From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk between Adipose Tissue and Metabolically Active Organs
Abstract
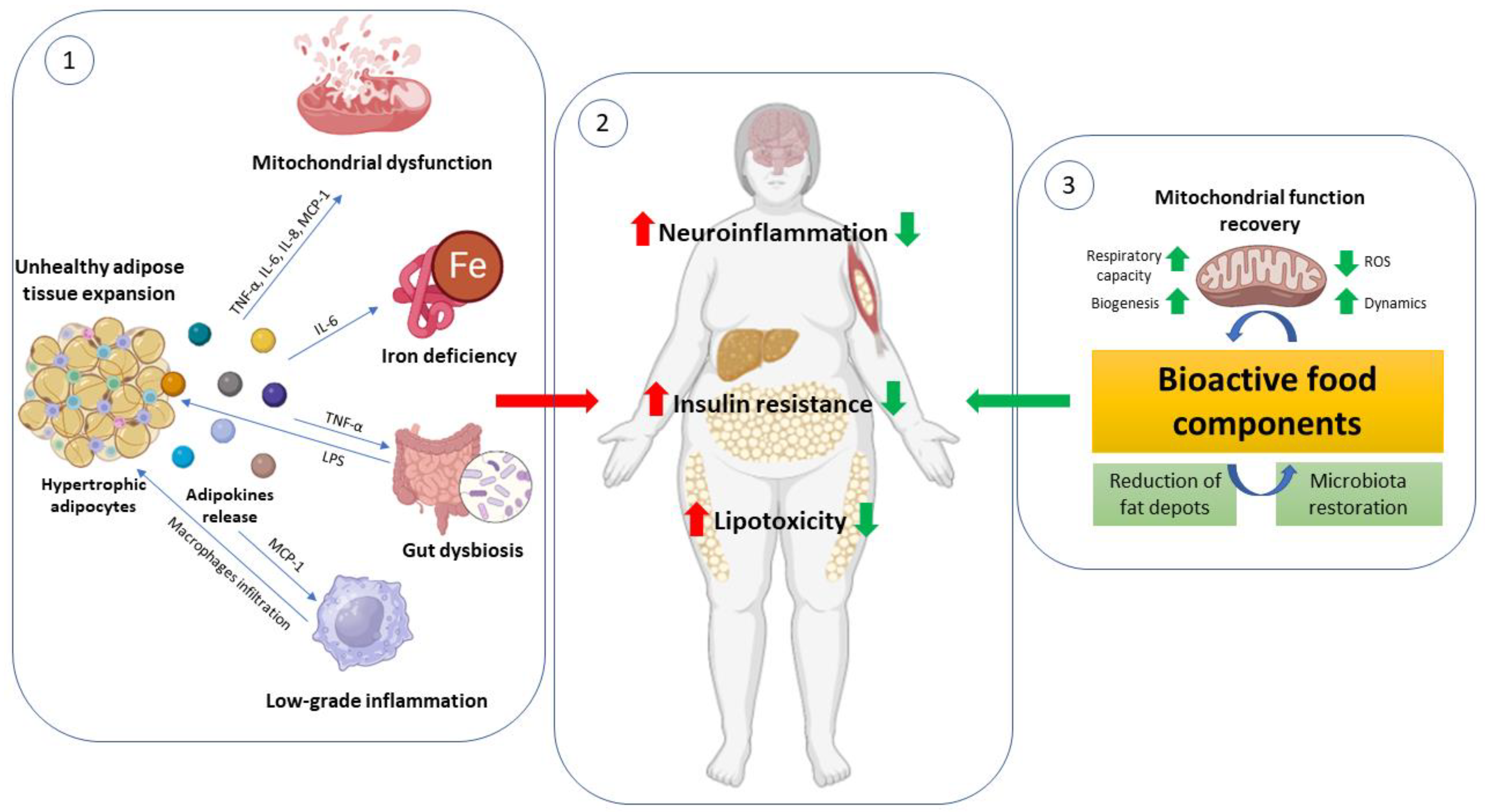
1. Introduction
2. Adipose Tissue: From Healthy to Pathologies
2.1. Adipose Tissues: Types and Functions
2.2. The Adipose Tissue Remodeling and the Unhealthy Hypertrophic Adipocyte
2.3. Adipocytes Dys (Function): Mitochondria Role
3. Peripheral Metabolic Complications Related to Adipose Tissue Dysfunction: Focus on Metabolic Flexibility and Mitochondrial Impairment
3.1. Adipose Tissue—Liver Crosstalk
3.2. Adipose Tissue—Skeletal Muscle Crosstalk
3.3. Adipose Tissue—Cardiac Muscle Crosstalk
3.4. Adipose Tissue—Brain Crosstalk
3.5. Adipose Tissue and Iron Deficiency during Obesity
4. Bioactive Food Components as a Target to Counteract Adipose Tissue Failure and Mitochondrial Dysfunction
4.1. Polyphenols
4.2. Fatty Acids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, C.D.; Lone, N.I.; Baillie, J.K. Comorbidities, Multimorbidity and COVID-19. Nat. Med. 2023, 29, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Chenchula, S.; Vidyasagar, K.; Pathan, S.; Sharma, S.; Chavan, M.R.; Bhagavathula, A.S.; Padmavathi, R.; Manjula, M.; Chhabra, M.; Gupta, R.; et al. Global Prevalence and Effect of Comorbidities and Smoking Status on Severity and Mortality of COVID-19 in Association with Age and Gender: A Systematic Review, Meta-Analysis and Meta-Regression. Sci. Rep. 2023, 13, 6415. [Google Scholar] [CrossRef] [PubMed]
- Sell, H.; Habich, C.; Eckel, J. Adaptive Immunity in Obesity and Insulin Resistance. Nat. Rev. Endocrinol. 2012, 8, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Kiani, L. Obesity-Associated Immune Exhaustion Accelerates AD Pathology. Nat. Rev. Neurol. 2023, 19, 257. [Google Scholar] [CrossRef]
- Paulo, E.; Wu, D.; Wang, Y.; Zhang, Y.; Wu, Y.; Swaney, D.L.; Soucheray, M.; Jimenez-Morales, D.; Chawla, A.; Krogan, N.J.; et al. Sympathetic Inputs Regulate Adaptive Thermogenesis in Brown Adipose Tissue through CAMP-Salt Inducible Kinase Axis. Sci. Rep. 2018, 8, 11001. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Kim, J.B. Dynamic Cross Talk between Metabolic Organs in Obesity and Metabolic Diseases. Exp. Mol. Med. 2016, 48, e214. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Javadov, S.; Kozlov, A.V.; Camara, A.K.S. Mitochondria in Health and Diseases. Cells 2020, 9, 1177. [Google Scholar] [CrossRef]
- Woo, C.-Y.; Jang, J.E.; Lee, S.E.; Koh, E.H.; Lee, K.-U. Mitochondrial Dysfunction in Adipocytes as a Primary Cause of Adipose Tissue Inflammation. Diabetes Metab. J. 2019, 43, 247. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.H.; Park, J.-Y.; Park, H.-S.; Jeon, M.J.; Ryu, J.W.; Kim, M.; Kim, S.Y.; Kim, M.-S.; Kim, S.-W.; Park, I.S.; et al. Essential Role of Mitochondrial Function in Adiponectin Synthesis in Adipocytes. Diabetes 2007, 56, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, R.; Pierce, N.; Koppe, S. Obesity and Nonalcoholic Fatty Liver Disease: Current Perspectives. Diabetes Metab. Syndr. Obes. 2018, 11, 533–542. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms Linking Obesity with Cardiovascular Disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef]
- Mazon, J.N.; de Mello, A.H.; Ferreira, G.K.; Rezin, G.T. The Impact of Obesity on Neurodegenerative Diseases. Life Sci. 2017, 182, 22–28. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. State of the Art Paper Biochemistry of Adipose Tissue: An Endocrine Organ. Arch. Med. Sci. 2013, 2, 191–200. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Giralt, M.; Villarroya, F. White, Brown, Beige/Brite: Different Adipose Cells for Different Functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef]
- Hausman, D.B.; DiGirolamo, M.; Bartness, T.J.; Hausman, G.J.; Martin, R.J. The Biology of White Adipocyte Proliferation. Obes. Rev. 2001, 2, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Deveaud, C.; Beauvoit, B.; Salin, B.; Schaeffer, J.; Rigoulet, M. Regional Differences in Oxidative Capacity of Rat White Adipose Tissue Are Linked to the Mitochondrial Content of Mature Adipocytes. Mol. Cell Biochem. 2004, 267, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, B.; Burri, L.; Staalesen, V.; Skorve, J.; Berge, R.K. Different Adipose Depots: Their Role in the Development of Metabolic Syndrome and Mitochondrial Response to Hypolipidemic Agents. J. Obes. 2011, 2011, 490650. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D. Role of Body Fat Distribution and the Metabolic Complications of Obesity. J. Clin. Endocrinol. Metab. 2008, 93, s57–s63. [Google Scholar] [CrossRef]
- Zhang, F.; Hao, G.; Shao, M.; Nham, K.; An, Y.; Wang, Q.; Zhu, Y.; Kusminski, C.M.; Hassan, G.; Gupta, R.K.; et al. An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab. 2018, 27, 252–262.e3. [Google Scholar] [CrossRef]
- Bahler, L.; Molenaars, R.J.; Verberne, H.J.; Holleman, F. Role of the Autonomic Nervous System in Activation of Human Brown Adipose Tissue: A Review of the Literature. Diabetes Metab. 2015, 41, 437–445. [Google Scholar] [CrossRef]
- Oelkrug, R.; Polymeropoulos, E.T.; Jastroch, M. Brown Adipose Tissue: Physiological Function and Evolutionary Significance. J. Comp. Physiol. B 2015, 185, 587–606. [Google Scholar] [CrossRef]
- Lowell, B.B.; S-Susulic, V.; Hamann, A.; Lawitts, J.A.; Himms-Hagen, J.; Boyer, B.B.; Kozak, L.P.; Flier, J.S. Development of Obesity in Transgenic Mice after Genetic Ablation of Brown Adipose Tissue. Nature 1993, 366, 740–742. [Google Scholar] [CrossRef]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.-E.; Kozak, L.P. Mice Lacking Mitochondrial Uncoupling Protein Are Cold-Sensitive but Not Obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Waldén, T.B.; Hansen, I.R.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Recruited vs. Nonrecruited Molecular Signatures of Brown, “Brite,” and White Adipose Tissues. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E19–E31. [Google Scholar] [CrossRef]
- Shao, M.; Wang, Q.A.; Song, A.; Vishvanath, L.; Busbuso, N.C.; Scherer, P.E.; Gupta, R.K. Cellular Origins of Beige Fat Cells Revisited. Diabetes 2019, 68, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Ishibashi, J.; Kusminski, C.M.; Wang, Q.A.; Hepler, C.; Vishvanath, L.; MacPherson, K.A.; Spurgin, S.B.; Sun, K.; Holland, W.L.; et al. Zfp423 Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program. Cell Metab. 2016, 23, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Vegiopoulos, A.; Rohm, M.; Herzig, S. Adipose Tissue: Between the Extremes. EMBO J. 2017, 36, 1999–2017. [Google Scholar] [CrossRef]
