Black Pepper (Piper nigrum) Alleviates Oxidative Stress, Exerts Potential Anti-Glycation and Anti-AChE Activity: A Multitargeting Neuroprotective Agent against Neurodegenerative Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Extraction
2.3. Gas Chromatography–Mass Spectrometry (GC–MS) Method
2.4. Determination of Total Phenolic Content
2.5. Determination of Total Flavonoids Content
2.6. Determination of Antioxidant Capacity
2.6.1. Free Radical Scavenging by 2,2-Diphenyl-1-picrylhydrazylhydrate (DPPH) Radical
2.6.2. Free Radical Scavenging by 2,2′-Azino-bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) [ABTS] Radical
2.6.3. Ferric Reducing Antioxidant Potential (FRAP) Assay
2.7. Acetylcholinesterase Inhibitory Activity
2.8. Thioflavin T (ThT) Assay
2.9. Advanced Glycation End-Product (AGE) Inhibition Activity
2.10. Cell Culture
2.10.1. Cell Viability Assay
2.10.2. Neuroprotective Activity Assay
2.10.3. Measurement of Intracellular Reactive Oxygen Species (ROS)
2.10.4. Mitochondrial Membrane Potential (ΔΨm) Assay
2.10.5. Statistical Analysis
3. Results and Discussion
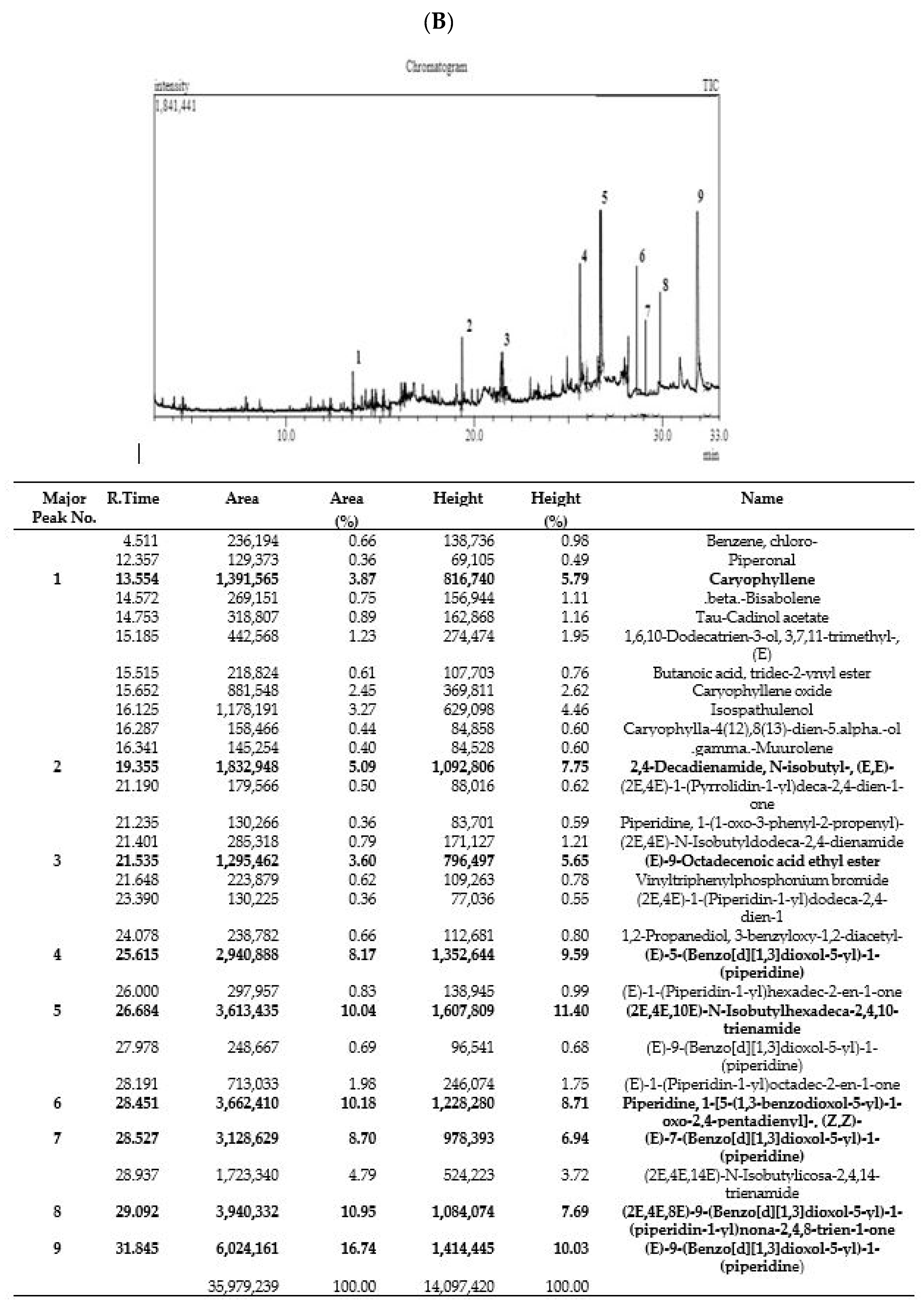
3.1. GC–MS Analysis
3.2. Phytochemical Estimation and Antioxidant Potential of Piper nigrum Extract
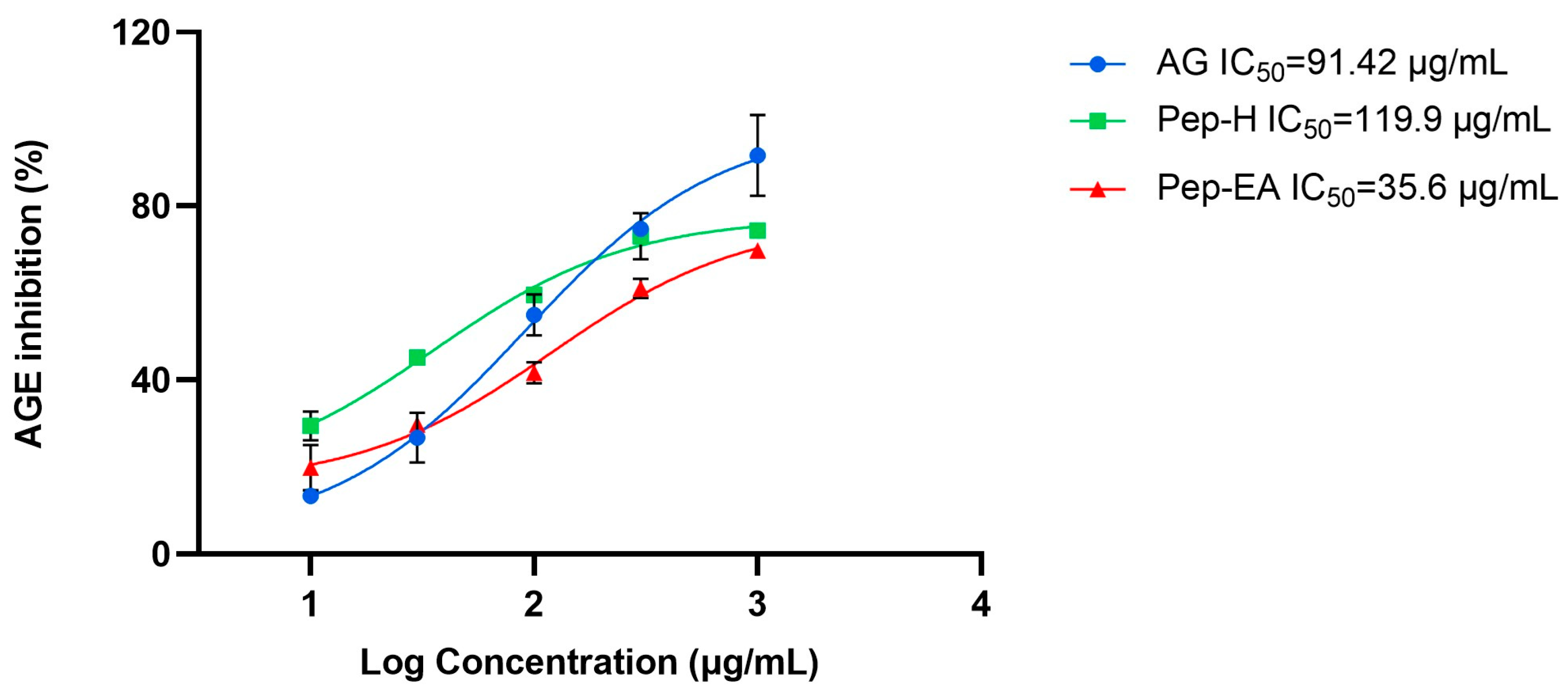
3.3. In Vitro Anti-Glycation Activity
3.4. Acetylcholinesterase Inhibitory Activity
3.5. Piper nigrum Extract Reduced Aβ Fibrilization
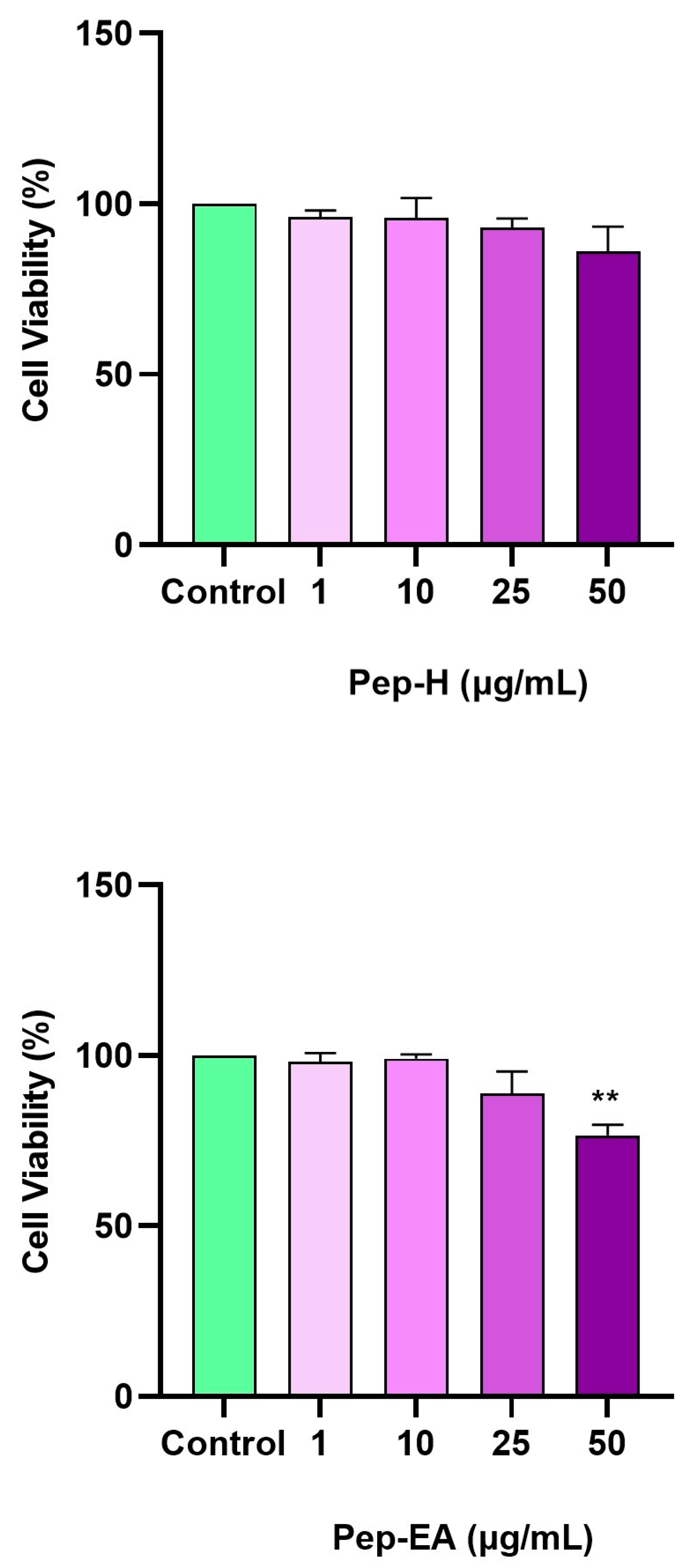
3.6. Cytotoxic Effect of Pepper Extracts on the SH-SY5Y Cell Line
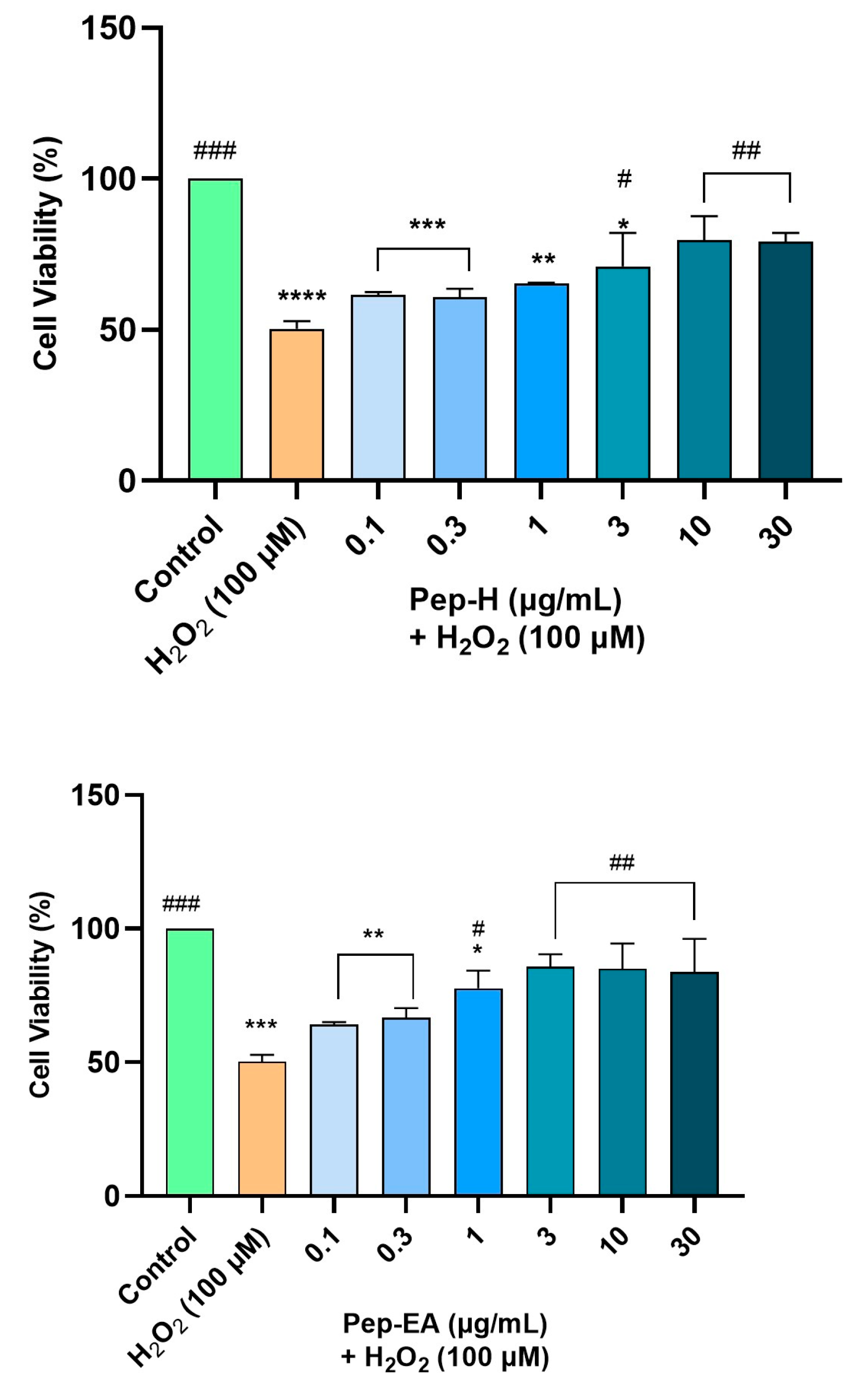
3.7. Piper nigrum Provided Neuroprotection against H2O2-Induced Oxidative Stress in SH-SY5Y
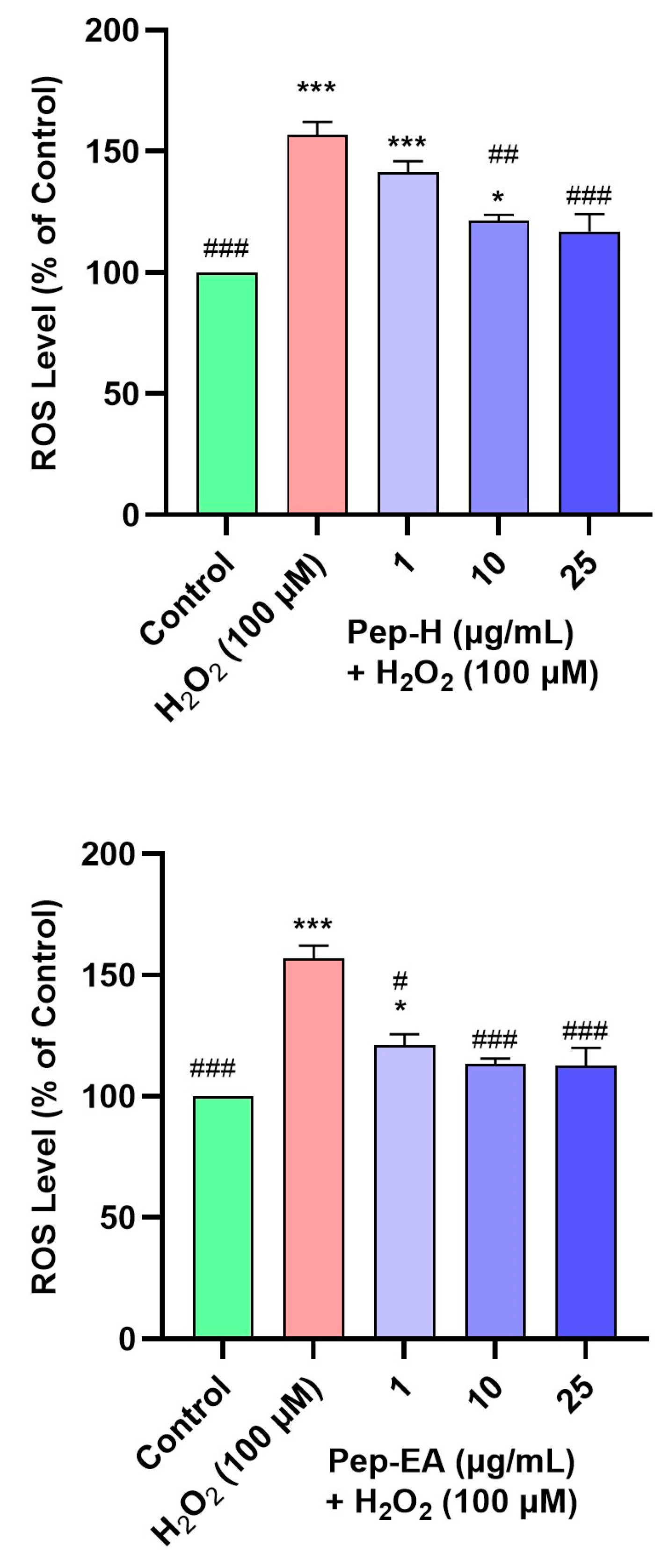
3.8. Piper nigrum Ameliorated H2O2-Induced ROS Generation
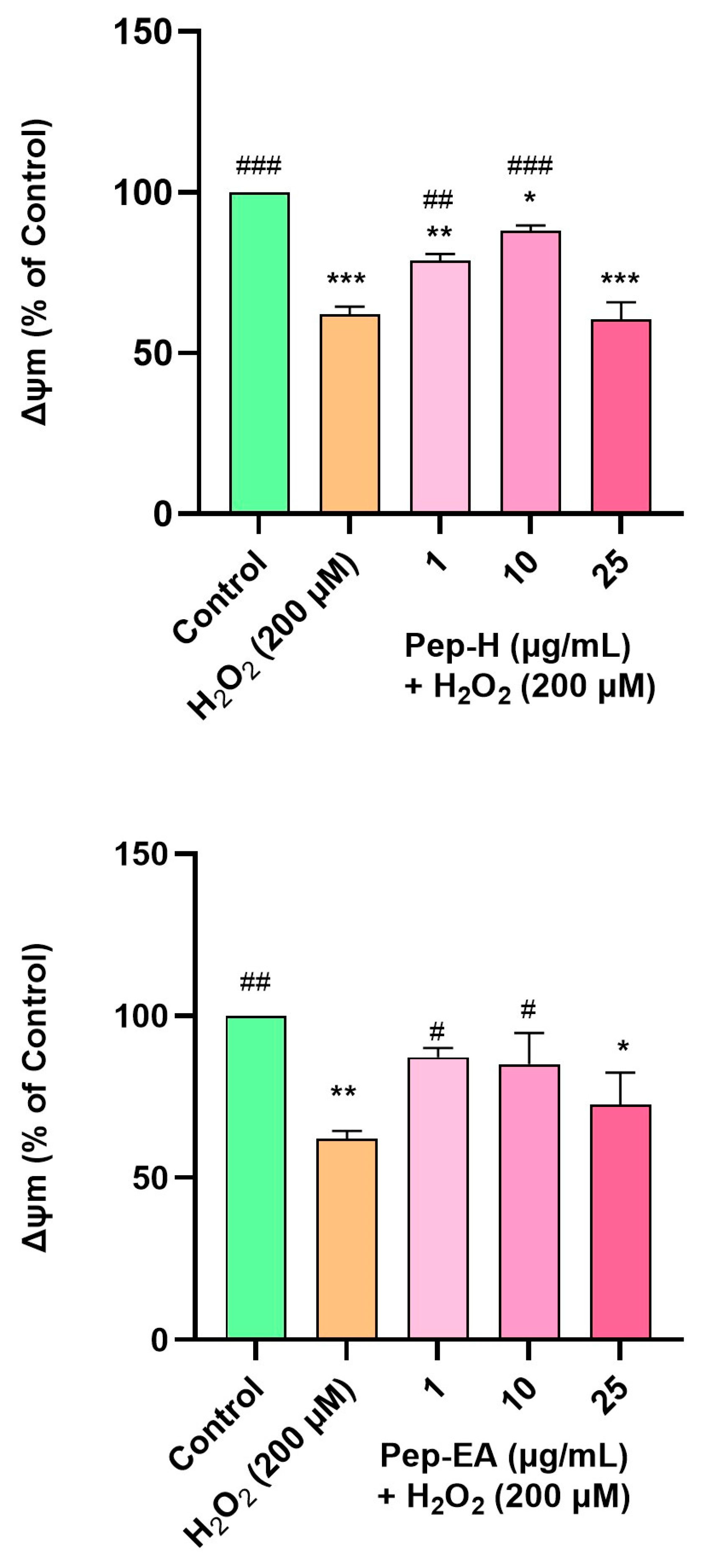
3.9. Piper nigrum Improved Mitochondrial Membrane Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taoufik, E.; Kouroupi, G.; Zygogianni, O.; Matsas, R. Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: An overview of induced plu-ripotent stem-cell-based disease models. Open Biol. 2018, 8, 180138. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lin, Q.; Liang, Y. Plant-Derived Antioxidants Protect the Nervous System From Aging by Inhibiting Oxidative Stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kunnumakkara, A.B. Molecular Targets and Therapeutic Uses of Spices: Modern Uses for Ancient Medicine; World Scientific: Singapore, 2009. [Google Scholar]
