Disruption of Bacterial Thiol-Dependent Redox Homeostasis by Magnolol and Honokiol as an Antibacterial Strategy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Strains and Reagents
2.2. Detection of the Minimal Inhibitory Concentration (MIC) Value of the TCM
2.3. Preparation of Bacterial Cell Lysates
2.4. Detection of Inhibition of the Bacterial Trx System
2.5. Nuclear Staining with Propidium Iodide
2.6. Inhibition of Bacterial TrxR In Vitro
2.7. Determination of ROS Levels
2.8. Detection of Bacterial Morphology by Electron Microscopies
2.9. Mild and Acute Peritonitis Mice Model Assay
2.10. Synergism Measurement
3. Results
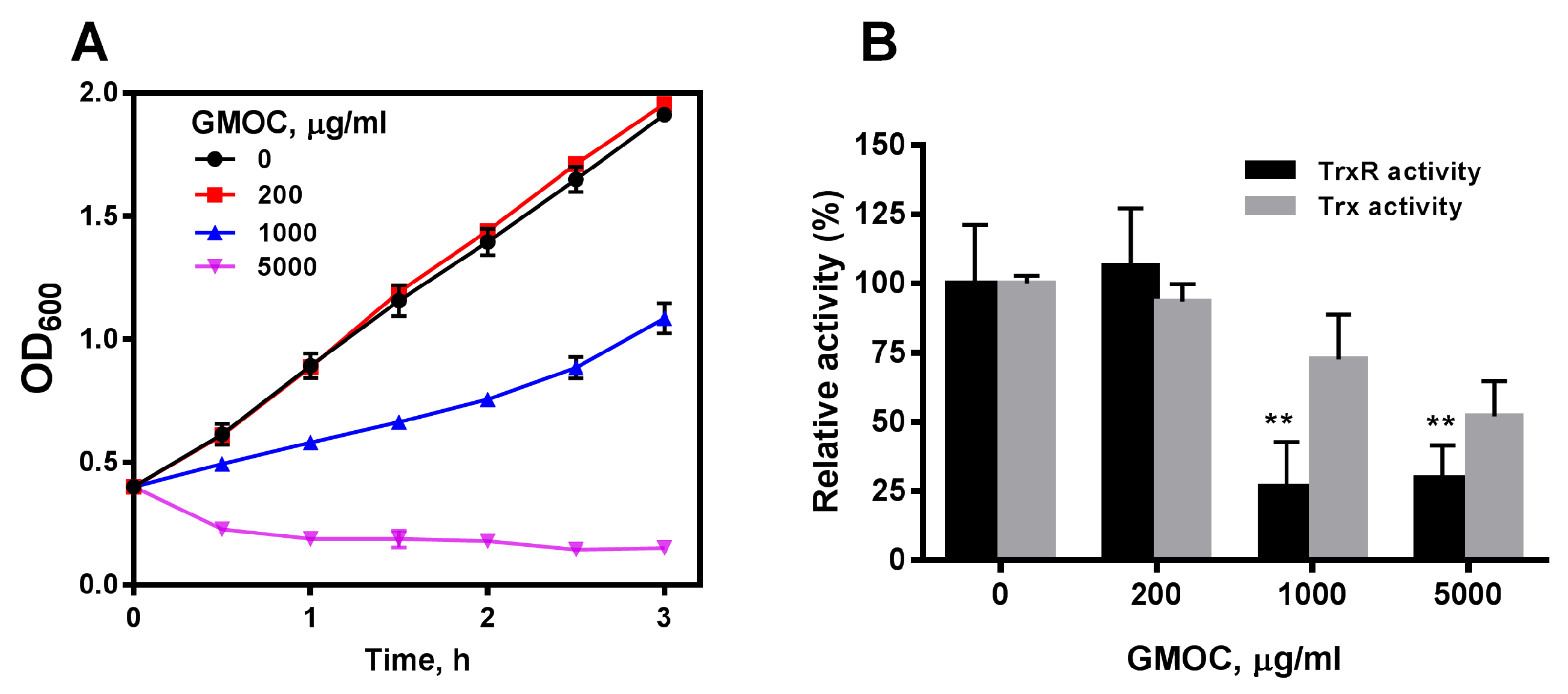
3.1. GMOC Was Screened from a TCM Library as the Most Efficient Antibacterial Agent
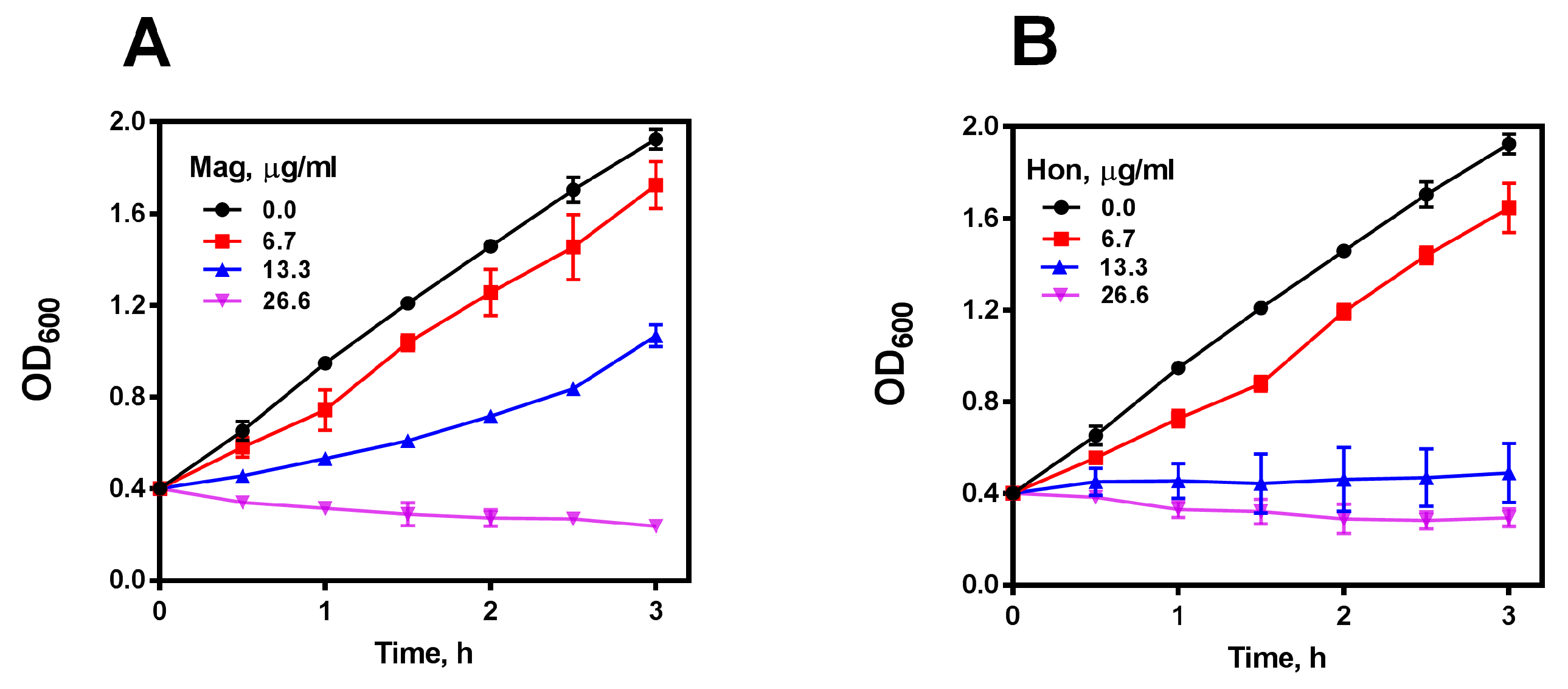
3.2. Magnolol and Honokiol Are the Main Active Ingredients in GMOC Exhibiting Antibacterial Effects
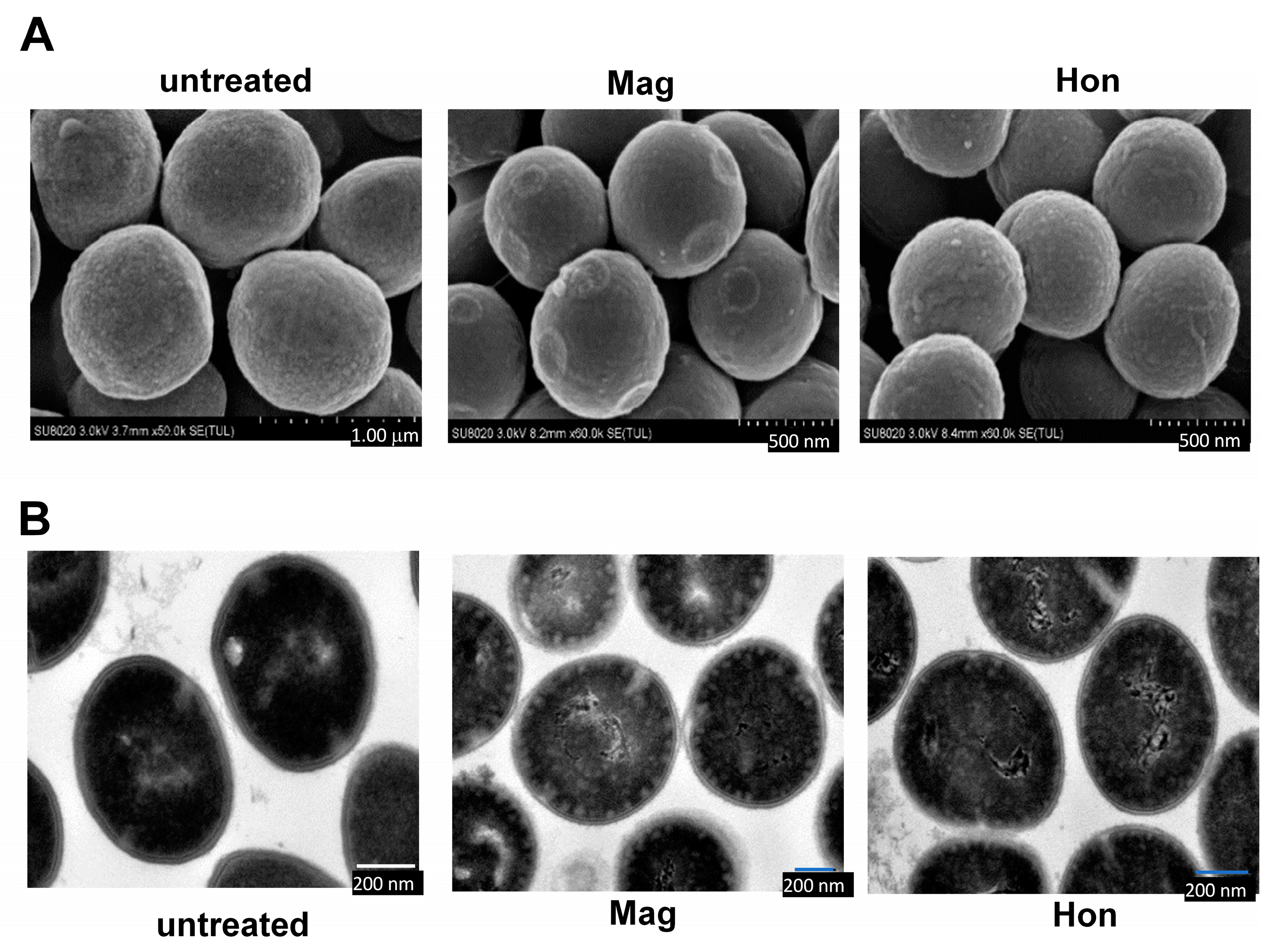
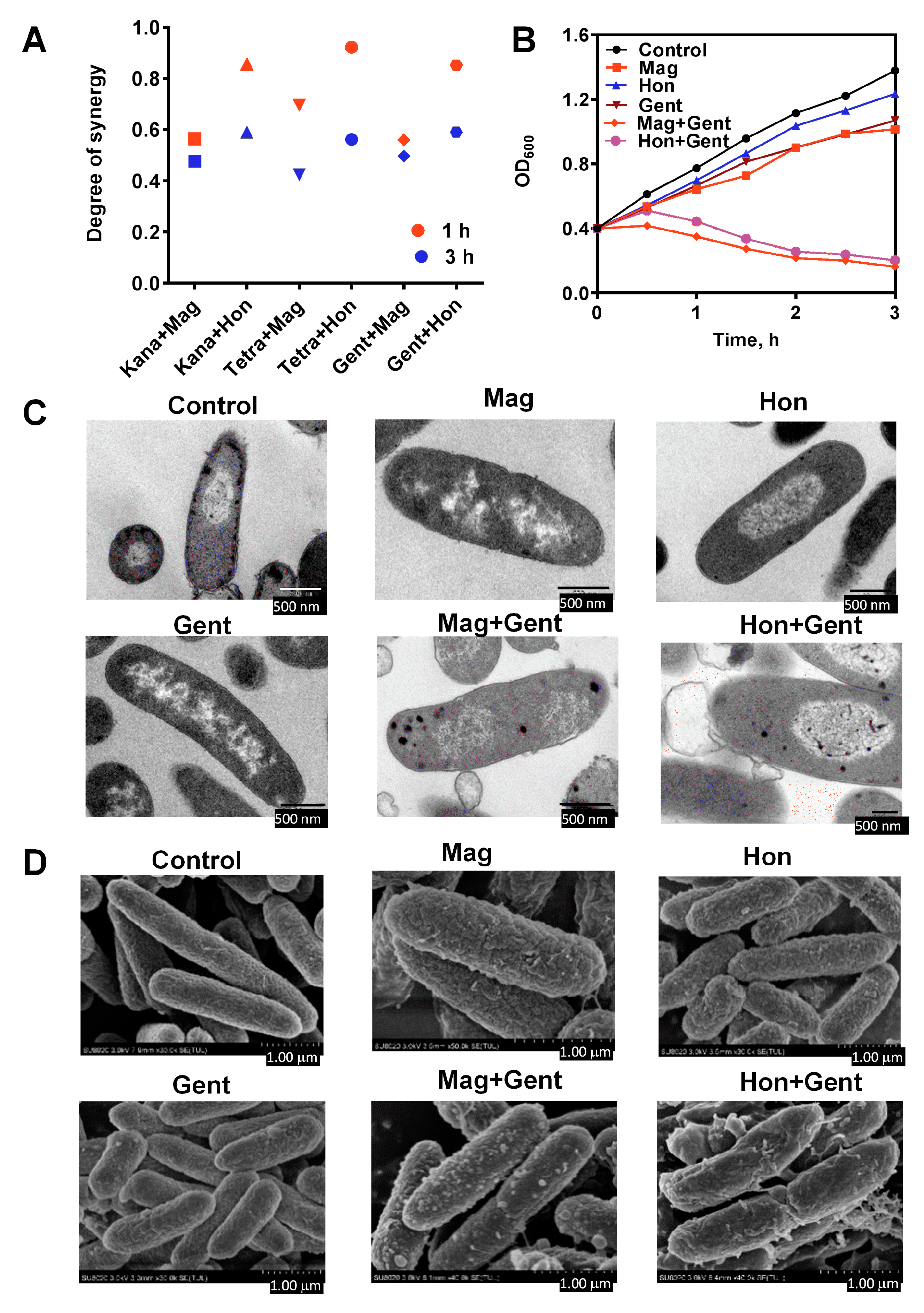
3.3. Magnolol and Honokiol Change the Morphology of S. aureus
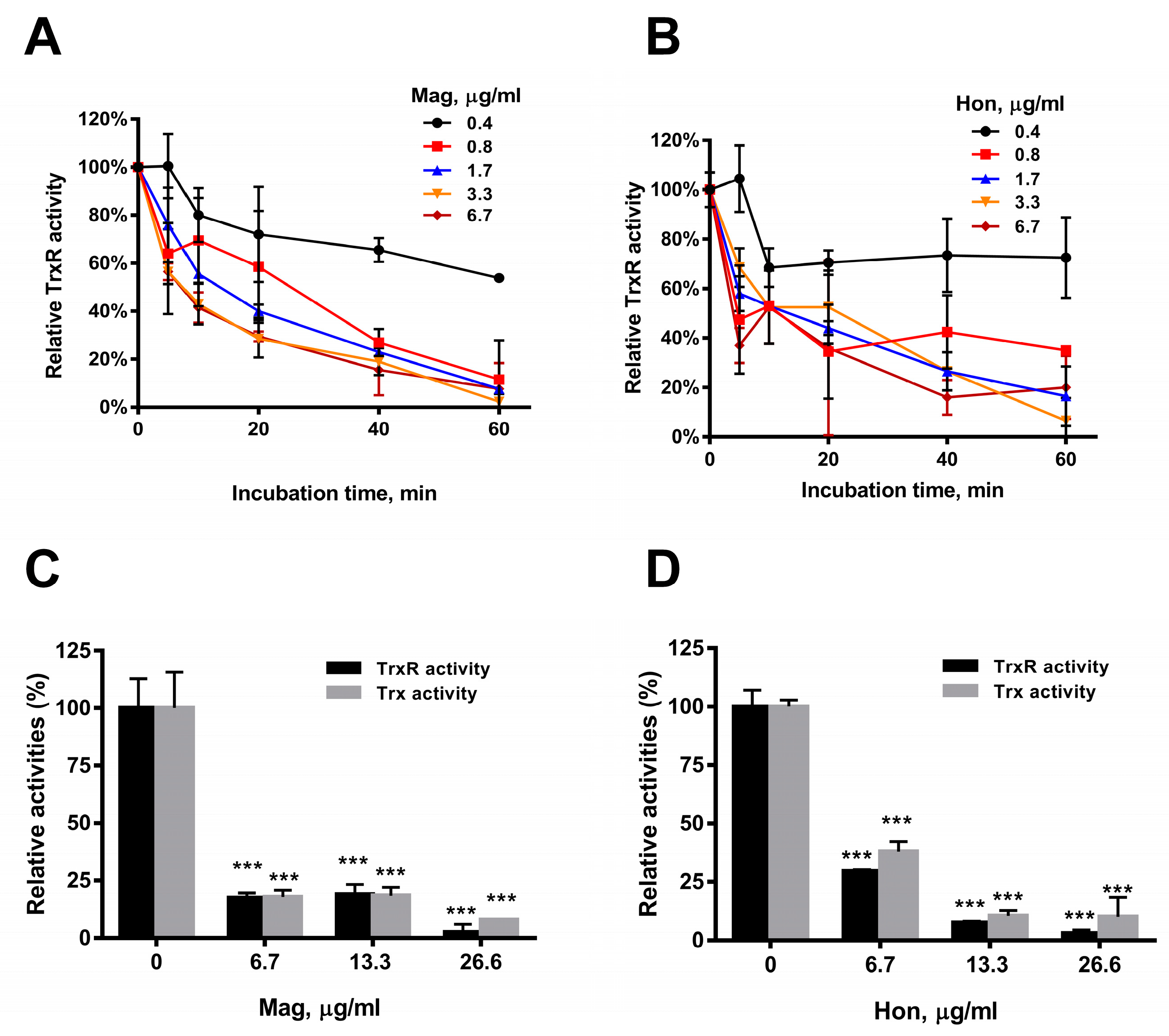
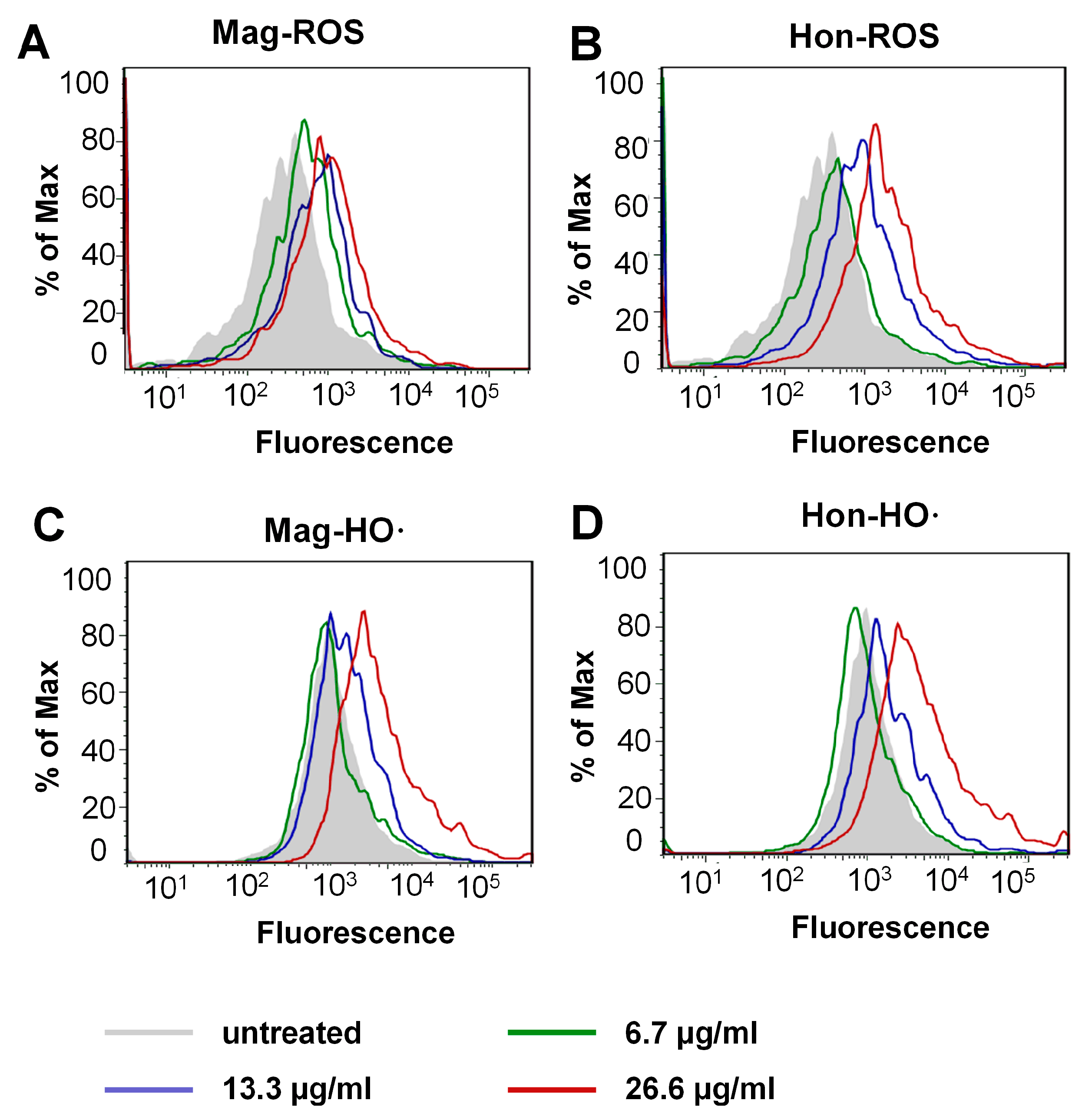
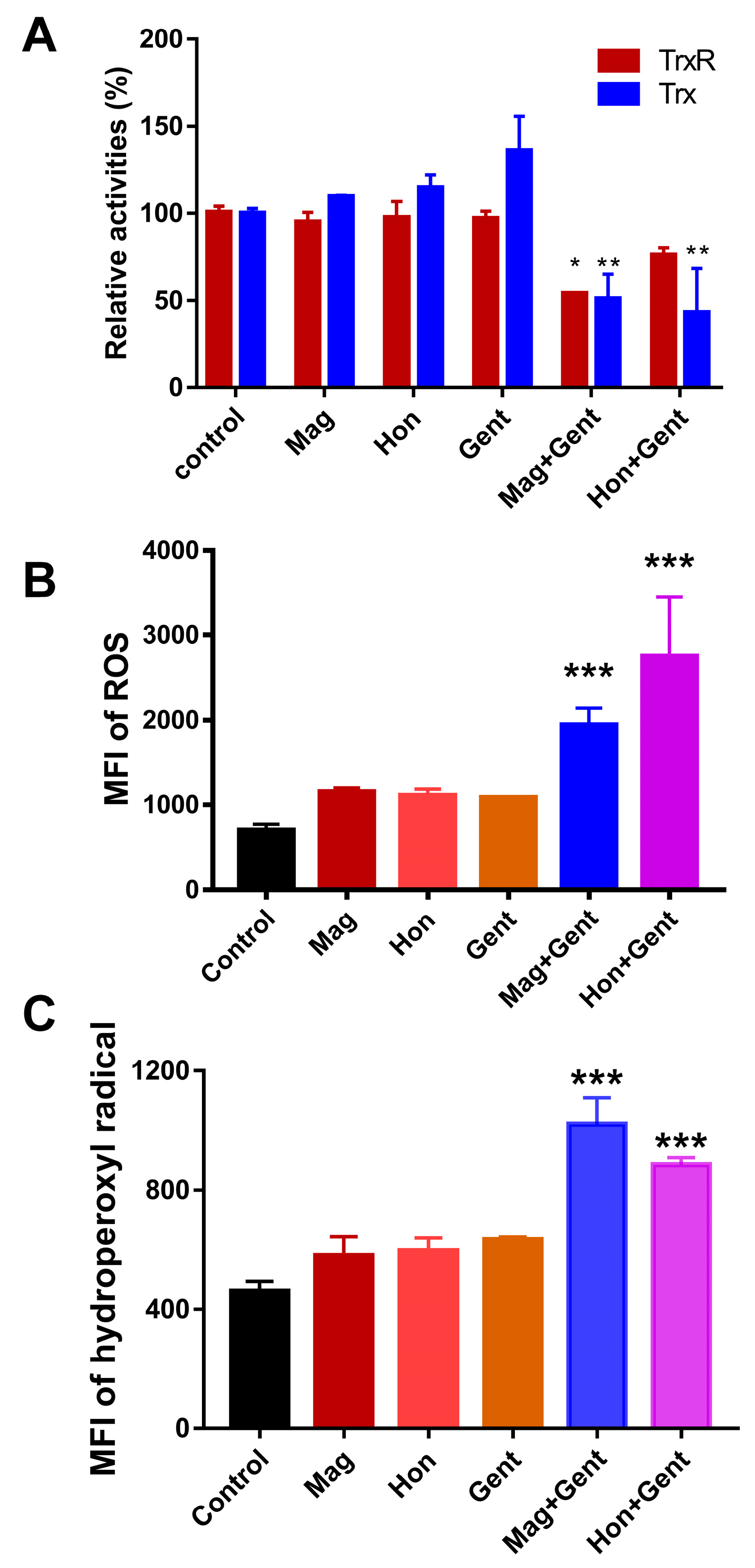
3.4. Magnolol and Honokiol Disrupt the Intracellular Redox Micro-Environment
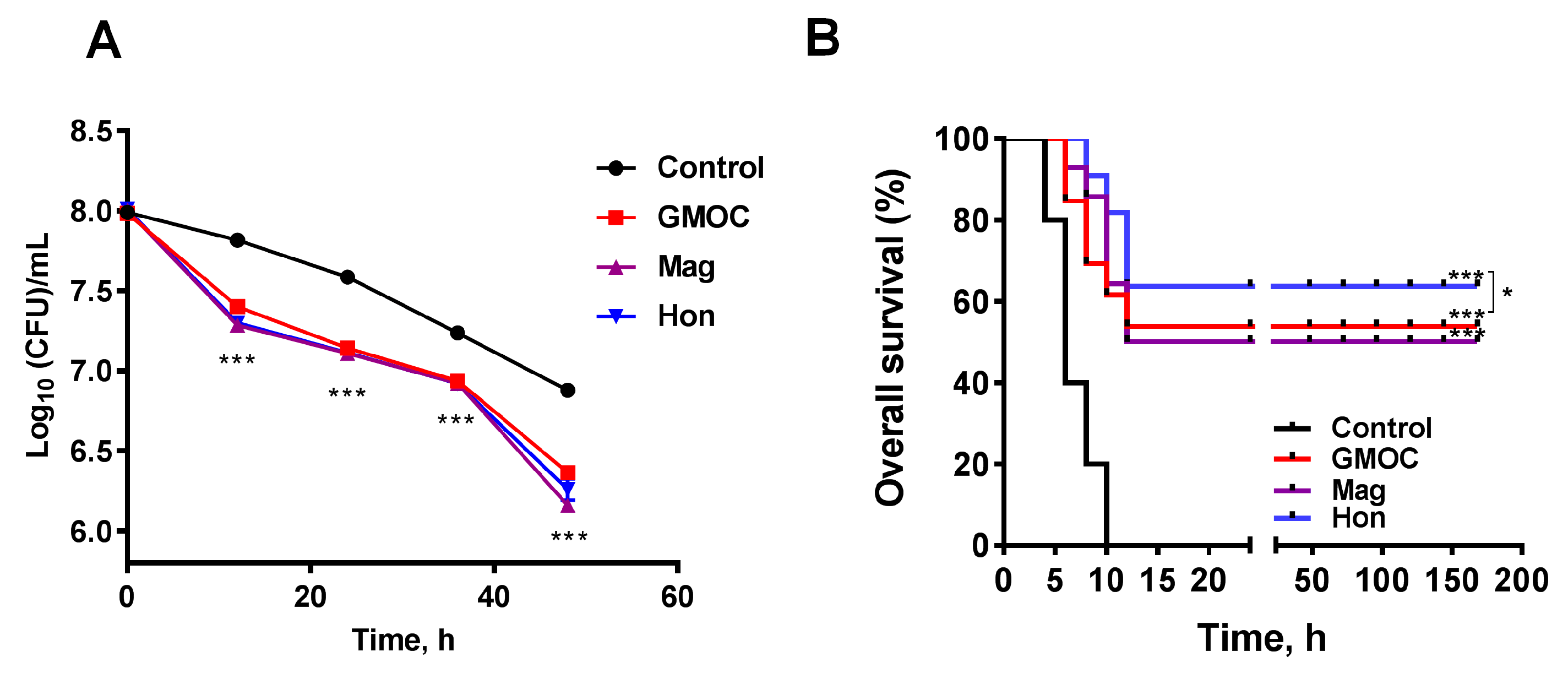
3.5. Therapeutic Efficacy of GMOC in Mice Staphylococcal Peritonitis Infections
3.6. Antibacterial Effects of the Combination of Magnolol, Honokiol with Classic Antibiotics against E. coli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhang, W.; Zhou, G.; Ma, B.; Mo, Q.; Chen, Y.; Wang, Y. Nine phenylethanoid glycosides from Magnolia officinalis var. biloba fruits and their protective effects against free radical-induced oxidative damage. Sci. Rep. 2017, 7, 45342. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, Q.; Shao, Y.; Yin, S.; Liu, C.; Liu, Y.; Wang, R.; Wang, T.; Qiu, Y.; Yu, H. Anticancer activities of TCM and their active components against tumor metastasis. Biomed. Pharmacother. 2021, 133, 111044. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Wang, Q.; Kuang, H.; Su, Y.; Sun, Y.; Feng, J.; Guo, R.; Chan, K. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J. Ethnopharmacol. 2013, 146, 9–39. [Google Scholar] [CrossRef]
- Li, J.; Feng, S.; Liu, X.; Jia, X.; Qiao, F.; Guo, J.; Deng, S. Effects of Traditional Chinese Medicine and its Active Ingredients on Drug-Resistant Bacteria. Front. Pharmacol. 2022, 13, 837907. [Google Scholar] [CrossRef]
- Stumpf, S.; Hostnik, G.; Primozic, M.; Leitgeb, M.; Salminen, J.P.; Bren, U. The Effect of Growth Medium Strength on Minimum Inhibitory Concentrations of Tannins and Tannin Extracts against E. coli. Molecules 2020, 25, 2947. [Google Scholar] [CrossRef]
- Stumpf, S.; Hostnik, G.; Primozic, M.; Leitgeb, M.; Bren, U. Generation Times of E. coli Prolong with Increasing Tannin Concentration while the Lag Phase Extends Exponentially. Plants 2020, 9, 1680. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Han, J.; Zuo, G.Y.; Wang, G.C.; Tang, H.S. Potentiation activity of multiple antibacterial agents by Salvianolate from the Chinese medicine Danshen against methicillin-resistant Staphylococcus aureus (MRSA). J. Pharmacol. Sci. 2016, 131, 13–17. [Google Scholar] [CrossRef]
- Xie, C.; Kokubun, T.; Houghton, P.J.; Simmonds, M.S. Antibacterial activity of the Chinese traditional medicine, Zi Hua Di Ding. Phytother. Res. 2004, 18, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, B.; Wu, F.; Li, T.; Wang, Y.; Ma, Q.; Liang, S. Bitterness and antibacterial activities of constituents from Evodia rutaecarpa. BMC Complement Altern Med. 