- Rasouli, N.; Molavi, B.; Elbein, S.C.; Kern, P.A. Ectopic Fat Accumulation and Metabolic Syndrome. Diabetes Obes. Metab. 2007, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of Fat Cell Turnover in Humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Sanjabi, B.; Dashty, M.; Özcan, B.; Akbarkhanzadeh, V.; Rahimi, M.; Vinciguerra, M.; van Rooij, F.; Al-Lahham, S.; Sheedfar, F.; van Kooten, T.G.; et al. Lipid Droplets Hypertrophy: A Crucial Determining Factor in Insulin Regulation by Adipocytes. Sci. Rep. 2015, 5, 8816. [Google Scholar] [CrossRef]
- Cotillard, A.; Poitou, C.; Torcivia, A.; Bouillot, J.-L.; Dietrich, A.; Klöting, N.; Grégoire, C.; Lolmede, K.; Blüher, M.; Clément, K. Adipocyte Size Threshold Matters: Link with Risk of Type 2 Diabetes and Improved Insulin Resistance After Gastric Bypass. J. Clin. Endocrinol. Metab. 2014, 99, E1466–E1470. [Google Scholar] [CrossRef]
- Lönn, M.; Mehlig, K.; Bengtsson, C.; Lissner, L. Adipocyte Size Predicts Incidence of Type 2 Diabetes in Women. FASEB J. 2010, 24, 326–331. [Google Scholar] [CrossRef]
- Gustafson, B.; Gogg, S.; Hedjazifar, S.; Jenndahl, L.; Hammarstedt, A.; Smith, U. Inflammation and Impaired Adipogenesis in Hypertrophic Obesity in Man. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E999–E1003. [Google Scholar] [CrossRef]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Wueest, S.; Rapold, R.A.; Rytka, J.M.; Schoenle, E.J.; Konrad, D. Basal Lipolysis, Not the Degree of Insulin Resistance, Differentiates Large from Small Isolated Adipocytes in High-Fat Fed Mice. Diabetologia 2009, 52, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Jurga, L.; van Vanessa, H.; Elisabet, A.N.; Andrea, D.; Lennart, B.; Erik, N.; Dominique, L.; Peter, A.; Mikael, R. NF-ΚB Is Important for TNF-α-Induced Lipolysis in Human Adipocytes. J. Lipid Res. 2007, 48, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Mollica, M.P.; Lombardi, A.; Cavaliere, G.; Gifuni, G.; Barletta, A. From Chronic Overnutrition to Insulin Resistance: The Role of Fat-Storing Capacity and Inflammation. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 146–152. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 Links Innate Immunity and Fatty Acid–Induced Insulin Resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Muir, L.A.; Neeley, C.K.; Meyer, K.A.; Baker, N.A.; Brosius, A.M.; Washabaugh, A.R.; Varban, O.A.; Finks, J.F.; Zamarron, B.F.; Flesher, C.G.; et al. Adipose Tissue Fibrosis, Hypertrophy, and Hyperplasia: Correlations with Diabetes in Human Obesity. Obesity 2016, 24, 597–605. [Google Scholar] [CrossRef]
- Haczeyni, F.; Bell-Anderson, K.S.; Farrell, G.C. Causes and Mechanisms of Adipocyte Enlargement and Adipose Expansion. Obes. Rev. 2018, 19, 406–420. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Scherer, P.E. Mitochondrial Dysfunction in White Adipose Tissue. Trends Endocrinol. Metab. 2012, 23, 435–443. [Google Scholar] [CrossRef]
- Kopecký, J.; Rossmeisl, M.; Flachs, P.; Bardová, K.; Brauner, P. Mitochondrial Uncoupling and Lipid Metabolism in Adipocytes. Biochem. Soc. Trans. 2001, 29, 791–797. [Google Scholar] [CrossRef]
- Boudina, S.; Graham, T.E. Mitochondrial Function/Dysfunction in White Adipose Tissue. Exp. Physiol. 2014, 99, 1168–1178. [Google Scholar] [CrossRef]
- Duchen, M.R. Mitochondria in Health and Disease: Perspectives on a New Mitochondrial Biology. Mol. Asp. Med. 2004, 25, 365–451. [Google Scholar] [CrossRef]
- Wilson-Fritch, L.; Nicoloro, S.; Chouinard, M.; Lazar, M.A.; Chui, P.C.; Leszyk, J.; Straubhaar, J.; Czech, M.P.; Corvera, S. Mitochondrial Remodeling in Adipose Tissue Associated with Obesity and Treatment with Rosiglitazone. J. Clin. Investig. 2004, 114, 1281–1289. [Google Scholar] [CrossRef]
- Wilson-Fritch, L.; Burkart, A.; Bell, G.; Mendelson, K.; Leszyk, J.; Nicoloro, S.; Czech, M.; Corvera, S. Mitochondrial Biogenesis and Remodeling during Adipogenesis and in Response to the Insulin Sensitizer Rosiglitazone. Mol. Cell Biol. 2003, 23, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.X.; Qiu, Y.; Hansen, M.K.; Zhu, L.; Zhang, V.; Xie, M.; Okamoto, Y.; Mattie, M.D.; Higashiyama, H.; Asano, S.; et al. Adipose Mitochondrial Biogenesis Is Suppressed in Db/Db and High-Fat Diet–Fed Mice and Improved by Rosiglitazone. Diabetes 2007, 56, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Pietiläinen, K.H.; Naukkarinen, J.; Rissanen, A.; Saharinen, J.; Ellonen, P.; Keränen, H.; Suomalainen, A.; Götz, A.; Suortti, T.; Yki-Järvinen, H.; et al. Global Transcript Profiles of Fat in Monozygotic Twins Discordant for BMI: Pathways behind Acquired Obesity. PLoS Med. 2008, 5, e51. [Google Scholar] [CrossRef]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef]
- Kaaman, M.; Sparks, L.M.; van Harmelen, V.; Smith, S.R.; Sjölin, E.; Dahlman, I.; Arner, P. Strong Association between Mitochondrial DNA Copy Number and Lipogenesis in Human White Adipose Tissue. Diabetologia 2007, 50, 2526–2533. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; GuhaThakurta, I.; Behera, P.; Ranjan, K.R.; Khanna, M.; Mukhopadhyay, S.; Chakrabarti, S. Mitochondrial Bioenergetics Is Not Impaired in Nonobese Subjects with Type 2 Diabetes Mellitus. Metabolism 2011, 60, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Vernochet, C.; Mourier, A.; Bezy, O.; Macotela, Y.; Boucher, J.; Rardin, M.J.; An, D.; Lee, K.Y.; Ilkayeva, O.R.; Zingaretti, C.M.; et al. Adipose-Specific Deletion of TFAM Increases Mitochondrial Oxidation and Protects Mice against Obesity and Insulin Resistance. Cell Metab. 2012, 16, 765–776. [Google Scholar] [CrossRef]
- Valerio, A. TNF- Downregulates ENOS Expression and Mitochondrial Biogenesis in Fat and Muscle of Obese Rodents. J. Clin. Investig. 2006, 116, 2791–2798. [Google Scholar] [CrossRef]
- Carrière, A.; Fernandez, Y.; Rigoulet, M.; Pénicaud, L.; Casteilla, L. Inhibition of Preadipocyte Proliferation by Mitochondrial Reactive Oxygen Species. FEBS Lett. 2003, 550, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Grune, T.; Speckmann, B. The Two Faces of Reactive Oxygen Species (ROS) in Adipocyte Function and Dysfunction. Biol. Chem. 2016, 397, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Baldini, F.; Fabbri, R.; Eberhagen, C.; Voci, A.; Portincasa, P.; Zischka, H.; Vergani, L. Adipocyte Hypertrophy Parallels Alterations of Mitochondrial Status in a Cell Model for Adipose Tissue Dysfunction in Obesity. Life Sci. 2021, 265, 118812. [Google Scholar] [CrossRef] [PubMed]