- Gülçin, I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Naureen, I.; Naeem, M.; Tasleem, G.; Ahmed, H.; Farooq, U. Therapeutic Role of Piper nigrum L (Black Pepper) and Pharmacological Activities. Sch. Int. J. Biochem. 2022, 5, 15–21. [Google Scholar] [CrossRef]
- Manap, A.S.A.; Tan, A.C.W.; Leong, W.H.; Chia, A.Y.Y.; Vijayabalan, S.; Arya, A.; Wong, E.H.; Rizwan, F.; Bindal, U.; Koshy, S.; et al. Synergistic Effects of Curcumin and Piperine as Potent Acetylcholine and Amyloidogenic Inhibitors With Significant Neuroprotective Activity in SH-SY5Y Cells via Computational Molecular Modeling and in vitro Assay. Front. Aging Neurosci. 2019, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Aluko, R.E. Food-derived Acetylcholinesterase Inhibitors as Potential Agents against Alzheimer’s Disease. Efood 2021, 2, 49–58. [Google Scholar] [CrossRef]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Postu, P.; Mihasan, M. Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1–42) rat model of Alzheimer’s disease. Behav. Brain Funct. 2015, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, Z.; Wang, W.; Wei, M.; Kou, D.; Li, T.; Yang, Z.; Guo, H.; Le, W.; Li, S. Piperine attenuates cognitive impairment in an experimental mouse model of sporadic Alzheimer’s disease. J. Nutr. Biochem. 2019, 70, 147–155. [Google Scholar] [CrossRef]
- Nazifi, M.; Oryan, S.; Esfahani, D.E.; Ashrafpoor, M. The functional effects of piperine and piperine plus donepezil on hippocampal synaptic plasticity impairment in rat model of Alzheimer’s disease. Life Sci. 2021, 265, 118802. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 152–178. [Google Scholar]
- Ribarova, F.; Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. JU Chem. Met. 2005, 40, 255–260. [Google Scholar]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.H.; De Groot, A.; Evstatieva, L.N. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three en-demic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Tan, M.A.; Ishikawa, H.; An, S.S.A. Pandanus amaryllifolius Exhibits In Vitro Anti-Amyloidogenic Activity and Promotes Neuroprotective Effects in Amyloid-β-Induced SH-SY5Y Cells. Nutrients 2022, 14, 3962. [Google Scholar] [CrossRef]
