Antioxidant Properties of Cranberry Leaves and Walnut Meal and Their Effect on Nutritional Quality and Oxidative Stability of Broiler Breast Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Chemical Analysis
2.3. Data Analysis
3. Results
3.1. Chemical Composition of Dietary Supplements
3.2. Proximate Composition of Breast Tissue
3.3. Trace Element Composition of Breast Tissue
3.4. Antioxidant Profile of Breast Meat
3.5. Oxidative Stability of Breast Meat
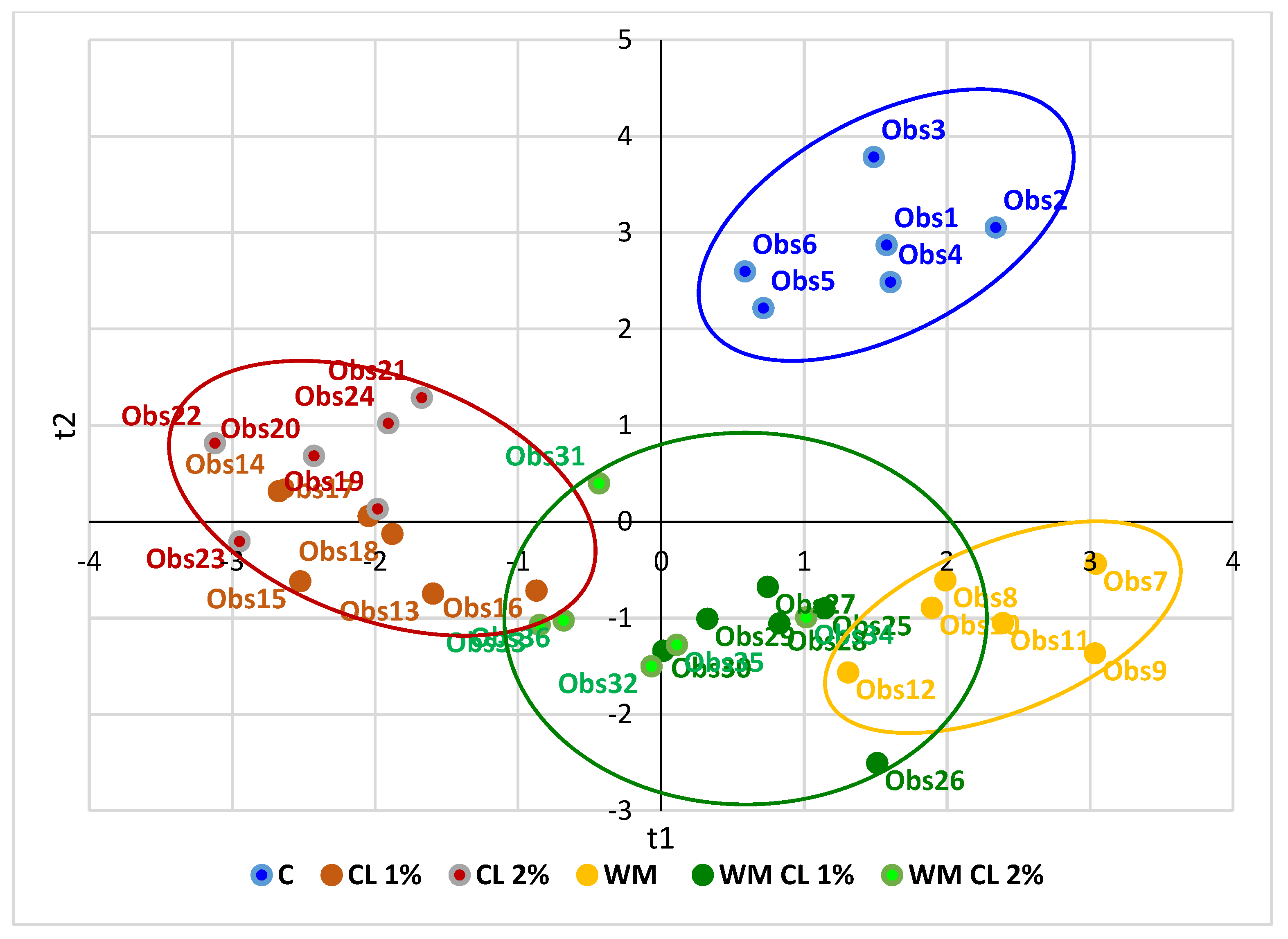
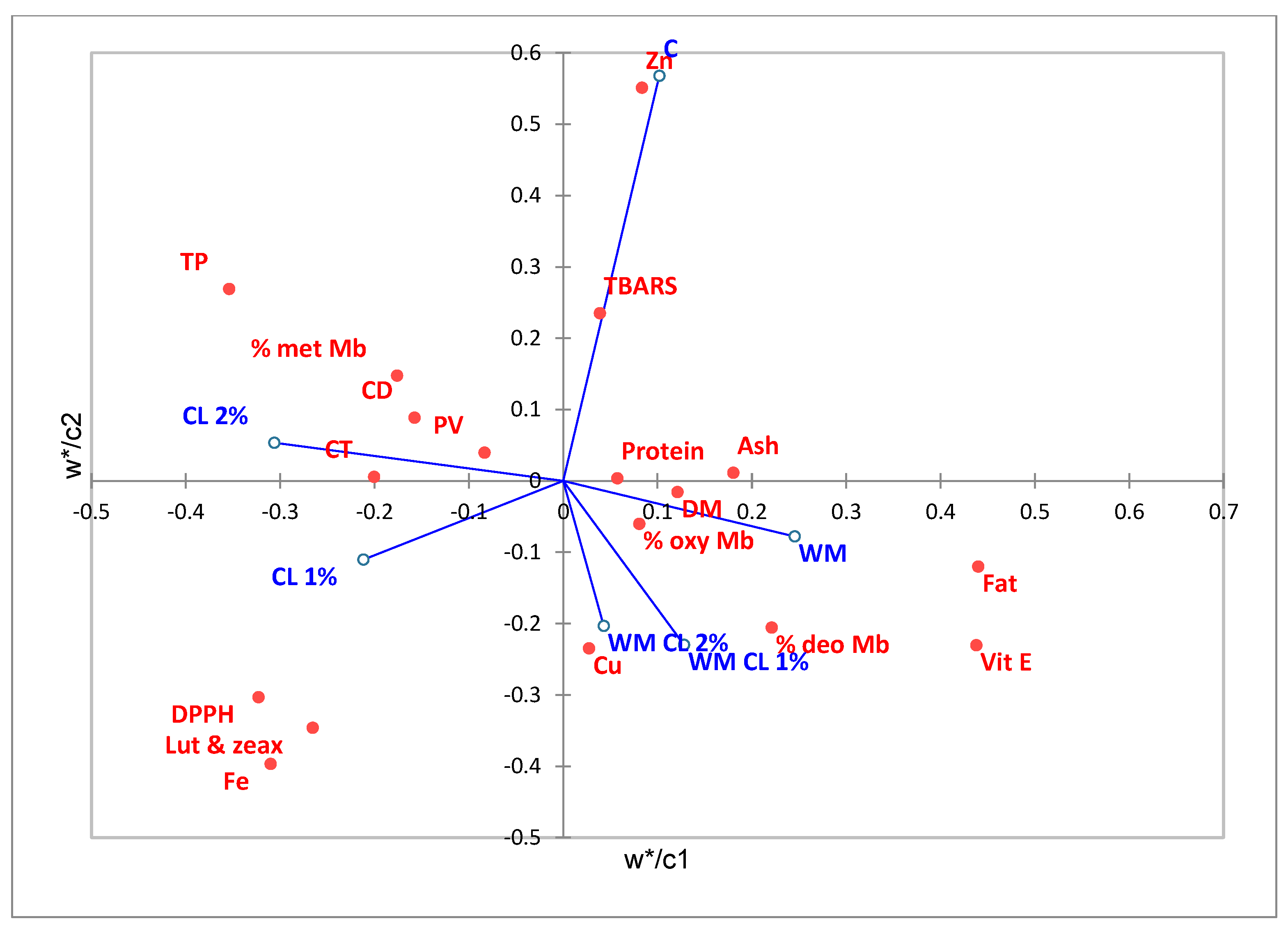
3.6. Partial Least Squares-Discriminant Analysis (PLS-DA)
4. Discussion
4.1. Chemical Composition of Dietary Supplements
4.2. Proximate Composition of Breast Tissue
4.3. Trace Element Composition of Breast Tissue
4.4. Antioxidant Profile of Breast Meat
4.5. Oxidative Stability of Breast Meat
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akbarirad, H.; Ardabili, A.G.; Kazemeini, S.M.; Khaneghah, A.M. An overview on some of important sources of natural antioxidants. Int. Food Res. J. 2016, 23, 928–933. [Google Scholar]
- Goliomytis, M.; Kartsonas, N.; Charismiadou, M.A.; Symeon, G.K.; Simitzis, P.E.; Deligeorgis, S.G. The influence of naringin or hesperidin dietary supplementation on broiler meat quality and oxidative stability. PLoS ONE 2015, 10, e0141652. [Google Scholar] [CrossRef]
- Oluwafemi, R.A.; Olawale, I.; Alagbe, J.O. Recent trends in the utilization of medicinal plants as growth promoters in poultry nutrition-A review. Res. Agric. Vet. Sci. 2020, 4, 5–11. [Google Scholar]
- Croitoru, A.; Ficai, D.; Craciun, L.; Ficai, A.; Andronescu, E. Evaluation and exploitation of bioactive compounds of walnut, Juglans regia. Curr. Pharm. Des. 2019, 25, 119–131. [Google Scholar] [CrossRef]
- Grosso, A.L.; Asensio, C.M.; Nepote, V.; Grosso, N.R. Antioxidant activity displayed by phenolic compounds obtained from walnut oil cake used for walnut oil preservation. J. Am. Oil Chem. Soc. 2018, 95, 1409–1419. [Google Scholar] [CrossRef]
- Wang, G.; Zhong, D.; Liu, H.; Yang, T.; Liang, Q.; Wang, J.; Zhang, Y. Water soluble dietary fiber from walnut meal as a prebiotic in preventing metabolic syndrome. J. Funct. Foods 2021, 78, 104358. [Google Scholar] [CrossRef]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Comparison of bioactive potential of cranberry fruit and fruit-based products versus leaves. J. Funct. Foods 2016, 22, 232–242. [Google Scholar] [CrossRef]