- Honecker, J.; Weidlich, D.; Heisz, S.; Lindgren, C.M.; Karampinos, D.C.; Claussnitzer, M.; Hauner, H. A Distribution-Centered Approach for Analyzing Human Adipocyte Size Estimates and Their Association with Obesity-Related Traits and Mitochondrial Function. Int. J. Obes. 2021, 45, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, J.; Zhou, H. Macrophage and Adipocyte Mitochondrial Dysfunction in Obesity-Induced Metabolic Diseases. World J. Men’s Health 2021, 39, 606. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Corpeleijn, E.; Saris, W.H.M.; Blaak, E.E. Metabolic Flexibility in the Development of Insulin Resistance and Type 2 Diabetes: Effects of Lifestyle. Obes. Rev. 2009, 10, 178–193. [Google Scholar] [CrossRef]
- FANTUZZI, G. Adipose Tissue, Adipokines, and Inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Sparks, L.M.; Ukropcova, B.; Smith, J.; Pasarica, M.; Hymel, D.; Xie, H.; Bray, G.A.; Miles, J.M.; Smith, S.R. Relation of Adipose Tissue to Metabolic Flexibility. Diabetes Res. Clin Pr. 2009, 83, 32–43. [Google Scholar] [CrossRef]
- Arner, P.; Kulyté, A. MicroRNA Regulatory Networks in Human Adipose Tissue and Obesity. Nat. Rev. Endocrinol. 2015, 11, 276–288. [Google Scholar] [CrossRef]
- Ukropcova, B.; McNeil, M.; Sereda, O.; de Jonge, L.; Xie, H.; Bray, G.A.; Smith, S.R. Dynamic Changes in Fat Oxidation in Human Primary Myocytes Mirror Metabolic Characteristics of the Donor. J. Clin. Investig. 2005, 115, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Ritov, V.B.; Menshikova, E.V.; He, J.; Ferrell, R.E.; Goodpaster, B.H.; Kelley, D.E. Deficiency of Subsarcolemmal Mitochondria in Obesity and Type 2 Diabetes. Diabetes 2005, 54, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Van de Weijer, T.; Sparks, L.M.; Phielix, E.; Meex, R.C.; van Herpen, N.A.; Hesselink, M.K.C.; Schrauwen, P.; Schrauwen-Hinderling, V.B. Relationships between Mitochondrial Function and Metabolic Flexibility in Type 2 Diabetes Mellitus. PLoS ONE 2013, 8, e51648. [Google Scholar] [CrossRef] [PubMed]
- Guo, S. Insulin Signaling, Resistance, and Metabolic Syndrome: Insights from Mouse Models into Disease Mechanisms. J. Endocrinol. 2014, 220, T1–T23. [Google Scholar] [CrossRef]
- Cavaliere, G.; Catapano, A.; Trinchese, G.; Cimmino, F.; Menale, C.; Petrella, L.; Mollica, M.P. Crosstalk between Adipose Tissue and Hepatic Mitochondria in the Development of the Inflammation and Liver Injury during Ageing in High-Fat Diet Fed Rats. Int. J. Mol. Sci. 2023, 24, 2967. [Google Scholar] [CrossRef]
- Wakil, S.J.; Abu-Elheiga, L.A. Fatty Acid Metabolism: Target for Metabolic Syndrome. J. Lipid Res. 2009, 50, S138–S143. [Google Scholar] [CrossRef]
- Kumashiro, N.; Erion, D.M.; Zhang, D.; Kahn, M.; Beddow, S.A.; Chu, X.; Still, C.D.; Gerhard, G.S.; Han, X.; Dziura, J.; et al. Cellular Mechanism of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Proc. Natl. Acad. Sci. USA 2011, 108, 16381–16385. [Google Scholar] [CrossRef]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The Role of Hepatic Lipids in Hepatic Insulin Resistance and Type 2 Diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Chavez, J.A.; Knotts, T.A.; Wang, L.-P.; Li, G.; Dobrowsky, R.T.; Florant, G.L.; Summers, S.A. A Role for Ceramide, but Not Diacylglycerol, in the Antagonism of Insulin Signal Transduction by Saturated Fatty Acids. J. Biol. Chem. 2003, 278, 10297–10303. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of Hepatic Mitochondrial Function in Humans with Non-Alcoholic Fatty Liver Is Lost in Steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Steatohepatitis. Free. Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.-Y.; Watt, M.J.; Rensen, S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial Dysfunction-Related Lipid Changes Occur in Nonalcoholic Fatty Liver Disease Progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.P.M.S.; Coelho, A.M.M.; Barbeiro, H.V.; Lima, V.M.R.; Soriano, F.; Ribeiro, C.; Molan, N.A.T.; Alves, V.A.F.; Souza, H.P.; Machado, M.C.C.; et al. Liver Mitochondrial Dysfunction and Oxidative Stress in the Pathogenesis of Experimental Nonalcoholic Fatty Liver Disease. Braz. J. Med. Biol. Res. 2006, 39, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Mollica, M.P.; Lionetti, L.; Putti, R.; Cavaliere, G.; Gaita, M.; Barletta, A. From Chronic Overfeeding to Hepatic Injury: Role of Endoplasmic Reticulum Stress and Inflammation. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 222–230. [Google Scholar] [CrossRef]
- Chavez, J.A.; Summers, S.A. Characterizing the Effects of Saturated Fatty Acids on Insulin Signaling and Ceramide and Diacylglycerol Accumulation in 3T3-L1 Adipocytes and C2C12 Myotubes. Arch. Biochem. Biophys. 2003, 419, 101–109. [Google Scholar] [CrossRef]
- Itani, S.I.; Ruderman, N.B.; Schmieder, F.; Boden, G. Lipid-Induced Insulin Resistance in Human Muscle Is Associated with Changes in Diacylglycerol, Protein Kinase C, and IκB-α. Diabetes 2002, 51, 2005–2011. [Google Scholar] [CrossRef]
- Montell, E.; Turini, M.; Marotta, M.; Roberts, M.; Noé, V.; Ciudad, C.J.; Macé, K.; Gómez-Foix, A.M. DAG Accumulation from Saturated Fatty Acids Desensitizes Insulin Stimulation of Glucose Uptake in Muscle Cells. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E229–E237. [Google Scholar] [CrossRef]
- Kraegen, E.; Cooney, G.; Ye, J.-M.; Thompson, A.; Furler, S. The Role of Lipids in the Pathogenesis of Muscle Insulin Resistance and Beta Cell Failure in Type II Diabetes and Obesity. Exp. Clin. Endocrinol. Diabetes 2001, 109, S189–S201. [Google Scholar] [CrossRef]
- Yu, C.; Chen, Y.; Cline, G.W.; Zhang, D.; Zong, H.; Wang, Y.; Bergeron, R.; Kim, J.K.; Cushman, S.W.; Cooney, G.J.; et al. Mechanism by Which Fatty Acids Inhibit Insulin Activation of Insulin Receptor Substrate-1 (IRS-1)-Associated Phosphatidylinositol 3-Kinase Activity in Muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef]
- Kim, J.K.; Fillmore, J.J.; Sunshine, M.J.; Albrecht, B.; Higashimori, T.; Kim, D.-W.; Liu, Z.-X.; Soos, T.J.; Cline, G.W.; O’Brien, W.R.; et al. PKC-θ Knockout Mice Are Protected from Fat-Induced Insulin Resistance. J. Clin. Investig. 2004, 114, 823–827. [Google Scholar] [CrossRef]