- Singh, P.; Jayaramaiah, R.H.; Agawane, S.B.; Vannuruswamy, G.; Korwar, A.M.; Anand, A.; Dhaygude, V.S.; Shaikh, M.L.; Joshi, R.S.; Boppana, R.; et al. Potential Dual Role of Eugenol in Inhibiting Advanced Glycation End Products in Diabetes: Proteomic and Mechanistic Insights. Sci. Rep. 2016, 6, 18798. [Google Scholar] [CrossRef]
- Alvariño, R.; Alonso, E.; Lacret, R.; Oves-Costales, D.; Genilloud, O.; Reyes, F.; Alfonso, A.; Botana, L.M. Caniferolide A, a Macrolide from Streptomyces caniferus, Attenuates Neuroinflammation, Oxidative Stress, Amyloid-Beta, and Tau Pathology in Vitro. Mol. Pharm. 2019, 16, 1456–1466. [Google Scholar] [CrossRef]
- Kozukue, N.; Park, M.-S.; Choi, S.-H.; Lee, S.-U.; Ohnishi-Kameyama, M.; Levin, C.E.; Friedman, M. Kinetics of Light-Induced Cis−TransIsomerization of Four Piperines and Their Levels in Ground Black Peppers as Determined by HPLC and LC/MS. J. Agric. Food Chem. 2007, 55, 7131–7139. [Google Scholar] [CrossRef]
- Iqbal, G.; Iqbal, A.; Mahboob, A.; Farhat, S.M.; Ahmed, T. Memory Enhancing Effect of Black Pepper in the AlCl3 Induced Neurotoxicity Mouse Model is Mediated Through Its Active Component Chavicine. Curr. Pharm. Biotechnol. 2016, 17, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, J.-R.; Yang, M.-S.; Lu, Y.; Chen, L.; Cao, H.-R. Study on the Extraction, Geometry Structure and Spectral Characterization of Piperine Alkaloid. Guang pu xue yu guang pu fen xi = Guang pu 2016, 36, 2082–2088. [Google Scholar] [PubMed]
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Chapter 53—Cannabis sativa and Hemp. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 735–754. [Google Scholar]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Min, B.-W.; Lee, S.; Jee, J.-G.; Kim, J.A.; Bae, J.-S. Vascular barrier protective effects of pellitorine in LPS-induced inflammation in vitro and in vivo. Fitoterapia 2014, 92, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.-K.; Lee, I.-C.; Kim, J.A.; Bae, J.-S. Anti-septic Effects of Pellitorine in HMGB1-Induced Inflammatory Responses In Vitro and In Vivo. Inflammation 2014, 37, 338–348. [Google Scholar] [CrossRef]
- Lieder, B.; Zaunschirm, M.; Holik, A.-K.; Ley, J.P.; Krammer, G.E.; Somoza, V. The alkamide trans-pellitorine targets PPARγ via TRPV1 and TRPA1 to reduce lipid accumulation in devel-oping 3T3-L1 adipocytes. Front. Pharmacol. 2017, 8, 316. [Google Scholar] [CrossRef]
