Chronic Vitamin E Deficiency Dysregulates Purine, Phospholipid, and Amino Acid Metabolism in Aging Zebrafish Skeletal Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
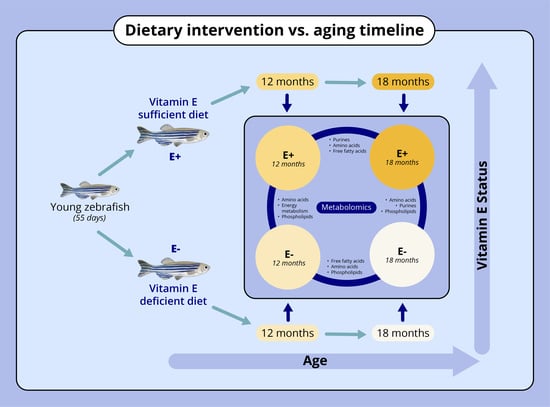
2.2. Zebrafish Husbandry and Dietary Conditions
2.3. Muscle Extraction
2.4. LC-MS/MS for Metabolomic Analysis
2.5. Quantitation and Statistical Analysis of α-Tocopherol in Tissues
2.6. Metabolomics Data Processing and Statistical Analyses
3. Results
3.1. Muscle Vitamin E Concentrations
3.2. Effect of Age on Muscle Metabolites
3.3. Effect at 12 Months of Vitamin E Deficiency on Muscle Metabolites
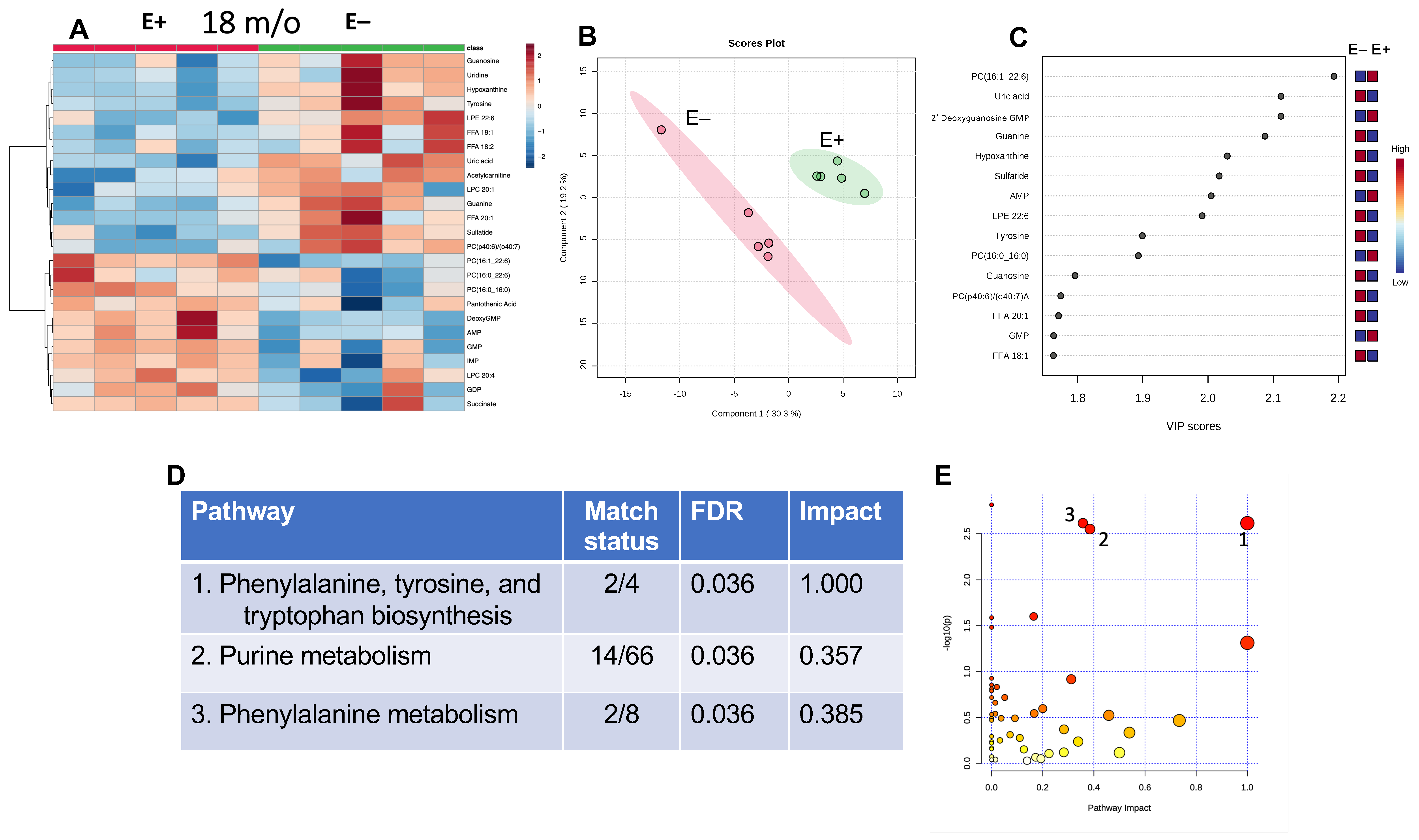
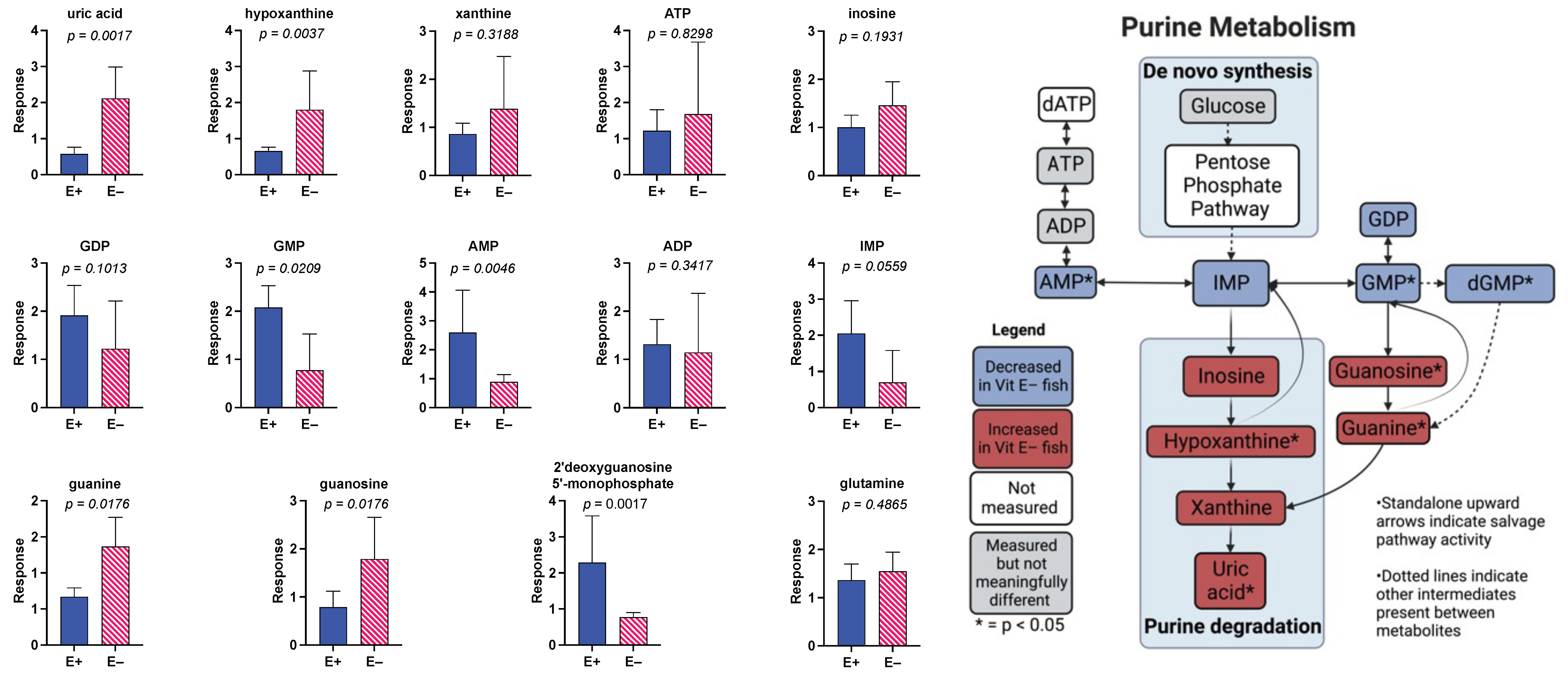
3.4. Effect of 18 Months of Vitamin E Deficiency of Muscle Metabolites
3.5. Effect of Prolonged Vitamin E Deficiency on Muscle Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [Green Version]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padron-Monedero, A.; Damian, J.; Pilar Martin, M.; Fernandez-Cuenca, R. Mortality trends for accidental falls in older people in Spain, 2000–2015. BMC Geriatr. 2017, 17, 276. [Google Scholar] [CrossRef] [Green Version]
- Terroso, M.; Rosa, N.; Torres Marques, A.; Simoes, R. Physical consequences of falls in the elderly: A literature review from 1995 to 2010. Eur. Rev. Aging Phys. Act. 2013, 11, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Ble, A.; Cherubini, A.; Volpato, S.; Bartali, B.; Walston, J.D.; Windham, B.G.; Bandinelli, S.; Lauretani, F.; Guralnik, J.M.; Ferrucci, L. Lower plasma vitamin E levels are associated with the frailty syndrome: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, A.A.; Hayhoe, R.P.G.; Luben, R.N.; Welch, A.A. Positive Associations of Dietary Intake and Plasma Concentrations of Vitamin E with Skeletal Muscle Mass, Heel Bone Ultrasound Attenuation and Fracture Risk in the EPIC-Norfolk Cohort. Antioxidants 2021, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Iidaka, T.; Horii, C.; Muraki, S.; Oka, H.; Nakamura, K.; Izumo, T.; Rogi, T.; Shibata, H.; Tanaka, S.; et al. Dietary Intake of Vitamin E and Fats Associated with Sarcopenia in Community-Dwelling Older Japanese People: A Cross-Sectional Study from the Fifth Survey of the ROAD Study. Nutrients 2021, 13, 1730. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Weinstein, S.J.; Yu, K.; Mannisto, S.; Albanes, D. Relationship between Serum Alpha-Tocopherol and Overall and Cause-Specific Mortality. Circ. Res. 2019, 125, 29–40. [Google Scholar] [CrossRef]
