; Kwiatkowski, S.C.; Lang, F.F.; Rao, G.; McCarty, J.H. Metastatic Brain Tumors Disrupt the Blood-Brain Barrier and Alter Lipid Metabolism by Inhibiting Expression of the Endothelial Cell Fatty Acid Transporter Mfsd2a. Sci. Rep. 2018, 8, 8267. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Macías-Islas, M.Á.; Flores-Alvarado, L.J.; Mireles-Ramírez, M.A.; González-Renovato, E.D.; Hernández-Navarro, V.E.; Sánchez-López, A.L.; Alatorre-Jiménez, M.A. Role of the blood-brain barrier in multiple sclerosis. Arch. Med. Res. 2014, 45, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue, K.M.; Kennedy, K.M.; Park, D.C. Beta-Amyloid Deposition and the Aging Brain. Neuropsychol. Rev. 2009, 19, 436–450. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2014, 277, 32–48. [Google Scholar] [CrossRef]
- Smith, G.S.; Protas, H.; Kuwabara, H.; Savonenko, A.; Nassery, N.; Gould, N.F.; Kraut, M.; Avramopoulos, D.; Holt, D.; Dannals, R.F.; et al. Molecular imaging of the association between serotonin degeneration and beta-amyloid deposition in mild cognitive impairment. NeuroImage. Clin. 2023, 37, 103322. [Google Scholar] [CrossRef]
- Yunes, R.A.; Poluektova, E.U.; Dyachkova, M.S.; Klimina, K.M.; Kovtun, A.S.; Averina, O.V.; Orlova, V.S.; Danilenko, V.N. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 2016, 42, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, J.S.; Solis, J.M.; Nicoll, R.A. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron 1993, 10, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural. Regen. Res. 2018, 13, 2055–2059. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Zhu, X.; Yang, Q.Q.; Lu, Q.; Wang, J.Y.; Li, H.P.; Wei, Y.Q.; Yin, J.L.; Yin, X.X. Suppression of methylglyoxal hyperactivity by mangiferin can prevent diabetes-associated cognitive decline in rats. Psychopharmacology 2013, 228, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, X.; Gou, L.; Sun, L.; Ling, X.; Yin, X. Luteolin attenuates diabetes-associated cognitive decline in rats. Brain Res. Bull. 2013, 94, 23–29. [Google Scholar] [CrossRef]
- Grossi, C.; Rigacci, S.; Ambrosini, S.; Dami, T.E.; Luccarini, I.; Traini, C.; Failli, P.; Berti, A.; Casamenti, F.; Stefani, M. The Polyphenol Oleuropein Aglycone Protects TgCRND8 Mice against Aß Plaque Pathology. PLoS ONE 2013, 8, e71702. [Google Scholar] [CrossRef]
- Cheng, D.; Xi, Y.; Cao, J.; Cao, D.; Ma, Y.; Jiang, W. Protective effect of apple (Ralls) polyphenol extract against aluminum-induced cognitive impairment and oxidative damage in rat. Neurotoxicology 2014, 45, 111–120. [Google Scholar] [CrossRef]
- Xia, S.F.; Xie, Z.X.; Qiao, Y.; Li, L.R.; Cheng, X.R.; Tang, X.; Shi, Y.H.; Le, G.W. Differential effects of quercetin on hippocampus-dependent learning and memory in mice fed with different diets related with oxidative stress. Physiol. Behav. 2014, 138, 325–331. [Google Scholar] [CrossRef]
- Xia, S.F.; Xie, Z.X.; Qiao, Y.; Li, L.R.; Cheng, X.R.; Duan, X.M.; Tang, X.; Shi, Y.H.; Le, G.W. Salvianolic acid B counteracts cognitive decline triggered by oxidative stress in mice fed with high-fat diets. J. Funct. Foods 2014, 11, 278–292. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Bi, W.; Ferruzzi, M.; Yemul, S.; Freire, D.; Mazzola, P.; Ho, L.; Dubner, L.; Pasinetti, G.M. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem. Int. 2015, 89, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; Antunes, M.S.; Filho, C.B.; Del Fabbro, L.; De Gomes, M.G.; Goes, A.T.R.; Donato, F.; Prigol, M.; Boeira, S.P.; Jesse, C.R. Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav. 2015, 134, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.H.; Lin, C.I.; Chen, Y.H.; Chiu, W.C.; Lin, S.H. A high-cholesterol diet enriched with polyphenols from Oriental plums (Prunus salicina) improves cognitive function and lowers brain cholesterol levels and neurodegenerative-related protein expression in mice. Br. J. Nutr. 2015, 113, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Bensalem, J.; Servant, L.; Alfos, S.; Gaudout, D.; Layé, S.; Pallet, V.; Lafenetre, P. Dietary polyphenol supplementation prevents alterations of spatial navigation in middle-aged mice. Front. Behav. Neurosci. 2016, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.M.; Lin, C.I.; Chen, Y.H.; Liao, H.; Lin, S.H. A diet containing grape powder ameliorates the cognitive decline in aged rats with a long-term high-fructose-high-fat dietary pattern. J. Nutr. Biochem. 2016, 34, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Dal-Pan, A.; Dudonné, S.; Bourassa, P.; Bourdoulous, M.; Tremblay, C.; Desjardins, Y.; Calon, F. Cognitive-Enhancing Effects of Polyphenols-Rich Extract from Fruits without Changes in Neuropathology in an Animal Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 55, 115–135. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Ren, G.; Yin, L.; Liang, X.; Geng, T.; Dang, H.; An, R. Resveratrol limits diabetes-associated cognitive decline in rats by preventing oxidative stress and inflammation and modulating hippocampal structural synaptic plasticity. Brain Res. 2016, 1650, 1–9. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.F.; Zhang, P.; Newell, K.A.; Wang, H.; Zheng, K.; Yu, Y. Dietary teasaponin ameliorates alteration of gut microbiota and cognitive decline in diet-induced obese mice. Sci. Rep. 2017, 7, 12203. [Google Scholar] [CrossRef]
- Sawmiller, D.; Li, S.; Mori, T.; Habib, A.; Rongo, D.; Delic, V.; Bradshaw, P.C.; Shytle, R.D.; Sanberg, C.; Bickford, P.; et al. Beneficial effects of a pyrroloquinolinequinone-containing dietary formulation on motor deficiency, cognitive decline and mitochondrial dysfunction in a mouse model of Alzheimer’s disease. Heliyon 2017, 3, e00279. [Google Scholar] [CrossRef]
- Abhijit, S.; Subramanyam, M.V.V.; Devi, S.A. Grape Seed Proanthocyanidin and Swimming Exercise Protects Against Cognitive Decline: A Study on M1 Acetylcholine Receptors in Aging Male Rat Brain. Neurochem. Res. 2017, 42, 3573–3586. [Google Scholar] [CrossRef]
- Cheng, J.; Rui, Y.; Qin, L.; Xu, J.; Han, S.; Yuan, L.; Yin, X.; Wan, Z. Vitamin D Combined with Resveratrol Prevents Cognitive Decline in SAMP8 Mice. Curr. Alzheimer Res. 2017, 14, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Fragua, V.; Lepoudère, A.; Leray, V.; Baron, C.; Araujo, J.A.; Nguyen, P.; Milgram, N.W. Effects of dietary supplementation with a mixed blueberry and grape extract on working memory in aged beagle dogs. J. Nutr. Sci. 2017, 6, e35. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.A.; Makboul, R.M.; Al-Mokhtar, M.A.; Nicola, M.A. Polyphenol-rich Boswellia serrata gum prevents cognitive impairment and insulin resistance of diabetic rats through inhibition of GSK3β activity, oxidative stress and pro-inflammatory cytokines. Biomed. Pharmacother. 2018, 109, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, S.; Singh, D. Apigenin reverses behavioural impairments and cognitive decline in kindled mice via CREB-BDNF upregulation in the hippocampus. Nutr. Neurosci. 2018, 23, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Sanna, R.S.; Muthangi, S.; Chandrasekhar, C.S.; Devi, S.A. Grape seed proanthocyanidin extract and insulin prevents cognitive decline in type 1 diabetic rat by impacting Bcl-2 and Bax in the prefrontal cortex. Metab. Brain Dis. 2018, 34, 103–117. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, R.; Jia, J.; Wang, L.; Li, K.; Li, Y.; Zhang, J. Oleanolic acid protects against cognitive decline and neuroinflammation-mediated neurotoxicity by blocking secretory phospholipase A2 IIA-activated calcium signals. Mol. Immunol. 2018, 99, 95–103. [Google Scholar] [CrossRef]