- Okumura, Y.; Narukawa, M.; Iwasaki, Y.; Ishikawa, A.; Matsuda, H.; Yoshikawa, M.; Watanabe, T. Activation of TRPV1 and TRPA1 by Black Pepper Components. Biosci. Biotechnol. Biochem. 2010, 74, 1068–1072. [Google Scholar] [CrossRef]
- Scott, I.M.; Puniani, E.; Jensen, H.; Livesey, J.F.; Poveda, L.; Sánchez-Vindas, P.; Durst, T.; Arnason, J.T. Analysis of Piperaceae Germplasm by HPLC and LCMS: A Method for Isolating and Identifying Unsaturated Amides from Piper spp Extracts. J. Agric. Food Chem. 2005, 53, 1907–1913. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, M.-S.; Jo, K.; Hwang, J.-K. Piperidine alkaloids from Piperretrofractum Vahl. protect against high-fat diet-induced obesity by regulating lipid metabolism and activating AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2011, 411, 219–225. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Upadhya, V.; Pai, S.R.; Naik, P.M.; Al-Mssallem, M.Q.; Alessa, F.M. Comparative Quantification of the Phenolic Compounds, Piperine Content, and Total Polyphenols along with the Antioxidant Activities in the Piper trichostachyon and P. nigrum. Molecules 2022, 27, 5965. [Google Scholar] [CrossRef]
- Zarai, Z.; Boujelbene, E.; Ben Salem, N.; Gargouri, Y.; Sayari, A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT Food Sci. Technol. 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Akbar, P.N.; Jahan, I.A.; Hossain, H.; Banik, R.; Nur, H.P.; Hossain, M.T. Antioxidant capacity of Piper longum and Piper nigrum fruits grown in Bangladesh. World J. Pharm. Sci. 2014, 2, 931–941. [Google Scholar]
- Starowicz, M.; Zieliński, H. Inhibition of Advanced Glycation End-Product Formation by High Antioxidant-Leveled Spices Commonly Used in European Cuisine. Antioxidants 2019, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Niaz, Z.; Gautam, S.; Adhikari, S.; Variyar, P.S.; Sharma, A. Antioxidant activity of some phenolic constituents from green pepper (Piper nigrum L.) and fresh nutmeg mace (Myristica fragrans). Food Chem. 2007, 101, 515–523. [Google Scholar] [CrossRef]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Téllez-Téllez, M.; Gupta, V.K.; Díaz-Godínez, G.; Soriano-Santos, J. Evaluation of the Antioxidant Activity of Aqueous and Methanol Extracts of Pleurotus ostreatus in Different Growth Stages. Front. Microbiol. 