- Senn, J.J. Toll-like Receptor-2 Is Essential for the Development of Palmitate-Induced Insulin Resistance in Myotubes. J. Biol. Chem. 2006, 281, 26865–26875. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and Endoplasmic Reticulum Stress in Obesity and Diabetes. Int. J. Obes. 2008, 32, S52–S54. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.K.; Kashyap, S.; Bajaj, M.; Cusi, K.; Mandarino, S.J.; Finlayson, J.; DeFronzo, R.A.; Jenkinson, C.P.; Mandarino, L.J. Lipid Infusion Decreases the Expression of Nuclear Encoded Mitochondrial Genes and Increases the Expression of Extracellular Matrix Genes in Human Skeletal Muscle. J. Biol. Chem. 2005, 280, 10290–10297. [Google Scholar] [CrossRef]
- Sparks, L.M.; Xie, H.; Koza, R.A.; Mynatt, R.; Hulver, M.W.; Bray, G.A.; Smith, S.R. A High-Fat Diet Coordinately Downregulates Genes Required for Mitochondrial Oxidative Phosphorylation in Skeletal Muscle. Diabetes 2005, 54, 1926–1933. [Google Scholar] [CrossRef]
- Reznick, R.M.; Shulman, G.I. The Role of AMP-Activated Protein Kinase in Mitochondrial Biogenesis. J. Physiol. 2006, 574, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Möhlig, M.; Isken, F.; Ristow, M. Impaired Mitochondrial Activity and Insulin-Resistant Offspring of Patients with Type 2 Diabetes. N. Engl. J. Med. 2004, 350, 2419–2421. [Google Scholar] [PubMed]
- Kim, J.-Y.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Houmard, J.A. Lipid Oxidation Is Reduced in Obese Human Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1039–E1044. [Google Scholar] [CrossRef]
- Morino, K.; Petersen, K.F.; Shulman, G.I. Molecular Mechanisms of Insulin Resistance in Humans and Their Potential Links with Mitochondrial Dysfunction. Diabetes 2006, 55, S9–S15. [Google Scholar] [CrossRef]
- Turner, N.; Bruce, C.R.; Beale, S.M.; Hoehn, K.L.; So, T.; Rolph, M.S.; Cooney, G.J. Excess Lipid Availability Increases Mitochondrial Fatty Acid Oxidative Capacity in Muscle. Diabetes 2007, 56, 2085–2092. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef]
- Hearse, D.J. Oxygen Deprivation and Early Myocardial Contractile Failure: A Reassessment of the Possible Role of Adenosine Triphosphate. Am. J. Cardiol. 1979, 44, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Nijtmans, L.G.J.; Henderson, N.S.; Attardi, G.; Holt, I.J. Impaired ATP Synthase Assembly Associated with a Mutation in the Human ATP Synthase Subunit 6 Gene. J. Biol. Chem. 2001, 276, 6755–6762. [Google Scholar] [CrossRef]
- Trinchese, G.; Cimmino, F.; Cavaliere, G.; Rosati, L.; Catapano, A.; Sorriento, D.; Murru, E.; Bernardo, L.; Pagani, L.; Bergamo, P.; et al. Heart Mitochondrial Metabolic Flexibility and Redox Status Are Improved by Donkey and Human Milk Intake. Antioxidants 2021, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Sorriento, D.; Ciccarelli, M.; Del Giudice, C.; Fiordelisi, A.; Napolitano, L.; Trimarco, B.; Iaccarino, G.; Santulli, G. Functional Role of Mitochondria in Arrhythmogenesis. Mitochondrial Dyn. Cardiovasc. Med. 2017, 982, 191–202. [Google Scholar]
- Scheuermann-Freestone, M.; Madsen, P.L.; Manners, D.; Blamire, A.M.; Buckingham, R.E.; Styles, P.; Radda, G.K.; Neubauer, S.; Clarke, K. Abnormal Cardiac and Skeletal Muscle Energy Metabolism in Patients with Type 2 Diabetes. Circulation 2003, 107, 3040–3046. [Google Scholar] [CrossRef]
- Peterson, L.R.; Herrero, P.; Schechtman, K.B.; Racette, S.B.; Waggoner, A.D.; Kisrieva-Ware, Z.; Dence, C.; Klein, S.; Marsala, J.; Meyer, T.; et al. Effect of Obesity and Insulin Resistance on Myocardial Substrate Metabolism and Efficiency in Young Women. Circulation 2004, 109, 2191–2196. [Google Scholar] [CrossRef]
- Sharma, S.; Adrogue, J.V.; Golfman, L.; Uray, I.; Lemm, J.; Youker, K.; Noon, G.P.; Frazier, O.H.; Taegtmeyer, H. Intramyocardial Lipid Accumulation in the Failing Human Heart Resembles the Lipotoxic Rat Heart. FASEB J. 2004, 18, 1692–1700. [Google Scholar] [CrossRef]
- Kuo, T.H.; Moore, K.H.; Giacomelli, F.; Wiener, J. Defective Oxidative Metabolism of Heart Mitochondria from Genetically Diabetic Mice. Diabetes 1983, 32, 781–787. [Google Scholar] [CrossRef]
- Boudina, S.; Sena, S.; O’Neill, B.T.; Tathireddy, P.; Young, M.E.; Abel, E.D. Reduced Mitochondrial Oxidative Capacity and Increased Mitochondrial Uncoupling Impair Myocardial Energetics in Obesity. Circulation 2005, 112, 2686–2695. [Google Scholar] [CrossRef]
- Buchanan, J.; Mazumder, P.K.; Hu, P.; Chakrabarti, G.; Roberts, M.W.; Yun, U.J.; Cooksey, R.C.; Litwin, S.E.; Abel, E.D. Reduced Cardiac Efficiency and Altered Substrate Metabolism Precedes the Onset of Hyperglycemia and Contractile Dysfunction in Two Mouse Models of Insulin Resistance and Obesity. Endocrinology 2005, 146, 5341–5349. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The Mechanisms of Action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Sena, S.; Theobald, H.; Sheng, X.; Wright, J.J.; Hu, X.X.; Aziz, S.; Johnson, J.I.; Bugger, H.; Zaha, V.G.; et al. Mitochondrial Energetics in the Heart in Obesity-Related Diabetes. Diabetes 2007, 56, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- How, O.-J.; Aasum, E.; Severson, D.L.; Chan, W.Y.A.; Essop, M.F.; Larsen, T.S. Increased Myocardial Oxygen Consumption Reduces Cardiac Efficiency in Diabetic Mice. Diabetes 2006, 55, 466–473. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide Activates Mitochondrial Uncoupling Proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial Dysfunction and Oxidative Stress in Heart Disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Calcagno, A.; Granzotto, M.; Calabrese, F.; Thiene, G.; Federspil, G.; Vettor, R. Heart Lipid Accumulation in Obese Non-Diabetic Rats: Effect of Weight Loss. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 189–197. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Kovacs, A.; Ford, D.A.; Hsu, F.-F.; Garcia, R.; Herrero, P.; Saffitz, J.E.; Schaffer, J.E. A Novel Mouse Model of Lipotoxic Cardiomyopathy. J. Clin. Investig. 2001, 107, 813–822. [Google Scholar] [CrossRef]
- Zhou, Y.-T.; Grayburn, P.; Karim, A.; Shimabukuro, M.; Higa, M.; Baetens, D.; Orci, L.; Unger, R.H. Lipotoxic Heart Disease in Obese Rats: Implications for Human Obesity. Proc. Natl. Acad. Sci. USA 2000, 97, 1784–1789. [Google Scholar] [CrossRef]
- Chess, D.J.; Stanley, W.C. Role of Diet and Fuel Overabundance in the Development and Progression of Heart Failure. Cardiovasc. Res. 2008, 79, 269–278. [Google Scholar] [CrossRef]
- Dhahri, W.; Drolet, M.-C.; Roussel, E.; Couet, J.; Arsenault, M. Chronic High-Fat Diet-Induced Obesity Decreased Survival and Increased Hypertrophy of Rats with Experimental Eccentric Hypertrophy from Chronic Aortic Regurgitation. BMC Cardiovasc. Disord. 2014, 14, 123. [Google Scholar] [CrossRef]