- Islam, M.R.; Hassan, Y.I.; Das, Q.; Lepp, D.; Hernandez, M.; Godfrey, D.V.; Diarra, M.S. Dietary organic cranberry pomace influences multiple blood biochemical parameters and cecal microbiota in pasture-raised broiler chickens. J. Funct. Foods 2020, 72, 104053. [Google Scholar] [CrossRef]
- Das, Q.; Tang, J.; Yin, X.; Ross, K.; Warriner, K.; Marcone, M.F.; Diarra, M.S. Organic cranberry pomace and its ethanolic extractives as feed supplement in broiler: Impacts on serum Ig titers, liver and bursal immunity. Poult. Sci. 2021, 100, 517–526. [Google Scholar] [CrossRef]
- Kithama, M.; Ross, K.; Diarra, M.S.; Kiarie, E.G. Utilization of grape (Vitis vinifera), cranberry (Vaccinium macrocarpon), wild blueberry (Vaccinium angustifolium), and apple (Malus pumila/domestica) pomaces in broiler chickens when fed without or with multi-enzyme supplement. Can. J. Anim. Sci. 2022, 1, 15–25. [Google Scholar] [CrossRef]
- Lee, H.J.; Yong, H.I.; Kim, M.; Choi, Y.S.; Jo, C. Status of meat alternatives and their potential role in the future meat market—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1533. [Google Scholar] [CrossRef]
- Ursachi, C.Ș.; Perța-Crișan, S.; Munteanu, F.D. Strategies to improve meat products’ quality. Foods 2020, 9, 1883. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Untea, A.E.; Turcu, R.P.; Saracila, M.; Panaite, T.D.; Cornescu, G.M. Nutritional Composition and Bioactive Compounds of Basil, Thyme and Sage Plant Additives and Their Functionality on Broiler Thigh Meat Quality. Foods 2022, 11, 1105. [Google Scholar] [CrossRef]
- Turcu, R.P.; Panaite, T.D.; Untea, A.E.; Șoica, C.; Iuga, M.; Mironeasa, S. Effects of Supplementing Grape Pomace to Broilers Fed Polyunsaturated Fatty Acids Enriched Diets on Meat Quality. Animals 2020, 10, 947. [Google Scholar] [CrossRef]
- Varzaru, I.; Untea, A.E.; Panaite, T.; Olteanu, M. Effect of dietary phytochemicals from tomato peels and rosehip meal on the lipid peroxidation of eggs from laying hens. Arch. Anim. Nutr. 2021, 75, 18–30. [Google Scholar] [CrossRef]
- Untea, A.E.; Panaite, T.D.; Dragomir, C.; Ropota, M.; Olteanu, M.; Varzaru, I. Effect of dietary chromium supplementation on meat nutritional quality and antioxidant status from broilers fed with Camelina-meal-supplemented diets. Animal 2019, 13, 2939–2947. [Google Scholar] [CrossRef]
- Untea, A.; Criste, R.C.; Vladescu, L. Development and validation of a microwave digestion–FAAS procedure for Cu, Mn and Zn determination in liver. Rev. De Chim. 2012, 63, 341–346. [Google Scholar]