2016, 7, 1099. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Sayed, A.A.; Abdeen, A.; Aleya, L.; Ali, D.; Alkahtane, A.A.; Alarifi, S.; Alkahtani, S. Piperine Enhances the Antioxidant and Anti-Inflammatory Activities of Thymoquinone against Microcystin-LR-Induced Hepatotoxicity and Neurotoxicity in Mice. Oxidative Med. Cell. Longev. 2019, 2019, 1309175. [Google Scholar] [CrossRef]
- Mittal, R.; Gupta, R. In vitro antioxidant activity of piperine. Methods Find. Exp. Clin. Pharmacol. 2000, 22, 271–274. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Glycotoxins: Dietary and Metabolic Origins; Possible Amelioration of Neurotoxicity by Carnosine, with Special Reference to Parkinson’s Disease. Neurotox. Res. 2018, 34, 164–172. [Google Scholar] [CrossRef]
- Kaewnarin, K.; Niamsup, H.; Shank, L.; Rakariyatham, N. Antioxidant and antiglycation activities of some edible and medicinal plants. Chiang Mai J. Sci. 2014, 41, 105–116. [Google Scholar]
- Dearlove, R.P.; Greenspan, P.; Hartle, D.K.; Swanson, R.B.; Hargrove, J.L. Inhibition of Protein Glycation by Extracts of Culinary Herbs and Spices. J. Med. Food 2008, 11, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, Y.; Lo, C.-Y.; Ho, C.-T. Methylglyoxal: Its presence and potential scavengers. Asia Pac. J. Clin. Nutr. 2008, 17, 261–264. [Google Scholar] [CrossRef]
- Bhattacherjee, A.; Datta, A. Mechanism of antiglycating properties of syringic and chlorogenic acids in in vitro glycation system. Food Res. Int. 2015, 77, 540–548. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G. Kinetics of Glycoxidation of Bovine Serum Albumin by Methylglyoxal and Glyoxal and its Prevention by Various Compounds. Molecules 2014, 19, 4880–4896. [Google Scholar] [CrossRef] [PubMed]
- Tupe, R.S.; Bangar, N.; Nisar, A.; Kulkarni, A.; Sankhe, N.; Chauhan, R.; Mistry, N.; Shaikh, S. Piperine exhibits preventive and curative effect on erythrocytes membrane modifications and oxidative stress against in vitro albumin glycation. J. Food Biochem. 2021, 45, e13846. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, F.M.; Sanders, R.A.; Watkins, J.B. Effects of piperine on antioxidant pathways in tissues from normal and streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxicol. 2000, 14, 329–334. [Google Scholar] [CrossRef]