- Pedditizi, E.; Peters, R.; Beckett, N. The Risk of Overweight/Obesity in Mid-Life and Late Life for the Development of Dementia: A Systematic Review and Meta-Analysis of Longitudinal Studies. Age Ageing 2016, 45, 14–21. [Google Scholar] [CrossRef]
- Freeman, L.R.; Granholm, A.-C.E. Vascular Changes in Rat Hippocampus Following a High Saturated Fat and Cholesterol Diet. J. Cereb. Blood Flow Metab. 2012, 32, 643–653. [Google Scholar] [CrossRef]
- Davidson, T.L.; Monnot, A.; Neal, A.U.; Martin, A.A.; Horton, J.J.; Zheng, W. The Effects of a High-Energy Diet on Hippocampal-Dependent Discrimination Performance and Blood–Brain Barrier Integrity Differ for Diet-Induced Obese and Diet-Resistant Rats. Physiol. Behav. 2012, 107, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity Is Associated with Hypothalamic Injury in Rodents and Humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, G.; Viggiano, E.; Trinchese, G.; De Filippo, C.; Messina, A.; Monda, V.; Valenzano, A.; Cincione, R.I.; Zammit, C.; Cimmino, F.; et al. Long Feeding High-Fat Diet Induces Hypothalamic Oxidative Stress and Inflammation, and Prolonged Hypothalamic AMPK Activation in Rat Animal Model. Front. Physiol. 2018, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Penna, E.; Pizzella, A.; Cimmino, F.; Trinchese, G.; Cavaliere, G.; Catapano, A.; Allocca, I.; Chun, J.T.; Campanozzi, A.; Messina, G.; et al. Neurodevelopmental Disorders: Effect of High-Fat Diet on Synaptic Plasticity and Mitochondrial Functions. Brain Sci. 2020, 10, 805. [Google Scholar] [CrossRef]
- Crispino, M.; Trinchese, G.; Penna, E.; Cimmino, F.; Catapano, A.; Villano, I.; Perrone-Capano, C.; Mollica, M.P. Interplay between Peripheral and Central Inflammation in Obesity-Promoted Disorders: The Impact on Synaptic Mitochondrial Functions. Int. J. Mol. Sci. 2020, 21, 5964. [Google Scholar] [CrossRef]
- Posey, K.A.; Clegg, D.J.; Printz, R.L.; Byun, J.; Morton, G.J.; Vivekanandan-Giri, A.; Pennathur, S.; Baskin, D.G.; Heinecke, J.W.; Woods, S.C.; et al. Hypothalamic Proinflammatory Lipid Accumulation, Inflammation, and Insulin Resistance in Rats Fed a High-Fat Diet. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1003–E1012. [Google Scholar] [CrossRef]
- Contreras, C.; González-García, I.; Martínez-Sánchez, N.; Seoane-Collazo, P.; Jacas, J.; Morgan, D.A.; Serra, D.; Gallego, R.; Gonzalez, F.; Casals, N.; et al. Central Ceramide-Induced Hypothalamic Lipotoxicity and ER Stress Regulate Energy Balance. Cell Rep. 2014, 9, 366–377. [Google Scholar] [CrossRef]
- McFadden, J.W.; Aja, S.; Li, Q.; Bandaru, V.V.R.; Kim, E.-K.; Haughey, N.J.; Kuhajda, F.P.; Ronnett, G.V. Increasing Fatty Acid Oxidation Remodels the Hypothalamic Neurometabolome to Mitigate Stress and Inflammation. PLoS ONE 2014, 9, e115642. [Google Scholar] [CrossRef]
- Martins, I.V.A.; Rivers-Auty, J.; Allan, S.M.; Lawrence, C.B. Mitochondrial Abnormalities and Synaptic Loss Underlie Memory Deficits Seen in Mouse Models of Obesity and Alzheimer’s Disease. J. Alzheimer Dis. 2016, 55, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Hantikainen, E.; Roos, E.; Bellocco, R.; D’Antonio, A.; Grotta, A.; Adami, H.-O.; Ye, W.; Trolle Lagerros, Y.; Bonn, S. Dietary Fat Intake and Risk of Parkinson Disease: Results from the Swedish National March Cohort. Eur. J. Epidemiol. 2022, 37, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.; Beal, M.F. Mitochondrial Dysfunction in Neurodegenerative Diseases. J. Pharmacol. Exp. Ther. 2012, 342, 619–630. [Google Scholar] [CrossRef]
- Trinchese, G.; Cimmino, F.; Cavaliere, G.; Catapano, A.; Fogliano, C.; Lama, A.; Pirozzi, C.; Cristiano, C.; Russo, R.; Petrella, L.; et al. The Hepatic Mitochondrial Alterations Exacerbate Meta-Inflammation in Autism Spectrum Disorders. Antioxidants 2022, 11, 1990. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.E.; Paek, S.H. Mitochondrial Dysfunction in Parkinson’s Disease. Exp. Neurobiol. 2015, 24, 103–116. [Google Scholar] [CrossRef]
- Perez Ortiz, J.M.; Swerdlow, R.H. Mitochondrial Dysfunction in Alzheimer’s Disease: Role in Pathogenesis and Novel Therapeutic Opportunities. Br. J. Pharm. 2019, 176, 3489–3507. [Google Scholar] [CrossRef] [PubMed]
- Devine, M.J.; Kittler, J.T. Mitochondria at the Neuronal Presynapse in Health and Disease. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Chen, H.; Chan, D.C. Mitochondrial Dynamics-Fusion, Fission, Movement, and Mitophagy-in Neurodegenerative Diseases. Hum. Mol. Genet. 2009, 18, R169–R176. [Google Scholar] [CrossRef]
- Li, Z.; Okamoto, K.-I.; Hayashi, Y.; Sheng, M. The Importance of Dendritic Mitochondria in the Morphogenesis and Plasticity of Spines and Synapses. Cell 2004, 119, 873–887. [Google Scholar] [CrossRef]
- Cavaliere, G.; Trinchese, G.; Penna, E.; Cimmino, F.; Pirozzi, C.; Lama, A.; Annunziata, C.; Catapano, A.; Mattace Raso, G.; Meli, R.; et al. High-Fat Diet Induces Neuroinflammation and Mitochondrial Impairment in Mice Cerebral Cortex and Synaptic Fraction. Front. Cell Neurosci. 2019, 13, 509. [Google Scholar] [CrossRef]
- Cavaliere, G.; Catapano, A.; Trinchese, G.; Cimmino, F.; Penna, E.; Pizzella, A.; Cristiano, C.; Lama, A.; Crispino, M.; Mollica, M.P. Butyrate Improves Neuroinflammation and Mitochondrial Impairment in Cerebral Cortex and Synaptic Fraction in an Animal Model of Diet-Induced Obesity. Antioxidants 2022, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Yiannikourides, A.; Latunde-Dada, G. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Current Understanding of Iron Homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef]
- Milto, I.V.; Suhodolo, I.V.; Prokopieva, V.D.; Klimenteva, T.K. Molecular and Cellular Bases of Iron Metabolism in Humans. Biochemistry 2016, 81, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, B.K.; Vulpe, C.D.; Anderson, G.J. Intestinal Iron Absorption. J. Trace Elem. Med. Biol. 2012, 26, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, B.J.; Stults, H.B.; Mayer, J. HYPOFERRÆMIA IN OBESE ADOLESCENTS. Lancet 1962, 280, 327–328. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Newfield, R.S.; Koren, I.; Agmon, A.; Lilos, P.; Phillip, M. Greater Prevalence of Iron Deficiency in Overweight and Obese Children and Adolescents. Int. J. Obes. 2003, 27, 416–418. [Google Scholar] [CrossRef]