- Varzaru, I.; Untea, A.E.; Saracila, M. In vitro antioxidant properties of berry leaves and their inhibitory effect on lipid peroxidation of thigh meat from broiler chickens. Eur. J. Lipid Sci. Technol. 2020, 122, 1900384. [Google Scholar] [CrossRef]
- Untea, A.E.; Varzaru, I.; Panaite, T.D.; Gavris, T.; Lupu, A.; Ropota, M. The effects of dietary inclusion of bilberry and walnut leaves in laying hens’ diets on the antioxidant properties of eggs. Animals 2020, 10, 191. [Google Scholar] [CrossRef]
- Orsavová, J.; Hlaváčová, I.; Mlček, J.; Snopek, L.; Mišurcová, L. Contribution of phenolic compounds, ascorbic acid and vitamin E to antioxidant activity of currant (Ribes L.) and gooseberry (Ribes uva-crispa L.) fruits. Food Chem. 2019, 284, 323–333. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Proximate and polyphenolic characterization of cranberry pomace. J. Agric. Food Chem. 2010, 58, 4030–4036. [Google Scholar] [CrossRef]
- Karlsons, A.; Osvalde, A.; Čekstere, G.; Pormale, J. Research on the mineral composition of cultivated and wild blueberries and cranberries. Agron. Res. 2018, 16, 454463. [Google Scholar]
- Arjomandi, M.A.; Salarmoini, M. Walnut Meal as an Excellent Source of Energy and Protein for Growing Japanese Quails. Iran. J. Appl. Anim. Sci. 2016, 6, 925–930. [Google Scholar]
- Vlaicu, P.A.; Panaite, T.D.; Voicu, I.; Turcu, R.P.; Olteanu, M.; Ropota, M. Determining the Feeding Value of some Food Industry By-Products. Sci. Pap. Anim. Sci. Biotechnol. 2018, 51, 62–69. [Google Scholar]
- Untea, A.E.; Turcu, R.P.; Saracila, M.; Vlaicu, P.A.; Panaite, T.D.; Oancea, A.G. Broiler meat fatty acids composition, lipid metabolism, and oxidative stability parameters as affected by cranberry leaves and walnut meal supplemented diets. Sci. Rep. 2022, 12, 21618. [Google Scholar] [CrossRef]
- Martău, G.A.; Bernadette-Emőke, T.; Odocheanu, R.; Soporan, D.A.; Bochiș, M.; Simon, E.; Vodnar, D.C. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef]
- Masek, A.; Latos-Brozio, M.; Chrzescijanska, E.; Podsedek, A. Polyphe-nolic profile and antioxidant activity of Juglans regia L. leaves and husk ex-tracts. Forests 2019, 10, 988. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic compounds from nuts: Extraction, chemical profiles, and bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef]