2017, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhou, Q.; Hao, Z.; Wang, F.; Zhu, Y.; Lin, Q.; Gao, J. The Discovery of Antibacterial Natural Compound Based on Peptide Deformylase. Comb Chem. High Throughput Screen 2018, 21, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.T.; Tan, Y.N.; Ee, K.Y.; Xiao, J.; Wong, F.C. Seeds, fermented foods, and agricultural by-products as sources of plant-derived antibacterial peptides. Crit Rev. Food Sci. Nutr. 2019, 59, S162–S177. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Lobritz, M.A.; Belenky, P.; Porter, C.B.; Gutierrez, A.; Yang, J.H.; Schwarz, E.G.; Dwyer, D.J.; Khalil, A.S.; Collins, J.J. Antibiotic efficacy is linked to bacterial cellular respiration. Proc. Natl. Acad. Sci. USA 2015, 112, 8173–8180. [Google Scholar] [CrossRef]
- Belenky, P.; Ye, J.D.; Porter, C.B.; Cohen, N.R.; Lobritz, M.A.; Ferrante, T.; Jain, S.; Korry, B.J.; Schwarz, E.G.; Walker, G.C.; et al. Bactericidal Antibiotics Induce Toxic Metabolic Perturbations that Lead to Cellular Damage. Cell Rep. 2015, 13, 968–980. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra181. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive Oxygen Species in Pathogen Clearance: The Killing Mechanisms, the Adaption Response, and the Side Effects. Front. Microbiol. 2020, 11, 622534. [Google Scholar] [CrossRef]
- Lu, J.; Vlamis-Gardikas, A.; Kandasamy, K.; Zhao, R.; Gustafsson, T.N.; Engstrand, L.; Hoffner, S.; Engman, L.; Holmgren, A. Inhibition of bacterial thioredoxin reductase: An antibiotic mechanism targeting bacteria lacking glutathione. FASEBJ. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1394–1403. [Google Scholar] [CrossRef]