- Nead, K.G.; Halterman, J.S.; Kaczorowski, J.M.; Auinger, P.; Weitzman, M. Overweight Children and Adolescents: A Risk Group for Iron Deficiency. Pediatrics 2004, 114, 104–108. [Google Scholar] [CrossRef]
- Yanoff, L.B.; Menzie, C.M.; Denkinger, B.; Sebring, N.G.; McHugh, T.; Remaley, A.T.; Yanovski, J.A. Inflammation and Iron Deficiency in the Hypoferremia of Obesity. Int. J. Obes. 2007, 31, 1412–1419. [Google Scholar] [CrossRef]
- Cheng, H.L.; Bryant, C.; Cook, R.; O’Connor, H.; Rooney, K.; Steinbeck, K. The Relationship between Obesity and Hypoferraemia in Adults: A Systematic Review. Obes. Rev. 2012, 13, 150–161. [Google Scholar] [CrossRef]
- Reichert, C.O.; da Cunha, J.; Levy, D.; Maselli, L.M.F.; Bydlowski, S.P.; Spada, C. Hepcidin: Homeostasis and Diseases Related to Iron Metabolism. Acta Haematol. 2017, 137, 220–236. [Google Scholar] [CrossRef] [PubMed]
- GANZ, T. Hepcidin—A Regulator of Intestinal Iron Absorption and Iron Recycling by Macrophages. Best Pr. Res. Clin. Haematol. 2005, 18, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.; Babitt, J.L. Overview of Iron Metabolism in Health and Disease. Hemodial. Int. 2017, 21, S6–S20. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef]
- Chaston, T.; Chung, B.; Mascarenhas, M.; Marks, J.; Patel, B.; Srai, S.K.; Sharp, P. Evidence for Differential Effects of Hepcidin in Macrophages and Intestinal Epithelial Cells. Gut 2008, 57, 374–382. [Google Scholar] [CrossRef]
- Gozzelino, R.; Arosio, P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 Induces Hepcidin Expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 Mediates Hypoferremia of Inflammation by Inducing the Synthesis of the Iron Regulatory Hormone Hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Stoffel, N.U.; El-Mallah, C.; Herter-Aeberli, I.; Bissani, N.; Wehbe, N.; Obeid, O.; Zimmermann, M.B. The Effect of Central Obesity on Inflammation, Hepcidin, and Iron Metabolism in Young Women. Int. J. Obes. 2020, 44, 1291–1300. [Google Scholar] [CrossRef]
- Walter, P.B.; Knutson, M.D.; Paler-Martinez, A.; Lee, S.; Xu, Y.; Viteri, F.E.; Ames, B.N. Iron Deficiency and Iron Excess Damage Mitochondria and Mitochondrial DNA in Rats. Proc. Natl. Acad. Sci. USA 2002, 99, 2264–2269. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Holland, W.L.; Sun, K.; Park, J.; Spurgin, S.B.; Lin, Y.; Askew, G.R.; Simcox, J.A.; McClain, D.A.; Li, C.; et al. MitoNEET-Driven Alterations in Adipocyte Mitochondrial Activity Reveal a Crucial Adaptive Process That Preserves Insulin Sensitivity in Obesity. Nat. Med. 2012, 18, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Park, J.; Scherer, P.E. MitoNEET-Mediated Effects on Browning of White Adipose Tissue. Nat. Commun. 2014, 5, 3962. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Li, J.; Kang, R.; Tang, D. Monitoring Autophagy-Dependent Ferroptosis. Methods Cell Biol. 2021, 165, 163–176. [Google Scholar] [PubMed]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni Zaitseva, A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef] [PubMed]
- Mele, L.; Bidault, G.; Mena, P.; Crozier, A.; Brighenti, F.; Vidal-Puig, A.; Del Rio, D. Dietary (Poly)Phenols, Brown Adipose Tissue Activation, and Energy Expenditure: A Narrative Review. Adv. Nutr. 2017, 8, 694–704. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Lama, A.; Pirozzi, C.; Mollica, M.P.; Trinchese, G.; Di Guida, F.; Cavaliere, G.; Calignano, A.; Mattace Raso, G.; Berni Canani, R.; Meli, R. Polyphenol-Rich Virgin Olive Oil Reduces Insulin Resistance and Liver Inflammation and Improves Mitochondrial Dysfunction in High-Fat Diet Fed Rats. Mol. Nutr. Food Res. 2017, 61, 1600418. [Google Scholar] [CrossRef]
- King, R.E.; Bomser, J.A.; Min, D.B. Bioactivity of Resveratrol. Compr. Rev. Food Sci. Food Saf. 2006, 5, 65–70. [Google Scholar] [CrossRef]
- Yu, H.; Yamashita, T.; Hu, X.; Bian, Z.; Hu, X.; Feng, T.; Tadokoro, K.; Morihara, R.; Abe, K. Protective and Anti-Oxidative Effects of Curcumin and Resveratrol on Aβ-Oligomer-Induced Damage in the SH-SY5Y Cell Line. J. Neurol. Sci. 2022, 441, 120356. [Google Scholar] [CrossRef]
- De Sá Coutinho, D.; Pacheco, M.; Frozza, R.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [PubMed]
- Huminiecki, L.; Horbańczuk, J. The Functional Genomic Studies of Resveratrol in Respect to Its Anti-Cancer Effects. Biotechnol. Adv. 2018, 36, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Bianchini, E.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Natural Compounds Modulating Mitochondrial Functions. Evid. Based Complement. Altern. Med. 2015, 2015, 527209. [Google Scholar] [CrossRef]
- Kairisalo, M.; Bonomo, A.; Hyrskyluoto, A.; Mudò, G.; Belluardo, N.; Korhonen, L.; Lindholm, D. Resveratrol Reduces Oxidative Stress and Cell Death and Increases Mitochondrial Antioxidants and XIAP in PC6.3-Cells. Neurosci. Lett. 2011, 488, 263–266. [Google Scholar] [CrossRef]
- Robb, E.L.; Page, M.M.; Wiens, B.E.; Stuart, J.A. Molecular Mechanisms of Oxidative Stress Resistance Induced by Resveratrol: Specific and Progressive Induction of MnSOD. Biochem. Biophys. Res. Commun. 2008, 367, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Selvaraj, K.; Khare, S.K.; Chaudhary, N. Superoxide Dismutase as Multipotent Therapeutic Antioxidant Enzyme: Role in Human Diseases. Biotechnol Lett 2022, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Um, J.-H.; Park, S.-J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. AMP-Activated Protein Kinase–Deficient Mice Are Resistant to the Metabolic Effects of Resveratrol. Diabetes 2010, 59, 554–563. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol Improves Health and Survival of Mice on a High-Calorie Diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial Action of Resveratrol: How and Why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef]
- Tennen, R.I.; Michishita-Kioi, E.; Chua, K.F. Finding a Target for Resveratrol. Cell 2012, 148, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol Induces Brown-like Adipocyte Formation in White Fat through Activation of AMP-Activated Protein Kinase (AMPK) A1. Int. J. Obes. 2015, 39, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Larsen, J.Ø.; Hamilton-Dutoit, S.; Clasen, B.F.; Jessen, N.; Paulsen, S.K.; Kjær, T.N.; Richelsen, B.; Pedersen, S.B. Resveratrol Up-Regulates Hepatic Uncoupling Protein 2 and Prevents Development of Nonalcoholic Fatty Liver Disease in Rats Fed a High-Fat Diet. Nutr. Res. 2012, 32, 701–708. [Google Scholar] [CrossRef]