- Xu, Q.; Si, W.; Mba, O.I.; Sienkiewicz, O.; Ngadi, M.; Ross, K.; Zhao, X. Research Note: Effects of supplementing cranberry and blueberry pomaces on meat quality and antioxidative capacity in broilers. Poult. Sci. 2021, 100, 100900. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, A.; Seidavi, A.; Phillips, C.J. Growth, carcass composition, haematology and immunity of broilers supplemented with sumac berries (Rhus coriaria L.) and thyme (Thymus vulgaris). Animals 2020, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Giromini, C.; Givens, D.I. Benefits and Risks Associated with Meat Consumption during Key Life Processes and in Relation to the Risk of Chronic Diseases. Foods 2022, 11, 2063. [Google Scholar] [CrossRef] [PubMed]
- Buzala, M.; Slomka, A.; Janicki, B. Heme iron in meat as the main source of iron in the human diet. J. Elem. 2016, 21, 303–314. [Google Scholar] [CrossRef]
- Saracila, M.; Untea, A.E.; Panaite, T.D.; Varzaru, I.; Oancea, A.; Turcu, R.P.; Vlaicu, P.A. Creeping wood sorrel and chromium picolinate effect on the nutritional composition and lipid oxidative stability of broiler meat. Antioxidants 2022, 11, 780. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, W.; Wang, D.; Xu, W. Effect of myoglobin, hemin, and ferric iron on quality of chicken breast meat. Anim. Biosci. 2021, 34, 1382. [Google Scholar] [CrossRef]
- Baron, C.P.; Andersen, H.J. Myoglobin-induced lipid oxidation. A review. J. Agric. Food Chem. 2002, 50, 3887–3897. [Google Scholar] [CrossRef]
- Zareian, M.; Tybussek, T.; Silcock, P.; Bremer, P.; Beauchamp, J.; Böhner, N. Interrelationship among myoglobin forms, lipid oxidation and protein carbonyls in minced pork packaged under modified atmosphere. Food Packag. Shelf Life 2019, 20, 100311. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Ramanathan, R.; Suman, S.P.; Faustman, C. Biomolecular interactions governing fresh meat color in post-mortem skeletal muscle: A review. J. Agric. Food Chem. 2020, 68, 12779–12787. [Google Scholar] [CrossRef]
| Ingredients | Grower Stage (12 to 22 Days) | Finisher Stage (23 to 42 Days) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WM 0% | WM 6% | WM 0% | WM 6% | |||||||||
| (%) | CL0% | CL1% | CL2% | CL0% | CL1% | CL2% | CL0% | CL1% | CL2% | CL0% | CL1% | CL2% |
| Maize | 42.00 | 42.00 | 42.00 | 42.00 | 42.00 | 42.00 | 42.00 | 42.00 | 42.00 | 42.00 | 42.00 | 42.00 |
| Wheat | 18.73 | 16.77 | 14.82 | 15.91 | 13.96 | 12.00 | 20.56 | 18.6 | 16.65 | 17.75 | 15.79 | 13.84 |
| SBM | 30.53 | 30.90 | 31.27 | 27.37 | 27.74 | 28.11 | 28.10 | 28.45 | 28.93 | 25.03 | 25.40 | 25.78 |