- Bensalem, J.; Dudonné, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Layé, S.; Lafenetre, P.; Pallet, V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef]
- Qi, Y.; Shang, L.; Liao, Z.; Su, H.; Jing, H.; Wu, B.; Bi, K.; Jia, Y. Intracerebroventricular injection of resveratrol ameliorated Aβ-induced learning and cognitive decline in mice. Metab. Brain Dis. 2018, 34, 257–266. [Google Scholar] [CrossRef]
- Yu, M.; Chen, X.; Liu, J.; Ma, Q.; Zhuo, Z.; Chen, H.; Zhou, L.; Yang, S.; Zheng, L.; Ning, C.; et al. Gallic acid disruption of Aβ1–42 aggregation rescues cognitive decline of APP/PS1 double transgenic mouse. Neurobiol. Dis. 2018, 124, 67–80. [Google Scholar] [CrossRef]
- González-Granillo, A.E.; Gnecco, D.; Díaz, A.; Garcés-Ramírez, L.; de la Cruz, F.; Juarez, I.; Morales-Medina, J.C.; Flores, G. Curcumin induces cortico-hippocampal neuronal reshaping and memory improvements in aged mice. J. Chem. Neuroanat. 2022, 121, 102091. [Google Scholar] [CrossRef]
- Ishida, K.; Yamamoto, M.; Misawa, K.; Nishimura, H.; Misawa, K.; Ota, N.; Shimotoyodome, A. Coffee polyphenols prevent cognitive dysfunction and suppress amyloid β plaques in APP/PS2 transgenic mouse. Neurosci. Res. 2019, 154, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, V.; Palomera-Ávalos, V.; López-Ruiz, S.; Canudas, A.M.; Pallàs, M.; Griñán-Ferré, C. Maternal resveratrol supplementation prevents cognitive decline in senescent mice offspring. Int. J. Mol. Sci. 2019, 20, 1134. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Lee, S.I.; Cho, E.J. Effect of Vigna angularis on High-Fat Diet-Induced Memory and Cognitive Impairments. J. Med. Food 2019, 23, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Kang, I.J.; Kim, B.; Sim, H.J.; Kim, D.W.; Ahn, J.H.; Lee, J.C.; Ryoo, S.; Shin, M.C.; Cho, J.H.; et al. Experimental pretreatment with chlorogenic acid prevents transient ischemia-induced cognitive decline and neuronal damage in the hippocampus through anti-oxidative and anti-inflammatory effects. Molecules 2020, 25, 3578. [Google Scholar] [CrossRef]
- Ishida, K.; Misawa, K.; Nishimura, H.; Hirata, T.; Yamamoto, M.; Ota, N. 5-Caffeoylquinic Acid Ameliorates Cognitive Decline and Reduces Aβ Deposition by Modulating Aβ Clearance Pathways in APP/PS2 Transgenic Mice. Nutrients 2020, 12, 494. [Google Scholar] [CrossRef]
- Ramis, M.R.; Sarubbo, F.; Moranta, D.; Tejada, S.; Lladó, J.; Miralles, A.; Esteban, S. Cognitive and neurochemical changes following polyphenol-enriched diet in rats. Nutrients 2021, 13, 59. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kayama, T.; Noguchi-Shinohara, M.; Hamaguchi, T.; Yamada, M.; Abe, K.; Kobayashi, S. Rosmarinic acid suppresses tau phosphorylation and cognitive decline by downregulating the JNK signaling pathway. Sci. Food 2021, 5, 1. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, M.; Song, C.; Cheng, L.; Li, X.; Yang, Q.; Zhang, Y.; Dong, R.; Kou, J.; Lv, C.; et al. Tea polyphenols improve the memory in aging ovariectomized rats by regulating brain glucose metabolism in vivo and in vitro. J. Funct. Foods 2021, 87, 104856. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Y.; Zhang, L.; Li, X.; Dong, R.; Song, C.; Cheng, L.; Shi, M.; Zhao, H. Combination of tea polyphenols and proanthocyanidins prevents menopause-related memory decline in rats via increased hippocampal synaptic plasticity by inhibiting p38 MAPK and TNF-α pathway. Nutr. Neurosci. 2021, 25, 1909–1927. [Google Scholar] [CrossRef]