- Zou, L.; Lu, J.; Wang, J.; Ren, X.; Zhang, L.; Gao, Y.; Rottenberg, M.E.; Holmgren, A. Synergistic antibacterial effect of silver and ebselen against multidrug-resistant Gram-negative bacterial infections. EMBO Mol. Med. 2017, 9, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Harbut, M.B.; Vilcheze, C.; Luo, X.; Hensler, M.E.; Guo, H.; Yang, B.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.R., Jr.; Schultz, P.G.; et al. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Gencheva, R.; Arner, E.S.J. Thioredoxin Reductase Inhibition for Cancer Therapy. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, B.; Li, X.; Han, X.; Liu, R.; Fang, J. Small molecule inhibitors of mammalian thioredoxin reductase as potential anticancer agents: An update. Med. Res. Rev. 2019, 39, 5–39. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Zou, L.; Peana, M.; Chasapis, C.T.; Hangan, T.; Lu, J.; Maes, M. The Role of the Thioredoxin System in Brain Diseases. Antioxidants 2022, 11, 2161. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin superfamily in oxidative protein folding. Antioxid. Redox Signal 2014, 21, 457–470. [Google Scholar] [CrossRef]
- Perez-Dominguez, S.; Caballero-Mancebo, S.; Marcuello, C.; Martinez-Julvez, M.; Medina, M.; Lostao, A. Nanomechanical Study of Enzyme: Coenzyme Complexes: Bipartite Sites in Plastidic Ferredoxin-NADP(+) Reductase for the Interaction with NADP(+). Antioxidants 2022, 11, 537. [Google Scholar] [CrossRef]
- Holmgren, A. Thioredoxin. Annu. Rev. Biochem. 1985, 54, 237–271. [Google Scholar] [CrossRef]
- Boschi-Muller, S. Molecular Mechanisms of the Methionine Sulfoxide Reductase System from Neisseria meningitidis. Antioxidants 2018, 7, 131. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, K.; Baum, K. The Role of Methionine Sulfoxide Reductases in Oxidative Stress Tolerance and Virulence of Staphylococcus aureus and Other Bacteria. Antioxidants 2018, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.S. Focus on mammalian thioredoxin reductases--important selenoproteins with versatile functions. Biochim. Biophys. Acta 2009, 1790, 495–526. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, J.; Peng, Y.; Huang, Z.; Ren, Q.; Lu, J. The Role and Mechanism of Thiol-Dependent Antioxidant System in Bacterial Drug Susceptibility and Resistance. Curr. Med. Chem. 2020, 27, 1940–1954. [Google Scholar] [CrossRef]
- Buey, R.M.; Fernandez-Justel, D.; De Pereda, J.M.; Revuelta, J.L.; Schurmann, P.; Buchanan, B.B.; Balsera, M. Ferredoxin-linked flavoenzyme defines a family of pyridine nucleotide-independent thioredoxin reductases. Proc. Natl. Acad. Sci. USA 2018, 115, 12967–12972. [Google Scholar] [CrossRef]
- Marcuello, C.; Frempong, G.A.; Balsera, M.; Medina, M.; Lostao, A. Atomic Force Microscopy to Elicit Conformational Transitions of Ferredoxin-Dependent Flavin Thioredoxin Reductases. Antioxidants 2021, 10, 1437. [Google Scholar] [CrossRef]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and Toxicology of Magnolol and Honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef]
- Poivre, M.; Duez, P. Biological activity and toxicity of the Chinese herb Magnolia officinalis Rehder & E. Wilson (Houpo) and its constituents. J. Zhejiang Univ. Sci. B 2017, 18, 194–214. [Google Scholar] [CrossRef]
- Jacobo-Salcedo Mdel, R.; Gonzalez-Espindola, L.A.; Alonso-Castro, A.J.; Gonzalez-Martinez Mdel, R.; Dominguez, F.; Garcia-Carranca, A. Antimicrobial activity and cytotoxic effects of Magnolia dealbata and its active compounds. Nat. Prod. Commun. 2011, 6, 1121–1124. [Google Scholar]