- De Ligt, M.; Bruls, Y.M.H.; Hansen, J.; Habets, M.-F.; Havekes, B.; Nascimento, E.B.M.; Moonen-Kornips, E.; Schaart, G.; Schrauwen-Hinderling, V.B.; van Marken Lichtenbelt, W.; et al. Resveratrol Improves Ex Vivo Mitochondrial Function but Does Not Affect Insulin Sensitivity or Brown Adipose Tissue in First Degree Relatives of Patients with Type 2 Diabetes. Mol. Metab. 2018, 12, 39–47. [Google Scholar] [CrossRef]
- Krogholm, K.S.; Bredsdorff, L.; Knuthsen, P.; Haraldsdóttir, J.; Rasmussen, S.E. Relative Bioavailability of the Flavonoids Quercetin, Hesperetin and Naringenin given Simultaneously through Diet. Eur. J. Clin. Nutr. 2010, 64, 432–435. [Google Scholar] [CrossRef]
- Rayamajhi, N.; Kim, S.-K.; Go, H.; Joe, Y.; Callaway, Z.; Kang, J.-G.; Ryter, S.W.; Chung, H.T. Quercetin Induces Mitochondrial Biogenesis through Activation of HO-1 in HepG2 Cells. Oxidative Med. Cell. Longev. 2013, 2013, 154279. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kim, Y. Effects of Isorhamnetin on Adipocyte Mitochondrial Biogenesis and AMPK Activation. Molecules 2018, 23, 1853. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, X.; Zhang, L.; Bian, H.-X.; Xu, N.; Bao, B.; Liu, J. Quercetin Reduces Obesity-Associated ATM Infiltration and Inflammation in Mice: A Mechanism Including AMPKα1/SIRT1. J. Lipid Res. 2014, 55, 363–374. [Google Scholar] [CrossRef]
- Kobori, M.; Takahashi, Y.; Sakurai, M.; Akimoto, Y.; Tsushida, T.; Oike, H.; Ippoushi, K. Quercetin Suppresses Immune Cell Accumulation and Improves Mitochondrial Gene Expression in Adipose Tissue of Diet-induced Obese Mice. Mol. Nutr. Food Res. 2016, 60, 300–312. [Google Scholar] [CrossRef]
- Henagan, T.M.; Cefalu, W.T.; Ribnicky, D.M.; Noland, R.C.; Dunville, K.; Campbell, W.W.; Stewart, L.K.; Forney, L.A.; Gettys, T.W.; Chang, J.S.; et al. In Vivo Effects of Dietary Quercetin and Quercetin-Rich Red Onion Extract on Skeletal Muscle Mitochondria, Metabolism, and Insulin Sensitivity. Genes Nutr. 2015, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Hamidie, R.D.R.; Shibaguchi, T.; Yamada, T.; Koma, R.; Ishizawa, R.; Saito, Y.; Hosoi, T.; Masuda, K. Curcumin Induces Mitochondrial Biogenesis by Increasing Cyclic AMP Levels via Phosphodiesterase 4A Inhibition in Skeletal Muscle. Br. J. Nutr. 2021, 126, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Aguilera, C.; Mesa, M.D.; Ramírez-Tortosa, M.C.; Baró, L.; Gil, A. An Ethanolic-Aqueous Extract of Curcuma Longa Decreases the Susceptibility of Liver Microsomes and Mitochondria to Lipid Peroxidation in Atherosclerotic Rabbits. BioFactors 1998, 8, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Revelo, X.; Shao, W.; Tian, L.; Zeng, K.; Lei, H.; Sun, H.-S.; Woo, M.; Winer, D.; Jin, T. Dietary Curcumin Intervention Targets Mouse White Adipose Tissue Inflammation and Brown Adipose Tissue UCP1 Expression. Obesity 2018, 26, 547–558. [Google Scholar] [CrossRef]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin Induces Brown Fat-like Phenotype in 3T3-L1 and Primary White Adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of Omega-3 Fatty Acids in Obesity, Metabolic Syndrome, and Cardiovascular Diseases: A Review of the Evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef]
- Mollica, M.; Trinchese, G.; Cimmino, F.; Penna, E.; Cavaliere, G.; Tudisco, R.; Musco, N.; Manca, C.; Catapano, A.; Monda, M.; et al. Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions. Nutrients 2021, 13, 1111. [Google Scholar] [CrossRef]
- Cimmino, F.; Catapano, A.; Villano, I.; Di Maio, G.; Petrella, L.; Traina, G.; Pizzella, A.; Tudisco, R.; Cavaliere, G. Invited Review: Human, Cow, and Donkey Milk Comparison: Focus on Metabolic Effects. J. Dairy Sci. 2023, 106, 3072–3085. [Google Scholar] [CrossRef]
- Buckley, J.D.; Howe, P.R.C. Long-Chain Omega-3 Polyunsaturated Fatty Acids May Be Beneficial for Reducing Obesity—A Review. Nutrients 2010, 2, 1212–1230. [Google Scholar] [CrossRef]
- Lionetti, L.; Cavaliere, G.; Bergamo, P.; Trinchese, G.; De Filippo, C.; Gifuni, G.; Gaita, M.; Pignalosa, A.; Donizzetti, I.; Putti, R.; et al. Diet Supplementation with Donkey Milk Upregulates Liver Mitochondrial Uncoupling, Reduces Energy Efficiency and Improves Antioxidant and Antiinflammatory Defences in Rats. Mol. Nutr. Food Res. 2012, 56, 1596–1600. [Google Scholar] [CrossRef]
- Viggiano, E.; Mollica, M.P.; Lionetti, L.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Chieffi, S.; Gaita, M.; Barletta, A.; De Luca, B.; et al. Effects of an High-Fat Diet Enriched in Lard or in Fish Oil on the Hypothalamic Amp-Activated Protein Kinase and Inflammatory Mediators. Front. Cell Neurosci. 2016, 10, 150. [Google Scholar] [CrossRef]
- Cavaliere, G.; Trinchese, G.; Bergamo, P.; De Filippo, C.; Mattace Raso, G.; Gifuni, G.; Putti, R.; Moni, B.H.; Canani, R.B.; Meli, R.; et al. Polyunsaturated Fatty Acids Attenuate Diet Induced Obesity and Insulin Resistance, Modulating Mitochondrial Respiratory Uncoupling in Rat Skeletal Muscle. PLoS ONE 2016, 11, e0149033. [Google Scholar] [CrossRef] [PubMed]
- Lanza, I.R.; Blachnio-Zabielska, A.; Johnson, M.L.; Schimke, J.M.; Jakaitis, D.R.; Lebrasseur, N.K.; Jensen, M.D.; Sreekumaran Nair, K.; Zabielski, P. Influence of Fish Oil on Skeletal Muscle Mitochondrial Energetics and Lipid Metabolites during High-Fat Diet. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1391–E1403. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.M.K.; Hashem, R.M.; Rashed, L.A.; El-Razek, S.M.A. Comparative Study between the Effect of the Peroxisome Proliferator Activated Receptor-α Ligands Fenofibrate and n-3 Polyunsaturated Fatty Acids on Activation of 5′-AMP-Activated Protein Kinase-A1 in High-Fat Fed Rats. J. Pharm. Pharmacol. 2010, 61, 1339–1346. [Google Scholar] [CrossRef]