| Oil | 4.10 | 4.68 | 5.25 | 3.96 | 4.54 | 5.12 | 5.11 | 5.67 | 6.26 | 4.97 | 5.55 | 6.12 |
| WM | 0 | 0 | 0 | 6.00 | 6.00 | 6.00 | 0 | 0 | 0 | 6.00 | 6.00 | 6.00 |
| CL | 0 | 1.00 | 2.00 | 0 | 1.00 | 2.00 | 0 | 1.00 | 2.00 | 0 | 1.00 | 2.00 |
| Lysine | 0.19 | 0.19 | 0.18 | 0.13 | 0.12 | 0.12 | 0.09 | 0.09 | 0.08 | 0.03 | 0.02 | 0.02 |
| Methionine | 0.24 | 0.25 | 0.25 | 0.30 | 0.30 | 0.31 | 0.20 | 0.20 | 0.20 | 0.25 | 0.26 | 0.26 |
| Threonine | 0.03 | 0.03 | 0.03 | 0.1 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.07 | 0.07 | 0.07 |
| CaCO3 | 1.29 | 1.29 | 1.29 | 1.30 | 1.29 | 1.29 | 1.17 | 1.17 | 1.17 | 1.18 | 1.17 | 1.17 |
| Ca(H2PO4)2 | 1.48 | 1.49 | 1.50 | 1.52 | 1.53 | 1.54 | 1.3 | 1.35 | 1.32 | 1.35 | 1.36 | 1.37 |
| Salt | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 | 0.37 | 0.33 | 0.33 | 0.33 | 0.33 | 0.34 | 0.34 |
| Choline | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 |
| Premix | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Chemical theoretical analysis | ||||||||||||
| ME, Kcal/kg | 3086 | 3086 | 3086 | 3086 | 3086 | 3086 | 3167 | 3167 | 3167 | 3167 | 3167 | 3167 |
| Crude protein, % | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 19.00 | 19.00 | 19.00 | 19.00 | 19.00 | 19.00 |
| Crude fat, % | 5.93 | 6.51 | 7.08 | 6.68 | 7.25 | 7.83 | 6.92 | 7.49 | 8.06 | 7.67 | 8.24 | 8.81 |
| Crude fibre, % | 3.78 | 3.94 | 4.10 | 4.62 | 4.78 | 4.94 | 3.75 | 3.87 | 4.03 | 4.55 | 4.71 | 4.87 |
| Ca (%) | 0.84 | 0.84 | 0.84 | 0.84 | 0.84 | 0.84 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 |
| Av. P, % | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 |
| Specification | CL | WM |
|---|---|---|
| Proximate composition | ||
| Dry matter (%) | 93.11 ± 0.72 | 92.88 ± 0.76 |
| Crude protein (%) | 6.63 ± 0.76 | 29.47 ± 1.03 |
| Crude fat (%) | 2.52 ± 0.46 | 16.24 ± 1.14 |
| Crude fibre (%) | 20.15 ± 1.27 | 18.41 ± 1.56 |
| Crude ash (%) | 3.0 ± 0.36 | 3.88 ± 0.75 |
| Mineral composition | ||
| Copper (mg/kg) | 2.182 ± 0.25 | 19.66 ± 1.18 |
| Iron (mg/kg) | 114.6 ± 14.7 | 225.35 ± 24.2 |
| Zinc (mg/kg) | 33.88 ± 3.45 | 185.21 ± 14.1 |
| Manganese (mg/kg) | 448.9 ± 52.1 | 72.57 ± 5.85 |
| Specification | CL | WM |
|---|---|---|
| Lipophilic Antioxidants and Antioxidant Capacity | ||
| Lutein and zeaxanthin (mg/kg) | 348.9 ± 44.5 | 2.903 ± 0.21 |
| Vitamin E (mg/kg) | 205.5 ± 23.4 | 69.32 ± 4.99 |
| DPPH (mM Trolox equiv) | 69.98 ± 3.92 | 32.77 ± 4.56 |