- Mikami, T.; Kim, J.; Park, J.; Lee, H.; Yaicharoen, P.; Suidasari, S.; Yokozawa, M.; Yamauchi, K. Olive leaf extract prevents obesity, cognitive decline, and depression and improves exercise capacity in mice. Sci. Rep. 2021, 11, 12495. [Google Scholar] [CrossRef]
- Kang, J.; Wang, Z.; Cremonini, E.; Le Gall, G.; Pontifex, M.G.; Muller, M.; Vauzour, D.; Oteiza, P.I. (-)-Epicatechin mitigates anxiety-related behavior in a mouse model of high fat diet-induced obesity. J. Nutr. Biochem. 2022, 110, 109158. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [PubMed]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Zhang, Y.; Wu, S.; Chen, Q.; Wang, L. The Role of NLRP3 Inflammasome in Alzheimer’s Disease and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 323. [Google Scholar] [CrossRef]
- Adamczak, S.E.; De Rivero Vaccari, J.P.; Dale, G.; Brand, F.J.; Nonner, D.; Bullock, M.; Dahl, G.P.; Dietrich, W.D.; Keane, R.W. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J. Cereb. Blood Flow Metab. 2013, 34, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bai, H.; Ma, X.; Shen, M.; Li, R.; Qiu, D.; Li, S.; Gao, L. Blockade of the NLRP3/caspase-1 axis attenuates ketamine-induced hippocampus pyroptosis and cognitive impairment in neonatal rats. J. Neuroinflamm. 2021, 18, 239. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wise, L.; Fukuchi, K.I. TLR4 Cross-Talk with NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer’s Disease. Front. Immunol. 2020, 11, 724. [Google Scholar] [CrossRef]
- Pellegrini, C.; Fornai, M.; Antonioli, L.; Blandizzi, C.; Calderone, V. Phytochemicals as Novel Therapeutic Strategies for NLRP3 Inflammasome-Related Neurological, Metabolic, and Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 2876. [Google Scholar] [CrossRef]
- Zhang, L.; Previn, R.; Lu, L.; Liao, R.F.; Jin, Y.; Wang, R.K. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res. Bull. 2018, 142, 352–359. [Google Scholar] [CrossRef]
- Azam, S.; Jakaria, M.; Kim, I.S.; Kim, J.; Ezazul Haque, M.; Choi, D.K. Regulation of toll-like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: Focus on TLR4 signaling. Front. Immunol. 2019, 10, 1000. [Google Scholar] [CrossRef]
- Hirao, K.; Yumoto, H.; Nakanishi, T.; Mukai, K.; Takahashi, K.; Takegawa, D.; Matsuo, T. Tea catechins reduce inflammatory reactions via mitogen-activated protein kinase pathways in toll-like receptor 2 ligand-stimulated dental pulp cells. Life Sci. 2010, 86, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Narasimhan, P.; Kim, G.S.; Jung, J.E.; Park, E.-H.; Chan, P.H. The role of Akt signaling in oxidative stress mediates NF-κB activation in mild transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2008, 28, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewicza, A.I.; Anderson, D.J. Cyclin D1 promotes neurogenesis in the developing spinal cord in a cell cycle-independent manner. Neuroscience 2011, 108, 11632–11637. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Shichiri, M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014, 54, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Chavarria, D.; Borges, F.; Wojtczak, L.; Wieckowski, M.R.; Karkucinska-Wieckowska, A.; Oliveira, P.J. Dietary Polyphenols and Mitochondrial Function: Role in Health and Disease. Curr. Med. Chem. 2019, 26, 3376–3406. [Google Scholar] [CrossRef]