- Yeggoni, D.P.; Rachamallu, A.; Kallubai, M.; Subramanyam, R. Cytotoxicity and comparative binding mechanism of piperine with human serum albumin and α-1-acid glycoprotein. J. Biomol. Struct. Dyn. 2015, 33, 1336–1351. [Google Scholar] [CrossRef]
- Tsakiris, S.; Kalafatakis, K.; Gkanti, V.; Scott, C.A.M.-G.; Zarros, A.; Baillie, G.S. Acetylcholinesterase activity as a neurotoxicity marker within the context of experimentally-simulated hyperprolinaemia: An in vitro approach. J. Nat. Sci. Biol. Med. 2015, 6, 98–S101. [Google Scholar] [CrossRef]
- Werawattanachai, N.; Kaewamatawong, R. Screening for Acetylcholinesterase Inhibitory Activity from the Piperaceae. วารสาร วิทยาศาสตร์ และ เทคโนโลยี มหาวิทยาลัย อุบลราชธานี 2016, 18, 25. [Google Scholar]
- Lomarat, P.; Sripha, K.; Phanthong, P.; Kitphati, W.; Thirapanmethee, K.; Bunyapraphatsara, N. In vitro biological activities of black pepper essential oil and its major components relevant to the prevention of Alzheimer’s disease. Thai J. Pharm. Sci. (TJPS) 2015, 39, 94–101. [Google Scholar]
- Balkrishna, A.; Pokhrel, S.; Tomer, M.; Verma, S.; Kumar, A.; Nain, P.; Gupta, A.; Varshney, A. Anti-acetylcholinesterase activities of mono-herbal extracts and exhibited synergistic effects of the phytoconstituents: A biochemical and computational study. Molecules 2019, 24, 4175. [Google Scholar] [CrossRef] [PubMed]
- Magaña-Barajas, E.; Buitimea-Cantúa, G.V.; Hernández-Morales, A.; Torres-Pelayo, V.D.R.; Vázquez-Martínez, J.; Buitimea-Cantúa, N.E. In vitro α-amylase and α-glucosidase enzyme inhibition and antioxidant activity by capsaicin and piperine from Capsicum chinense and Piper nigrum fruits. J. Environ. Sci. Health Part B 2021, 56, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Khatami, Z.; Sarkheil, P.; Adhami, H. Isolation and characterization of acetylcholinesterase inhibitors from Piper longum Linn. Planta Medica 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Ikawati, S.; Himawan, T.; Abadi, A.; Sarno, H.; Fajarudin, A. In Silico Study of Eugenol and trans-Caryophyllene also Clove Oil Fumigant Toxicity on Tribolium castaneum. J. Trop. Life Sci. 2022, 12, 339–349. [Google Scholar] [CrossRef]
- Hung, N.H.; Quan, P.M.; Satyal, P.; Dai, D.N.; Van Hoa, V.; Huy, N.G.; Giang, L.D.; Ha, N.T.; Huong, L.T.; Hien, V.T.; et al. Acetylcholinesterase Inhibitory Activities of Essential Oils from Vietnamese Traditional Medicinal Plants. Molecules 2022, 27, 7092. [Google Scholar] [CrossRef]