| Specification | Retention Time (min.) | λ (nm) | CL (mg/100 g) | WM (mg/100 g) |
|---|---|---|---|---|
| Phenolic acids | 535.86 | 971.27 | ||
| Hydroxybenzoic acids | 358.28 | 150.73 | ||
| Gallic acid (3,4,5-Trihydroxybenzoic acid) | 6.29 | 270 | 161.6 ± 3.89 | 70.83 ± 1.84 |
| Vanillic acid (4-Hydroxy-3-methoxybenzoic acid) | 23.86 | 270 | 15.98 ± 2.81 | 12.06 ± 1.38 |
| Syringic acid (3,5-Dimethoxy-4-hydroxybenzoic acid) | 25.58 | 270 | 11.22 ± 1.57 | 11.93 ± 1.51 |
| 3-Hydroxybenzoic acid | 25.90 | 270 | 149.38 ± 8.75 | 48.86 ± 2.73 |
| Ellagic acid (Benzoaric acid) | 29.84 | 270 | 14.54 ± 1.09 | n.d. |
| Protocatechuic acid (3,4-Dihydroxybenzoic acid) | 34.11 | 254 | 5.53 ± 0.37 | 7.05 ± 0.25 |
| Hydroxycinnamic acids | 177.58 | 820.54 | ||
| Chlorogenic acid (5-Caffeoylquinic acid) | 19.19 | 280 | 25.08 ± 1.47 | 69.34 ± 2.89 |
| Caffeic acid (3,4-Dihydroxycinnamic acid) | 24.75 | 270 | 16.21 ± 2.27 | 15.63 ± 1.75 |
| Methoxycinnamic acid | 30.45 | 270 | 38.07 ± 2.17 | 37.05 ± 2.16 |
| Ferulic acid (3-Methoxy-4-Hydroxycinnamic acid) | 31.62 | 320 | 71.88 ± 4.86 | 698.52 ± 34.3 |
| Cinnamic acid | 37.65 | 270 | 5.87 ± 0.62 | n.d. |
| Flavonoids | 445.16 | 169.02 | ||
| Flavanols | 415.09 | 154.72 | ||
| Epigallocatechin | 14.99 | 270 | 54.96 ± 3.51 | 79.79 ± 6.41 |
| Catechin (3,5,7,3′,4′-Pentahydroxyflavane) | 18.24 | 280 | 52.47 ± 3.87 | n.d. |
| Epicatechin (3,5,7,3′,4′-Pentahydroxyflavane) | 26.37 | 270 | 307.65 ± 16.1 | 74.93 ± 3.49 |
| Flavonols | 30.07 | 14.30 | ||
| Rutin (Quercetin 3-O-rutinoside) | 29.28 | 270 | 26.39 ± 1.69 | 14.30 ± 1.63 |
| Quercetin (3,5,7,3′,4′-Pentahydroxyflavone) | 35.70 | 254 | 3.69 ± 0.49 | n.d. |
| Stilbene | 11.38 | 11.89 | ||
| Resveratrol (3,5,4′-Trihydroxystilbene) | 34.24 | 254 | 11.38 ± 1.12 | 11.89 ± 0.98 |
| WM | CL | Dry Matter (%) | Protein (%) | Fat (%) | Ash (%) |
|---|---|---|---|---|---|
| 0 | 0 | 22.77 | 20.21 | 1.558 b | 0.961 |
| 1 | 22.58 | 20.31 | 1.299 c | 0.924 | |
| 2 | 21.43 | 19.25 | 1.203 c | 0.884 | |
| 6 | 0 | 22.69 | 19.94 | 1.775 a | 0.958 |
| 1 | 22.73 | 20.01 | 1.724 a | 0.944 | |
| 2 | 22.79 | 20.09 | 1.653 a | 0.966 | |
| Main effects | |||||
| WM | |||||
| 0% | 22.26 | 19.93 | 1.353 b | 0.923 | |
| 6% | 22.74 | 20.01 | 1.717 a | 0.956 | |
| CL | |||||
| 0% | 22.73 | 20.08 | 1.667 a | 0.959 | |
| 1% | 22.65 | 20.16 | 1.511 b | 0.934 | |
| 2% | 22.11 | 19.67 | 1.428 b | 0.925 | |
| p value | |||||
| CL | 0.599 | 0.675 | 0.0001 | 0.474 | |