- Machlin, L.J.; Filipski, R.; Nelson, J.; Horn, L.R.; Brin, M. Effects of a prolonged vitamin E deficiency in the rat. J. Nutr. 1977, 107, 1200–1208. [Google Scholar] [CrossRef]
- Thomas, P.K.; Cooper, J.M.; King, R.H.; Workman, J.M.; Schapira, A.H.; Goss-Sampson, M.A.; Muller, D.P. Myopathy in vitamin E deficient rats: Muscle fibre necrosis associated with disturbances of mitochondrial function. J. Anat. 1993, 183 Pt 3, 451–461. [Google Scholar] [PubMed]
- Lebold, K.M.; Lohr, C.V.; Barton, C.L.; Miller, G.W.; Labut, E.M.; Tanguay, R.L.; Traber, M.G. Chronic vitamin E deficiency promotes vitamin C deficiency in zebrafish leading to degenerative myopathy and impaired swimming behavior. Comp. Biochem. Physiol. C Toxicol. Pharm. 2013, 157, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Daya, A.; Donaka, R.; Karasik, D. Zebrafish models of sarcopenia. Dis. Model. Mech. 2020, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, M.; Jagadeeswaran, P. Annual fish as a genetic model for aging. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 101–107. [Google Scholar] [CrossRef] [Green Version]
- McDougall, M.; Choi, J.; Kim, H.K.; Bobe, G.; Stevens, J.F.; Cadenas, E.; Tanguay, R.; Traber, M.G. Lethal dysregulation of energy metabolism during embryonic vitamin E deficiency. Free Radic. Biol. Med. 2017, 104, 324–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDougall, M.; Choi, J.; Kim, H.K.; Bobe, G.; Stevens, J.F.; Cadenas, E.; Tanguay, R.; Traber, M.G. Lipid quantitation and metabolomics data from vitamin E-deficient and -sufficient zebrafish embryos from 0 to 120 hours-post-fertilization. Data Brief 2017, 11, 432–441. [Google Scholar] [CrossRef]
- Zhang, J.; Head, B.; Leonard, S.W.; Choi, J.; Tanguay, R.L.; Traber, M.G. Vitamin E deficiency dysregulates thiols, amino acids and related molecules during zebrafish embryogenesis. Redox Biol. 2021, 38, 101784. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Leonard, S.W.; Kasper, K.; McDougall, M.; Stevens, J.F.; Tanguay, R.L.; Traber, M.G. Novel function of vitamin E in regulation of zebrafish (Danio rerio) brain lysophospholipids discovered using lipidomics. J. Lipid Res. 2015, 56, 1182–1190. [Google Scholar] [CrossRef] [Green Version]