- Schmitt, L.O.; Gaspar, J.M. Obesity-Induced Brain Neuroinflammatory and Mitochondrial Changes. Metabolites 2023, 13, 86. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Choi, J.; Arvas, M.I.; Salimian, M.; Singh, S.; Xu, S.; Gullapalli, R.P.; Kristian, T.; Russell, J.W. Nicotinamide Mononucleotide Administration Prevents Experimental Diabetes-Induced Cognitive Impairment and Loss of Hippocampal Neurons. Int. J. Mol. Sci. 2020, 21, 3756. [Google Scholar] [CrossRef]
- Ng, F.; Wijaya, L.; Tang, B.L. SIRT1 in the brain—Connections with aging-associated disorders and lifespan. Front. Cell. Neurosci. 2015, 9, 64. [Google Scholar] [CrossRef]
- Prozorovski, T.; Schulze-Topphoff, U.; Glumm, R.; Baumgart, J.; Schröter, F.; Ninnemann, O.; Siegert, E.; Bendix, I.; Brüstle, O.; Nitsch, R.; et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat. Cell Biol. 2008, 10, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Esbensen, Y.; Kunke, D.; Suganthan, R.; Rachek, L.; Bjørås, M.; Eide, L. Mitochondrial DNA damage level determines neural stem cell differentiation fate. J. Neurosci. 2011, 31, 9746–9751. [Google Scholar] [CrossRef] [PubMed]
- Ignatenko, O.; Chilov, D.; Paetau, I.; De Miguel, E.; Jackson, C.B.; Capin, G.; Paetau, A.; Terzioglu, M.; Euro, L.; Suomalainen, A. Loss of mtDNA activates astrocytes and leads to spongiotic encephalopathy. Nat. Commun. 2017, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef]
- Elliott, P.J.; Jirousek, M. Sirtuins: Novel targets for metabolic disease. Curr. Opin. Investig. Drugs 2008, 9, 371–378. [Google Scholar]
- Hussain, B.; Fang, C.; Chang, J. Blood–Brain Barrier Breakdown: An Emerging Biomarker of Cognitive Impairment in Normal Aging and Dementia. Front. Neurosci. 2021, 15, 978. [Google Scholar] [CrossRef]
- Al-Bachari, S.; Naish, J.H.; Parker, G.J.M.; Emsley, H.C.A.; Parkes, L.M. Blood–Brain Barrier Leakage Is Increased in Parkinson’s Disease. Front. Physiol. 2020, 11, 26. [Google Scholar] [CrossRef]
- Memis, I.; Mittal, R.; Furar, E.; White, I.; Eshraghi, R.S.; Mittal, J.; Eshraghi, A.A. Altered Blood Brain Barrier Permeability and Oxidative Stress in Cntnap2 Knockout Rat Model. J. Clin. Med. 2022, 11, 2725. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.; Li, J.; Sun, Y.; Hasimu, H.; Liu, R.; Zhang, T. Quercetin protects human brain microvascular endothelial cells from fibrillar β-amyloid1-40-induced toxicity. Acta Pharm. Sin. B 2014, 5, 47–54. [Google Scholar] [CrossRef]
- Taïlé, J.; Arcambal, A.; Clerc, P.; Gauvin-Bialecki, A.; Gonthier, M.P. Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants 2020, 9, 573. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wan, S.F.; Yao, N.N.; Lin, Z.J.; Mao, Y.G.; Yu, X.H.; Wang, Y.Z. Inhibition of the immunoproteasome LMP2 ameliorates ischemia/hypoxia-induced blood–brain barrier injury through the Wnt/β-catenin signalling pathway. Mil. Med. Res. 2021, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Taïlé, J.; Patché, J.; Veeren, B.; Gonthier, M.P. Hyperglycemic Condition Causes Pro-Inflammatory and Permeability Alterations Associated with Monocyte Recruitment and Deregulated NFκB/PPARγ Pathways on Cerebral Endothelial Cells: Evidence for Polyphenols Uptake and Protective Effect. Int. J. Mol. Sci. 2021, 22, 1385. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, T.; Weiss, E.; Marksteiner, J.; Humpel, C. Soluble cell adhesion molecules in monocytes of Alzheimer’s disease and mild cognitive impairment. Exp. Gerontol. 2009, 45, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Xing, J.G.; Wang, L.L.; Jiang, H.L.; Guo, S.L.; Liu, R. Luteolin Inhibits Fibrillary β-Amyloid1-40-Induced Inflammation in a Human Blood-Brain Barrier Model by Suppressing the p38 MAPK-Mediated NF-κB Signaling Pathways. Molecules 2017, 22, 334. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.; Tarantini, S.; Tucsek, Z.; Ashpole, N.M.; Sosnowska, D.; Gautam, T.; Ballabh, P.; Koller, A.; Sonntag, W.E.; Csiszar, A.; et al. Resveratrol treatment rescues neurovascular coupling in aged mice: Role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am. J. Physiol. 2013, 306, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Polyphenols and brain health. OCL 2017, 24, A202. [Google Scholar] [CrossRef]