| WM | 0.384 | 0.849 | 0.0001 | 0.167 | |
| CL × WM | 0.513 | 0.546 | 0.061 | 0.318 | |
| SEM | |||||
| CL | 0.382 | 0.337 | 0.030 | 0.016 | |
| WM | 0.468 | 0.412 | 0.036 | 0.020 | |
| CL × WM | 0.662 | 0.583 | 0.051 | 0.029 | |
| WM | CL | Cu (mg/kg) | Fe (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|
| 0 | 0 | 24.98 d | 22.75 d | 37.17 a |
| 1 | 26.66 b | 30.66 ab | 30.54 d | |
| 2 | 26.62 b | 33.40 a | 34.24 b | |
| 6 | 0 | 28.29 a | 26.86 c | 32.65 bc |
| 1 | 25.74 c | 30.20 abc | 33.17 bc | |
| 2 | 25.47 cd | 29.04 bc | 31.48 cd | |
| Main effects | ||||
| WM | ||||
| 0% | 26.09 b | 28.94 | 33.98 a | |
| 6% | 26.50 a | 28.70 | 32.43 b | |
| CL | ||||
| 0% | 26.63 a | 24.80 b | 34.91 a | |
| 1% | 26.20 b | 30.43 a | 31.86 b | |
| 2% | 26.05 b | 31.22 a | 32.86 b | |
| p value | ||||
| CL | 0.006 | 0.0001 | 0.0001 | |
| WM | 0.008 | 0.732 | 0.0001 | |
| CL × WM | 0.0001 | 0.0001 | 0.0001 | |
| SEM | ||||
| CL | 0.102 | 0.492 | 0.238 | |
| WM | 0.125 | 0.603 | 0.292 | |
| CL × WM | 0.176 | 0.852 | 0.413 | |
| WM | CL | Total Polyphenols | Vitamin E | Lutein and Zeaxanthin | DPPH |
|---|---|---|---|---|---|
| 0 | 0 | 1.000 b | 70.71 cd | 0.756 d | 1.363 c |
| 1 | 1.058 b | 59.18 de | 1.045 bc | 1.836 a | |
| 2 | 1.301 a | 47.44 e | 1.260 ab | 1.846 a | |
| 6 | 0 | 0.859 bc | 107.31 a | 0.845 cd | 1.443 bc |
| 1 | 0.767 c | 91.71 ab | 1.021 bc | 1.720 ab | |
| 2 | 0.718 c | 86.11 bc | 1.398 a | 1.630 ab | |
| Main effects | |||||
| WM | |||||
| 0% | 1.120 a | 59.11 b | 1.020 | 1.682 | |
| 6% | 0.782 b | 95.04 a | 1.088 | 1.598 | |
| CL | |||||
| 0% | 0.930 | 89.01 a | 0.801 c | 1.403 b | |
| 1% | 0.913 | 75.45 b | 1.033 b | 1.778 a | |
| 2% | 1.010 | 66.78 b | 1.329 a | 1.738 a | |
| p value | |||||
| CL | 0.167 | 0.0001 | 0.0001 | 0.0001 | |
| WM | 0.0001 | 0.0001 | 0.140 | 0.121 | |
| CL × WM | 0.001 | 0.719 | 0.329 | 0.082 | |
| SEM | |||||
| CL | 0.030 | 2.205 | 0.031 | 0.037 | |
| WM | 0.037 | 2.700 | 0.038 | 0.046 | |
| CL × WM | 0.053 | 3.819 | 0.054 | 0.065 | |
| WM | CL | PV | Diene | Triene | VA | TBARS | metMyo | deoxyMyo | oxyMyo |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0.218 | 19.28 | 5.619 ab | 26.38 | 173.2 a | 59.58 a | 23.64 b | 16.88 |
| 1 | 0.216 | 19.86 | 6.140 a | 24.66 | 145.7 ab | 61.03 a | 23.46 b | 15.66 | |
| 2 | 0.222 | 19.35 | 5.825 ab | 21.19 | 167.2 a | 59.84 a | 23.71 ab | 16.55 | |
| 6 | 0 | 0.201 | 15.59 | 4.214 b | 23.26 | 194.7 a | 57.86 b | 24.63 a | 16.20 |