- Tu, Y.; Zhong, Y.; Du, H.; Luo, W.; Wen, Y.; Li, Q.; Zhu, C.; Li, Y. Anticholinesterases and antioxidant alkamides from Piper nigrum fruits. Nat. Prod. Res. 2016, 30, 1945–1949. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.A.; Zakharova, E.; An, S.S.A. Diaportheone A Analogues Instigate a Neuroprotective Effect by Protecting Neuro-blastoma SH-SY5Y Cells from Oxidative Stress. Biology 2021, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E. Mechanisms of amyloid fibril self-assembly and inhibition: Model short peptides as a key research tool. FEBS J. 2005, 272, 5971–5978. [Google Scholar] [CrossRef] [PubMed]
- Kotormán, M.; Varga, A.; Kasi, P.B.; Nemcsók, J. Inhibition of the formation of amyloid-like fibrils with spices, especially cloves. Acta Biol. Hung. 2018, 69, 385–394. [Google Scholar] [CrossRef]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A mini review. Oxidative Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Ghaffari, H.; Ghassam, B.J.; Nayaka, S.C.; Kini, K.R.; Prakash, H.S. Antioxidant and Neuroprotective Activities of Hyptis suaveolens (L.) Poit. Against Oxidative Stress-Induced Neurotoxicity. Cell. Mol. Neurobiol. 2014, 34, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Polster, B.M.; Fiskum, G. Mitochondrial mechanisms of neural cell apoptosis. J. Neurochem. 2004, 90, 1281–1289. [Google Scholar] [CrossRef]
- Beal, M.F. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2005, 58, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Starkov, A.A. The Role of Mitochondria in Reactive Oxygen Species Metabolism and Signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Ali, M.; Salman, M.; Tabassum, H.; Parvez, S. Harnessing the mitochondrial integrity for neuroprotection: Therapeutic role of piperine against experimental ischemic stroke. Neurochem. Int. 2021, 149, 105138. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Liu, J.; Zhang, Y.; Li, J.; Zhang, X.; Dong, L.; Zhao, Y.; Fu, X. Piperine as a neuroprotective functional component in rats with cerebral ischemic injury. Food Sci. Nutr. 2019, 7, 3443–3451. [Google Scholar] [CrossRef]