- McDougall, M.; Choi, J.; Magnusson, K.; Truong, L.; Tanguay, R.; Traber, M.G. Chronic vitamin E deficiency impairs cognitive function in adult zebrafish via dysregulation of brain lipids and energy metabolism. Free Radic. Biol. Med. 2017, 112, 308–317. [Google Scholar] [CrossRef]
- Miller, G.W.; Labut, E.M.; Lebold, K.M.; Floeter, A.; Tanguay, R.L.; Traber, M.G. Zebrafish (Danio rerio) fed vitamin E-deficient diets produce embryos with increased morphologic abnormalities and mortality. J. Nutr. Biochem. 2012, 23, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Lebold, K.M.; Jump, D.B.; Miller, G.W.; Wright, C.L.; Labut, E.M.; Barton, C.L.; Tanguay, R.L.; Traber, M.G. Vitamin E deficiency decreases long-chain PUFA in zebrafish (Danio rerio). J. Nutr. 2011, 141, 2113–2118. [Google Scholar] [CrossRef] [Green Version]
- Watt, A.T.; Head, B.; Leonard, S.W.; Tanguay, R.L.; Traber, M.G. Gene Expression of CRAL_TRIO Family Proteins modulated by Vitamin E Deficiency in Zebrafish (Danio rerio). J. Nutr. Biochem. 2021, 97, 108801. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- McDougall, M.Q.; Choi, J.; Stevens, J.F.; Truong, L.; Tanguay, R.L.; Traber, M.G. Lipidomics and H2(18)O labeling techniques reveal increased remodeling of DHA-containing membrane phospholipids associated with abnormal locomotor responses in alpha-tocopherol deficient zebrafish (Danio rerio) embryos. Redox Biol. 2016, 8, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Head, B.; Traber, M.G. Expanding role of vitamin E in protection against metabolic dysregulation: Insights gained from model systems, especially the developing nervous system of zebrafish embryos. Free Radic. Biol. Med. 2021, 176, 80–91. [Google Scholar] [CrossRef]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protti, A.; Carre, J.; Frost, M.T.; Taylor, V.; Stidwill, R.; Rudiger, A.; Singer, M. Succinate recovers mitochondrial oxygen consumption in septic rat skeletal muscle. Crit. Care Med. 2007, 35, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Veledo, S.; Ceperuelo-Mallafre, V.; Vendrell, J. Rethinking succinate: An unexpected hormone-like metabolite in energy homeostasis. Trends Endocrinol. Metab. 2021, 32, 680–692. [Google Scholar] [CrossRef]