- Monserrat Hernández-Hernández, E.; Serrano-García, C.; Antonio Vázquez-Roque, R.; Díaz, A.; Monroy, E.; Rodríguez-Moreno, A.; Florán, B.; Flores, G. Chronic administration of resveratrol prevents morphological changes in prefrontal cortex and hippocampus of aged rats. Synapse 2016, 70, 206–217. [Google Scholar] [CrossRef]
- Trovò, L.; Fuchs, C.; De Rosa, R.; Barbiero, I.; Tramarin, M.; Ciani, E.; Rusconi, L.; Kilstrup-Nielsen, C. The green tea polyphenol epigallocatechin-3-gallate (EGCG) restores CDKL5-dependent synaptic defects in vitro and in vivo. Neurobiol. Dis. 2020, 138, 104791. [Google Scholar] [CrossRef]
- Cong, L.; Cao, C.; Cheng, Y.; Qin, X.Y. Green Tea Polyphenols Attenuated Glutamate Excitotoxicity via Antioxidative and Antiapoptotic Pathway in the Primary Cultured Cortical Neurons. Oxid. Med. Cell. Longev. 2016, 2016, 435. [Google Scholar] [CrossRef]
- Vauzour, D. Dietary Polyphenols as Modulators of Brain Functions: Biological Actions and Molecular Mechanisms Underpinning Their Beneficial Effects. Oxid. Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef]
- Rahn, K.A.; Slusher, B.S.; I. Kaplin, A. Glutamate in CNS neurodegeneration and cognition and its regulation by GCPII inhibition. Curr. Med. Chem. 2012, 19, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.K.; Ou, Y.C.; Raung, S.L.; Lai, C.Y.; Liao, S.L.; Chen, C.J. Inhibition of nitric oxide production by quercetin in endotoxin/cytokine-stimulated microglia. Life Sci. 2009, 86, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Lau, F.C.; Carey, A.N.; Galli, R.L.; SpanglerIngram, E.L.; Ingram, D.K.; Joseph, J.A. Blueberry polyphenols attenuate kainic acid-induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutr. Neurosci. 2008, 11, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Roubaud-baudron, C.; Krolak-salmon, P.; Quadrio, I. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: Preliminary results. Neurobiol. Aging 2011, 33, 1009.e11–1009.e19. [Google Scholar] [CrossRef]
- Hennessey, C.; Keogh, C.E.; Barboza, M.; Brust-Mascher, I.; Knotts, T.A.; Sladek, J.A.; Pusceddu, M.M.; Stokes, P.; Rabasa, G.; Honeycutt, M.; et al. Neonatal enteropathogenic Escherichia coli infection disrupts microbiota-gut-brain axis signaling. Infect. Immun. 2021, 89, e00059-21. [Google Scholar] [CrossRef]
- Cerovic, M.; Forloni, G.; Balducci, C. Neuroinflammation and the Gut Microbiota: Possible Alternative Therapeutic Targets to Counteract Alzheimer ’ s Disease? Front. Aging Neurosci. 2019, 11, 284. [Google Scholar] [CrossRef]
- Wioland, L.; Dupont, J.L.; Bossu, J.L.; Popoff, M.R.; Poulain, B. Attack of the nervous system by Clostridium perfringens Epsilon toxin: From disease to mode of action on neural cells. Toxicon 2013, 75, 122–135. [Google Scholar] [CrossRef]