- Khalili-Fomeshi, M.; Azizi, M.G.; Esmaeili, M.R.; Gol, M.; Kazemi, S.; Ashrafpour, M.; Moghadamnia, A.A.; Hosseinzadeh, S. Piperine restores streptozotocin-induced cognitive impairments: Insights into oxidative balance in cerebrospinal fluid and hippocampus. Behav. Brain Res. 2017, 337, 131–138. [Google Scholar] [CrossRef]
- Ren, T.; Wang, Q.; Li, C.; Yang, M.; Zuo, Z. Efficient brain uptake of piperine and its pharmacokinetics characterization after oral administration. Xenobiotica 2017, 48, 1249–1257. [Google Scholar] [CrossRef]
- Itharat, A.; Kanokkangsadal, P.; Khemawoot, P.; Wanichsetakul, P.; Davies, N.M. Pharmacokinetics of piperine after oral administration of Sahastara remedy capsules in healthy volunteers. Res. Pharm. Sci. 2020, 15, 410–417. [Google Scholar] [CrossRef]


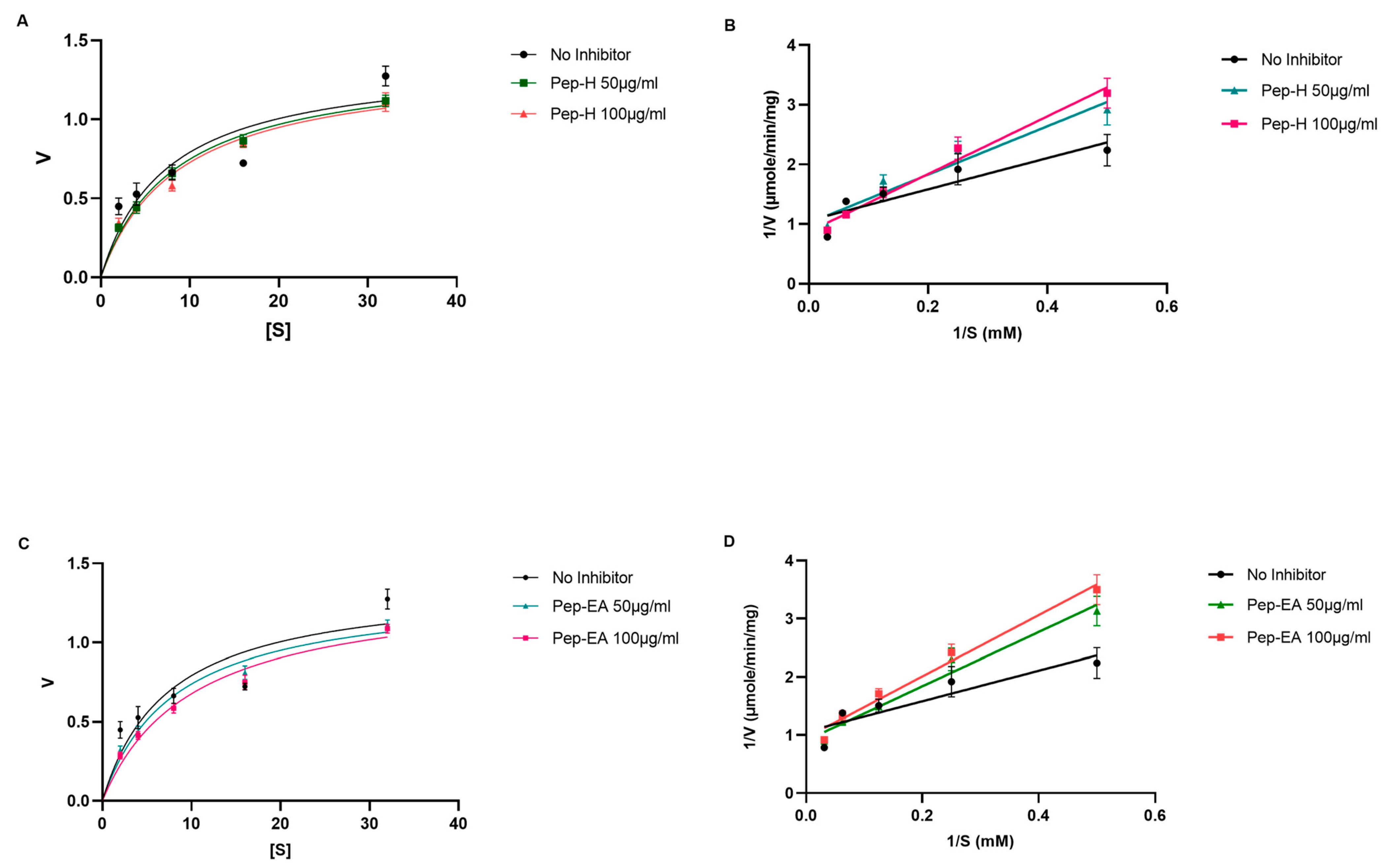

| Vmax (μmole/min/mg) | Km (mM) | Type of Inhibition | |
|---|---|---|---|
| No Inhibitor | 1.375 | 7.371 | |
| Pep-H (50 μg/mL) | 1.396 | 8.379 | Competitive |
| Pep-H (100 μg/mL) | 1.364 | 8.755 | Competitive |
| Pep-EA (50 μg/mL) | 1.333 | 8.052 | Competitive |
| Pep-EA (100 μg/mL) | 1.360 | 10.080 | Competitive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, H.; Sharma, N.; An, S.S.A. Black Pepper (Piper nigrum) Alleviates Oxidative Stress, Exerts Potential Anti-Glycation and Anti-AChE Activity: A Multitargeting Neuroprotective Agent against Neurodegenerative Diseases. Antioxidants 2023, 12, 1089. https://doi.org/10.3390/antiox12051089
Sharma H, Sharma N, An SSA. Black Pepper (Piper nigrum) Alleviates Oxidative Stress, Exerts Potential Anti-Glycation and Anti-AChE Activity: A Multitargeting Neuroprotective Agent against Neurodegenerative Diseases. Antioxidants. 2023; 12(5):1089. https://doi.org/10.3390/antiox12051089
Chicago/Turabian StyleSharma, Himadri, Niti Sharma, and Seong Soo A. An. 2023. "Black Pepper (Piper nigrum) Alleviates Oxidative Stress, Exerts Potential Anti-Glycation and Anti-AChE Activity: A Multitargeting Neuroprotective Agent against Neurodegenerative Diseases" Antioxidants 12, no. 5: 1089. https://doi.org/10.3390/antiox12051089
APA StyleSharma, H., Sharma, N., & An, S. S. A. (2023). Black Pepper (Piper nigrum) Alleviates Oxidative Stress, Exerts Potential Anti-Glycation and Anti-AChE Activity: A Multitargeting Neuroprotective Agent against Neurodegenerative Diseases. Antioxidants, 12(5), 1089. https://doi.org/10.3390/antiox12051089