- Zhang, J.; Chen, Z.; Huang, X.; Shi, W.; Zhang, R.; Chen, M.; Huang, H.; Wu, L. Insights on the Multifunctional Activities of Magnolol. Biomed. Res. Int. 2019, 2019, 1847130. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Zeng, Y.; Tian, B.; Qu, X.; Yuan, Q.; Song, Y. Pharmacology, Toxicity, Bioavailability, and Formulation of Magnolol: An Update. Front. Pharmacol. 2021, 12, 632767. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fu, W.; Chen, H.; You, S.; Liu, X.; Yang, Y.; Wei, Y.; Huang, J.; Rui, W. Magnolol attenuates the inflammation and enhances phagocytosis through the activation of MAPK, NF-kappaB signal pathways in vitro and in vivo. Mol. Immunol. 2019, 105, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.; Spina, C.S.; Costello, J.C.; Liesa, M.; Morones-Ramirez, J.R.; Slomovic, S.; Molina, A.; Shirihai, O.S.; Collins, J.J. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells. Sci. Transl. Med. 2013, 5, 192ra185. [Google Scholar] [CrossRef] [PubMed]
- Hegreness, M.; Shoresh, N.; Damian, D.; Hartl, D.; Kishony, R. Accelerated evolution of resistance in multidrug environments. Proc. Natl. Acad. Sci. USA 2008, 105, 13977–13981. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.; Tu, X.; Wu, X.; Wang, G.X.; Ling, F. Isolation of anti-Saprolegnia lignans from Magnolia officinalis and SAR evaluation of honokiol/magnolol analogs. Bioorg Med. Chem. Lett. 2019, 29, 389–395. [Google Scholar] [CrossRef]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant Activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their Reaction with Peroxyl Radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef]
- Deng, Y.; Han, X.; Tang, S.; Xiao, W.; Tan, Z.; Zhou, C.; Wang, M.; Kang, J. Magnolol and honokiol regulate the calcium-activated potassium channels signaling pathway in Enterotoxigenic Escherichia coli-induced diarrhea mice. Eur. J. Pharmacol. 2015, 755, 66–73. [Google Scholar] [CrossRef]