- Vance, D.E. Physiological roles of phosphatidylethanolamine N-methyltransferase. Biochim. Biophys. Acta 2013, 1831, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Vendelbo, M.H.; Moller, A.B.; Christensen, B.; Nellemann, B.; Clasen, B.F.; Nair, K.S.; Jorgensen, J.O.; Jessen, N.; Moller, N. Fasting increases human skeletal muscle net phenylalanine release and this is associated with decreased mTOR signaling. PLoS ONE 2014, 9, e102031. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.E.; Won, C.W.; Kim, M. Metabolomic profiles to explore biomarkers of severe sarcopenia in older men: A pilot study. Exp. Gerontol. 2022, 167, 111924. [Google Scholar] [CrossRef] [PubMed]
- Czibik, G.; Mezdari, Z.; Murat Altintas, D.; Brehat, J.; Pini, M.; d’Humieres, T.; Delmont, T.; Radu, C.; Breau, M.; Liang, H.; et al. Dysregulated Phenylalanine Catabolism Plays a Key Role in the Trajectory of Cardiac Aging. Circulation 2021, 144, 559–574. [Google Scholar] [CrossRef]
- Kouchiwa, T.; Wada, K.; Uchiyama, M.; Kasezawa, N.; Niisato, M.; Murakami, H.; Fukuyama, K.; Yokogoshi, H. Age-related changes in serum amino acids concentrations in healthy individuals. Clin. Chem. Lab. Med. 2012, 50, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Bancel, E.; Strubel, D.; Bellet, H.; Polge, A.; Peray, P.; Magnan de Bornier, B. Effect of the age and the sex on plasma concentration of amino acids. Ann. Biol. Clin. 1994, 52, 667–670. [Google Scholar]
- Forman, H.J. Glutathione—From antioxidant to post-translational modifier. Arch. Biochem. Biophys. 2016, 595, 64–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.W.; Lee, C.C.; Muzny, D.M.; Caskey, C.T. Urate oxidase: Primary structure and evolutionary implications. Proc. Natl. Acad. Sci. USA 1989, 86, 9412–9416. [Google Scholar] [CrossRef] [Green Version]
- KEGG PATHWAY: Purine Metabolism—Danio Rerio (Zebrafish). Available online: https://www.genome.jp/kegg-bin/show_pathway?dre00230 (accessed on 24 May 2023).