- Saji, N.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Niida, S.; Toba, K.; Sakurai, T. The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: A cross-sectional study conducted in Japan. Sci. Rep. 2019, 9, 19227. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Judaki, A.; Rahmani, A.; Feizi, J.; Asadollahi, K.; Hafezi Ahmadi, M.R. Curcumin in combination with triple therapy regimes ameliorates oxidative stress and histopathologic changes in chronic gastritis– associated Helicobacter pylori infection. Arq. Gastroenterol. 2017, 54, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.G.; Chen, Y.J.; Tong, J.W.; Huang, J.A.; Li, J.; Gong, Y.S.; Liu, Z.H. Tea polyphenol epigallocatechin gallate inhibits Escherichia coli by increasing endogenous oxidative stress. Food Chem. 2016, 217, 196–204. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Masella, R.; Di Benedetto, R.; Varı, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, T.P.M.; Sies, H. Transport of Glutathione, Glutathione Disulfide, and Glutathione Conjugates across the Hepatocyte Plasma Membrane. Methods Enzym. 1989, 173, 523–534. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sport. Med. 2018, 49 (Suppl. S1), 3–23. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2013, 66, 24–35. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Su, Z.Y.; Kong, A.N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef]
- Grosso, G.; Stepaniak, U.; Topor-madry, R.; Szafraniec, K.; Pajak, A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 2014, 30, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Ray, S.K.; Mukherjee, S. Evolving Interplay Between Dietary Polyphenols and Gut Microbiota-An Emerging Importance in Healthcare. Front. Nutr. 2021, 8, 634944. [Google Scholar] [CrossRef] [PubMed]
- Shivashankara, K.S.; Acharya, S.N. Bioavailability of Dietary Polyphenols and the Cardiovascular Diseases. Open Nutraceuticals J. 2010, 3, 227–241. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Basu, S.; Meng, S.; Wang, X.; Hu, M. Regioselective sulfation and glucuronidation of phenolics: Insights into the structural basis. Curr. Drug Metab. 2011, 12, 900–916. [Google Scholar] [CrossRef]
- Rowland, A.; Miners, J.O.; Mackenzie, P.I. The UDP-glucuronosyltransferases: Their role in drug metabolism and detoxification. Int. J. Biochem. Cell Biol. 2013, 45, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Kulkarni, K.; Basu, S.; Zhang, S.; Hu, M. First-pass metabolism via UDP-glucuronosyltransferase: A barrier to oral bioavailability of phenolics. J. Pharm. Sci. 2011, 100, 3655–3681. [Google Scholar] [CrossRef]
- Singh, A.K.; Cabral, C.; Kumar, R.; Ganguly, R.; Rana, H.K.; Gupta, A.; Lauro, M.R.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Roquim, M.; Dudonné, S.; Pilon, G.; Levy, E.; Marette, A.; Roy, D.; Desjardins, Y. Berry Polyphenols and Fibers Modulate Distinct Microbial Metabolic Functions and Gut Microbiota Enterotype-Like Clustering in Obese Mice. Front. Microbiol. 2020, 11, 2032. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Ruscica, M.; Banach, M. Resveratrol and cognitive decline: A clinician perspective. Arch. Med. Sci. 2019, 15, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Pandey, A.; Jahan, S.; Shukla, R.K.; Kumar, D.; Srivastava, A.; Singh, S.; Rajpurohit, C.S.; Yadav, S.; Khanna, V.K.; et al. Differential responses of Trans-Resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci. Rep. 2016, 6, 28142. [Google Scholar] [CrossRef] [PubMed]
- Elanthendral, G.; Shobana, N.; Meena, R.; Prakash, P.; Samrot, A.V. Utilizing pharmacological properties of polyphenolic curcumin in nanotechnology. Biocatal. Agric. Biotechnol. 2021, 38, 102212. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Nakagawa, A.; Takahashi, Y.; Iguchi, K.; Yamamoto, H.; Hoshino, M.; Hara, A.; Takagaki, A.; Nanjo, F.; et al. Blood brain barrier permeability of (−)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem. Biophys. Rep. 2016, 9, 180. [Google Scholar] [CrossRef]
- Perry, M. What Are Polyphenols and How Much You Need. Available online: https://blog.zonediet.com/lifestyletips/what-are-polyphenols-and-how-much-you-need (accessed on 9 March 2023).