| 1 | 0.201 | 14.85 | 5.478 ab | 19.46 | 109.7 b | 57.37 b | 24.95 a | 17.84 | |
| 2 | 0.228 | 21.13 | 6.066 a | 18.89 | 142.5 ab | 58.19 ab | 23.95 ab | 17.95 | |
| Main effects | |||||||||
| WM | |||||||||
| 0% | 0.219 | 19.49 | 5.862 | 24.08 | 162.1 | 60.15 a | 23.60 b | 16.36 | |
| 6% | 0.210 | 17.18 | 5.253 | 20.54 | 149.0 | 58.29 b | 24.51 a | 17.33 | |
| CL | |||||||||
| 0% | 0.209 | 17.43 | 4.917 | 24.82 | 183.9 a | 59.45 | 24.13 | 16.54 | |
| 1% | 0.208 | 17.35 | 5.809 | 22.06 | 127.7 b | 59.20 | 24.20 | 16.74 | |
| 2% | 0.225 | 20.24 | 5.946 | 20.04 | 154.9 ab | 59.02 | 23.83 | 17.25 | |
| p value | |||||||||
| CL | 0.497 | 0.147 | 0.041 | 0.116 | 0.001 | 0.830 | 0.449 | 0.671 | |
| WM | 0.496 | 0.092 | 0.085 | 0.061 | 0.229 | 0.003 | 0.001 | 0.153 | |
| CL × WM | 0.727 | 0.102 | 0.161 | 0.796 | 0.082 | 0.072 | 0.141 | 0.204 | |
| SEM | |||||||||
| CL | 0.009 | 0.938 | 0.242 | 1.276 | 7.524 | 0.412 | 0.178 | 0.466 | |
| WM | 0.011 | 1.149 | 0.296 | 1.562 | 9.215 | 0.505 | 0.217 | 0.571 | |
| CL × WM | 0.016 | 1.624 | 0.418 | 1.987 | 13.03 | 0.714 | 0.307 | 0.808 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Untea, A.E.; Varzaru, I.; Saracila, M.; Panaite, T.D.; Oancea, A.G.; Vlaicu, P.A.; Grosu, I.A. Antioxidant Properties of Cranberry Leaves and Walnut Meal and Their Effect on Nutritional Quality and Oxidative Stability of Broiler Breast Meat. Antioxidants 2023, 12, 1084. https://doi.org/10.3390/antiox12051084
Untea AE, Varzaru I, Saracila M, Panaite TD, Oancea AG, Vlaicu PA, Grosu IA. Antioxidant Properties of Cranberry Leaves and Walnut Meal and Their Effect on Nutritional Quality and Oxidative Stability of Broiler Breast Meat. Antioxidants. 2023; 12(5):1084. https://doi.org/10.3390/antiox12051084
Chicago/Turabian StyleUntea, Arabela Elena, Iulia Varzaru, Mihaela Saracila, Tatiana Dumitra Panaite, Alexandra Gabriela Oancea, Petru Alexandru Vlaicu, and Iulian Alexandru Grosu. 2023. "Antioxidant Properties of Cranberry Leaves and Walnut Meal and Their Effect on Nutritional Quality and Oxidative Stability of Broiler Breast Meat" Antioxidants 12, no. 5: 1084. https://doi.org/10.3390/antiox12051084
APA StyleUntea, A. E., Varzaru, I., Saracila, M., Panaite, T. D., Oancea, A. G., Vlaicu, P. A., & Grosu, I. A. (2023). Antioxidant Properties of Cranberry Leaves and Walnut Meal and Their Effect on Nutritional Quality and Oxidative Stability of Broiler Breast Meat. Antioxidants, 12(5), 1084. https://doi.org/10.3390/antiox12051084