- Xu, M.; Ye, J.; Wang, Y.; Chu, K.; Pere, M.; Xu, M.; Tang, X.; Fu, J. Vitamin E performs antioxidant effect via PAP retrograde signaling pathway in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022, 127, 918–924. [Google Scholar] [CrossRef]
- Young, J.M.; Dinning, J.S. A relationship of vitamin E to nucleic acid metabolism. J. Biol. Chem. 1951, 193, 743–747. [Google Scholar] [CrossRef]
- Dinning, J.S.; Day, P.L. Vitamin E deficiency in the monkey. I. Muscular dystrophy, hematologic changes, and the excretion of urinary nitrogenous constituents. J. Exp. Med. 1957, 105, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Czerska, M.; Mikolajewska, K.; Zielinski, M.; Gromadzinska, J.; Wasowicz, W. Today’s oxidative stress markers. Med. Pr. 2015, 66, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.R.; Toews, C.J.; Ward, G.R.; Fox, I.H. Purine metabolism during strenuous muscular exercise in man. Metabolism 1980, 29, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Kirkwood, J.S.; Lebold, K.M.; Miranda, C.L.; Wright, C.L.; Miller, G.W.; Tanguay, R.L.; Barton, C.L.; Traber, M.G.; Stevens, J.F. Vitamin C deficiency activates the purine nucleotide cycle in zebrafish. J. Biol. Chem. 2012, 287, 3833–3841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [Green Version]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass. Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [Green Version]
- McDougall, M.; Choi, J.; Truong, L.; Tanguay, R.; Traber, M.G. Vitamin E deficiency during embryogenesis in zebrafish causes lasting metabolic and cognitive impairments despite refeeding adequate diets. Free Radic. Biol. Med. 2017, 110, 250–260. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, T.D.; Choi, J.; Leonard, S.W.; Head, B.; Tanguay, R.L.; Barton, C.L.; Traber, M.G. Chronic Vitamin E Deficiency Dysregulates Purine, Phospholipid, and Amino Acid Metabolism in Aging Zebrafish Skeletal Muscle. Antioxidants 2023, 12, 1160. https://doi.org/10.3390/antiox12061160
Henderson TD, Choi J, Leonard SW, Head B, Tanguay RL, Barton CL, Traber MG. Chronic Vitamin E Deficiency Dysregulates Purine, Phospholipid, and Amino Acid Metabolism in Aging Zebrafish Skeletal Muscle. Antioxidants. 2023; 12(6):1160. https://doi.org/10.3390/antiox12061160
Chicago/Turabian StyleHenderson, Trent D., Jaewoo Choi, Scott W. Leonard, Brian Head, Robyn L. Tanguay, Carrie L. Barton, and Maret G. Traber. 2023. "Chronic Vitamin E Deficiency Dysregulates Purine, Phospholipid, and Amino Acid Metabolism in Aging Zebrafish Skeletal Muscle" Antioxidants 12, no. 6: 1160. https://doi.org/10.3390/antiox12061160
APA StyleHenderson, T. D., Choi, J., Leonard, S. W., Head, B., Tanguay, R. L., Barton, C. L., & Traber, M. G. (2023). Chronic Vitamin E Deficiency Dysregulates Purine, Phospholipid, and Amino Acid Metabolism in Aging Zebrafish Skeletal Muscle. Antioxidants, 12(6), 1160. https://doi.org/10.3390/antiox12061160