- Kapolou, A.; Karantonis, H.C.; Rigopoulos, N.; Koutelidakis, A.E. Association of Mean Daily Polyphenols Intake with Mediterranean Diet Adherence and Anthropometric Indices in Healthy Greek Adults: A Retrospective Study. Appl. Sci. 2021, 11, 4664. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Salinas-Roca, B.; Rubió-Piqué, L.; Montull-López, A. Polyphenol Intake in Pregnant Women on Gestational Diabetes Risk and Neurodevelopmental Disorders in Offspring: A Systematic Review. Nutrients 2022, 14, 3753. [Google Scholar] [CrossRef]
- Garrigue, P.; Mounien, L.; Champion, S.; Mouhajir, Y.; Pechere, L.; Guillet, B.; Landrier, J.F.; Seree, E. Long-term administration of resveratrol at low doses improves neurocognitive performance as well as cerebral blood flow and modulates the inflammatory pathways in the brain. J. Nutr. Biochem. 2021, 97, 108786. [Google Scholar] [CrossRef]
- Park, H.R.; Kong, K.H.; Yu, B.P.; Mattson, M.P.; Lee, J. Resveratrol inhibits the proliferation of neural progenitor cells and hippocampal neurogenesis. J. Biol. Chem. 2012, 287, 42588–42600. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry Supplementation Improves Memory in Older Adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef]
- Onishi, S.; Meguro, S.; Pervin, M.; Kitazawa, H.; Yoto, A.; Ishino, M.; Shimba, Y.; Mochizuki, Y.; Miura, S.; Tokimitsu, I.; et al. Green tea extracts attenuate brain dysfunction in high-fat-diet-fed SAMP8 mice. Nutrients 2019, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Dunnick, J.K.; Halley, J.R. Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Toxicol. Sci. 1992, 19, 423–431. [Google Scholar] [CrossRef]
- Jones, E.; Hughes, R. Quercetin, flavonoids and the life-span of mice. Exp. Gerontol. 1982, 17, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Hirose, M.; Takahashi, S.; Ogawa, K.; Shirai, T.; Ito, N. Forestomach and kidney carcinogenicity of caffeic acid in F344 rats and C57BL/6N x C3H/HeN F1 mice. Cancer Res. 1991, 51, 5655–5660. [Google Scholar] [PubMed]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012, 3, 635. [Google Scholar] [CrossRef]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326–329. [Google Scholar] [CrossRef]
- Hirose, M.; Hoshiya, T.; Mizoguchi, Y.; Nakamura, A.; Akagi, K.; Shirai, T. Green tea catechins enhance tumor development in the colon without effects in the lung or thyroid after pretreatment with 1,2-dimethylhydrazine or 2,2′-dihydroxy-di-n-propylnitrosamine in male F344 rats. Cancer Lett. 2001, 168, 23–29. [Google Scholar] [CrossRef]
- Temme, E.H.M.; Van Hoydonck, P.G.A. Tea consumption and iron status. Eur. J. Clin. Nutr. 2002, 56, 379–386. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.M.; Elahi, F.; Chelliah, R.; Lee, B.H.; Oh, D.H. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Front. Sustain. Food Syst. 2020, 4, 223. [Google Scholar] [CrossRef]
- Kiani, J.; Imam, S.Z. Medicinal importance of grapefruit juice and its interaction with various drugs. Nutr. J. 2007, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).