- Lionetti, L.; Mollica, M.P.; Donizzetti, I.; Gifuni, G.; Sica, R.; Pignalosa, A.; Cavaliere, G.; Gaita, M.; De Filippo, C.; Zorzano, A.; et al. High-Lard and High-Fish-Oil Diets Differ in Their Effects on Function and Dynamic Behaviour of Rat Hepatic Mitochondria. PLoS ONE 2014, 9, e92753. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Mollica, M.; Sica, R.; Donizzetti, I.; Gifuni, G.; Pignalosa, A.; Cavaliere, G.; Putti, R. Differential Effects of High-Fish Oil and High-Lard Diets on Cells and Cytokines Involved in the Inflammatory Process in Rat Insulin-Sensitive Tissues. Int. J. Mol. Sci. 2014, 15, 3040–3063. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, X. Eicosapentaenoic Acid Promotes Thermogenic and Fatty Acid Storage Capacity in Mouse Subcutaneous Adipocytes. Biochem. Biophys. Res. Commun. 2014, 450, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Oudart, H.; Groscolas, R.; Calgari, C.; Nibbelink, M.; Leray, C.; Le Maho, Y.; Malan, A. Brown Fat Thermogenesis in Rats Fed High-Fat Diets Enriched with n-3 Polyunsaturated Fatty Acids. Int. J. Obes. 1997, 21, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Banu, J.; Rahman, M.; Causey, J.; Fernandes, G. Biological Effects of Conjugated Linoleic Acids in Health and Disease. J. Nutr. Biochem. 2006, 17, 789–810. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, G.; Trinchese, G.; Musco, N.; Infascelli, F.; De Filippo, C.; Mastellone, V.; Morittu, V.M.; Lombardi, P.; Tudisco, R.; Grossi, M.; et al. Milk from Cows Fed a Diet with a High Forage:Concentrate Ratio Improves Inflammatory State, Oxidative Stress, and Mitochondrial Function in Rats. J. Dairy Sci. 2018, 101, 1843–1851. [Google Scholar] [CrossRef]
- Trinchese, G.; Cavaliere, G.; Penna, E.; De Filippo, C.; Cimmino, F.; Catapano, A.; Musco, N.; Tudisco, R.; Lombardi, P.; Infascelli, F.; et al. Milk from Cow Fed with High Forage/Concentrate Ratio Diet: Beneficial Effect on Rat Skeletal Muscle Inflammatory State and Oxidative Stress Through Modulation of Mitochondrial Functions and AMPK Activity. Front. Physiol. 2019, 9, 1969. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Conn, C.A.; Trujillo, K.A. Conjugated Linoleic Acid or Omega 3 Fatty Acids Increase Mitochondrial Biosynthesis and Metabolism in Skeletal Muscle Cells. Lipids Health Dis. 2012, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Mollica, M.P.; Trinchese, G.; Cavaliere, G.; De Filippo, C.; Cocca, E.; Gaita, M.; Della-Gatta, A.; Marano, A.; Mazzarella, G.; Bergamo, P. C9,T11-Conjugated Linoleic Acid Ameliorates Steatosis by Modulating Mitochondrial Uncoupling and Nrf2 Pathway. J. Lipid Res. 2014, 55, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Cavaliere, G.; Cimmino, F.; Catapano, A.; Carta, G.; Pirozzi, C.; Murru, E.; Lama, A.; Meli, R.; Bergamo, P.; et al. Decreased Metabolic Flexibility in Skeletal Muscle of Rat Fed with a High-Fat Diet Is Recovered by Individual CLA Isomer Supplementation via Converging Protective Mechanisms. Cells 2020, 9, 823. [Google Scholar] [CrossRef]
- Aydın, B.; Güler Şahin, C.; Şekeroğlu, V.; Atlı Şekeroğlu, Z. Conjugated Linoleic Acid Protects Brain Mitochondrial Function in Acrolein Induced Male Rats. Toxicol Mech Methods 2021, 31, 674–679. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Cristiano, C.; Cuozzo, M.; Coretti, L.; Liguori, F.M.; Cimmino, F.; Turco, L.; Avagliano, C.; Aviello, G.; Mollica, M.P.; Lembo, F.; et al. Oral Sodium Butyrate Supplementation Ameliorates Paclitaxel-Induced Behavioral and Intestinal Dysfunction. Biomed. Pharmacother. 2022, 153, 113528. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik Jr, E.J.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Cerdá, B.; Pérez, M.; Pérez-Santiago, J.D.; Tornero-Aguilera, J.F.; González-Soltero, R.; Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front. Physiol. 2016, 7, 51. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C.; et al. Butyrate as a Bioactive Human Milk Protective Component against Food Allergy. Allergy 2021, 76, 1398–1415. [Google Scholar] [CrossRef]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, C.; Lama, A.; Annunziata, C.; Cavaliere, G.; De Caro, C.; Citraro, R.; Russo, E.; Tallarico, M.; Iannone, M.; Ferrante, M.C.; et al. Butyrate Prevents Valproate-induced Liver Injury: In Vitro and in Vivo Evidence. FASEB J. 2020, 34, 676–690. [Google Scholar] [CrossRef]
- Mock, E.D.; Gagestein, B.; van der Stelt, M. Anandamide and Other N-Acylethanolamines: A Class of Signaling Lipids with Therapeutic Opportunities. Prog. Lipid Res. 2023, 89, 101194. [Google Scholar] [CrossRef] [PubMed]
- Tovar, R.; Gavito, A.L.; Vargas, A.; Soverchia, L.; Hernandez-Folgado, L.; Jagerovic, N.; Baixeras, E.; Ciccocioppo, R.; Rodríguez de Fonseca, F.; Decara, J. Palmitoleoylethanolamide Is an Efficient Anti-Obesity Endogenous Compound: Comparison with Oleylethanolamide in Diet-Induced Obesity. Nutrients 2021, 13, 2589. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, C.; Lama, A.; Pirozzi, C.; Cavaliere, G.; Trinchese, G.; Di Guida, F.; Nitrato Izzo, A.; Cimmino, F.; Paciello, O.; De Biase, D.; et al. Palmitoylethanolamide Counteracts Hepatic Metabolic Inflexibility Modulating Mitochondrial Function and Efficiency in Diet-induced Obese Mice. FASEB J. 2020, 34, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, H.; Saghafi-Asl, M.; Ostadrahimi, A. A Systematic Review of the Effects of Oleoylethanolamide, a High-affinity Endogenous Ligand of PPAR-α, on the Management and Prevention of Obesity. Clin. Exp. Pharm. Physiol. 2020, 47, 543–552. [Google Scholar] [CrossRef]
- Trinchese, G.; Cavaliere, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Chun, J.T.; Penna, E.; Negri, R.; Muredda, L.; Demurtas, A.; et al. Human Milk and Donkey Milk, Compared to Cow Milk, Reduce Inflammatory Mediators and Modulate Glucose and Lipid Metabolism, Acting on Mitochondrial Function and Oleylethanolamide Levels in Rat Skeletal Muscle. Front. Physiol. 2018, 9, 32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaliere, G.; Cimmino, F.; Trinchese, G.; Catapano, A.; Petrella, L.; D’Angelo, M.; Lucchin, L.; Mollica, M.P. From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk between Adipose Tissue and Metabolically Active Organs. Antioxidants 2023, 12, 1172. https://doi.org/10.3390/antiox12061172
Cavaliere G, Cimmino F, Trinchese G, Catapano A, Petrella L, D’Angelo M, Lucchin L, Mollica MP. From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk between Adipose Tissue and Metabolically Active Organs. Antioxidants. 2023; 12(6):1172. https://doi.org/10.3390/antiox12061172
Chicago/Turabian StyleCavaliere, Gina, Fabiano Cimmino, Giovanna Trinchese, Angela Catapano, Lidia Petrella, Margherita D’Angelo, Lucio Lucchin, and Maria Pina Mollica. 2023. "From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk between Adipose Tissue and Metabolically Active Organs" Antioxidants 12, no. 6: 1172. https://doi.org/10.3390/antiox12061172
APA StyleCavaliere, G., Cimmino, F., Trinchese, G., Catapano, A., Petrella, L., D’Angelo, M., Lucchin, L., & Mollica, M. P. (2023). From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk between Adipose Tissue and Metabolically Active Organs. Antioxidants, 12(6), 1172. https://doi.org/10.3390/antiox12061172