- Zuo, G.Y.; Zhang, X.J.; Han, J.; Li, Y.Q.; Wang, G.C. In vitro synergism of magnolol and honokiol in combination with antibacterial agents against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). BMC Complement Altern Med. 2015, 15, 425. [Google Scholar] [CrossRef]
- Chang, S.; Sievert, D.M.; Hageman, J.C.; Boulton, M.L.; Tenover, F.C.; Downes, F.P.; Shah, S.; Rudrik, J.T.; Pupp, G.R.; Brown, W.J.; et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 2003, 348, 1342–1347. [Google Scholar] [CrossRef]
- Freire-Moran, L.; Aronsson, B.; Manz, C.; Gyssens, I.C.; So, A.D.; Monnet, D.L.; Cars, O.; Group, E.-E.W. Critical shortage of new antibiotics in development against multidrug-resistant bacteria-Time to react is now. Drug Resist Updat. 2011, 14, 118–124. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Syu, W.J.; Shen, C.C.; Lu, J.J.; Lee, G.H.; Sun, C.M. Antimicrobial and cytotoxic activities of neolignans from Magnolia officinalis. Chem. Biodivers 2004, 1, 530–537. [Google Scholar] [CrossRef]
- Sakaue, Y.; Domon, H.; Oda, M.; Takenaka, S.; Kubo, M.; Fukuyama, Y.; Okiji, T.; Terao, Y. Anti-biofilm and bactericidal effects of magnolia bark-derived magnolol and honokiol on Streptococcus mutans. Microbiol. Immunol. 2016, 60, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Qiao, J.; Zhang, X.; Ge, C. Antimicrobial effect of Magnolia officinalis extract against Staphylococcus aureus. J. Sci. Food Agric. 2011, 91, 1050–1056. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, J.; Wang, P.; Li, T.; Zhao, Y.; Ren, X.; Lu, J.; Wang, J.; Holmgren, A.; Zou, L. Topical Therapeutic Efficacy of Ebselen Against Multidrug-Resistant Staphylococcus aureus LT-1 Targeting Thioredoxin Reductase. Front. Microbiol. 2020, 10, 3016. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, Q.; An, H.; Li, J.; Shen, S.; Zheng, X.; Chen, W.; Wang, L.; Li, J.; Du, Y.; et al. The synergistic activity of SBC3 in combination with Ebselen against Escherichia coli infection. Front. Pharmacol. 2022, 13, 1080281. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, J.; Chen, H.; Wang, P.; Zhou, J.; Zhao, Y.; Zou, L. Synergistic therapeutic efficacy of ebselen and silver ions against multidrug-resistant Acinetobacter baumannii-induced urinary tract infections. Metallomics 2020, 12, 860–867. [Google Scholar] [CrossRef]
- Dong, C.; Chen, W.; Zou, L.; Liu, B.; Deng, K.; Guo, D.; Wang, P.; Chen, H.; Wang, H.; Wang, J. The Assessment on Synergistic Activity of Ebselen and Silver Ion Against Yersinia pseudotuberculosis. Front. Microbiol. 2022, 13, 963901. [Google Scholar] [CrossRef]
- Holmgren, A.; Johansson, C.; Berndt, C.; Lonn, M.E.; Hudemann, C.; Lillig, C.H. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem. Soc. T 2005, 33, 1375–1377. [Google Scholar] [CrossRef]
- Hashemy, S.I.; Holmgren, A. Thioredoxin and glutaredoxin systems in cellular thiol redox homeostasis. Free Radic. Res. 2007, 41, S7. [Google Scholar]
- Holmgren, A. Thioredoxin and Glutaredoxin Systems. J. Biol. Chem. 1989, 264, 13963–13966. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, Y.; Tang, X.; Zhao, Y.; Zuo, X.; Ren, X.; Wang, J.; Zou, L.; Lu, J. Disruption of Bacterial Thiol-Dependent Redox Homeostasis by Magnolol and Honokiol as an Antibacterial Strategy. Antioxidants 2023, 12, 1180. https://doi.org/10.3390/antiox12061180
Ouyang Y, Tang X, Zhao Y, Zuo X, Ren X, Wang J, Zou L, Lu J. Disruption of Bacterial Thiol-Dependent Redox Homeostasis by Magnolol and Honokiol as an Antibacterial Strategy. Antioxidants. 2023; 12(6):1180. https://doi.org/10.3390/antiox12061180
Chicago/Turabian StyleOuyang, Yanfang, Xuewen Tang, Ying Zhao, Xin Zuo, Xiaoyuan Ren, Jun Wang, Lili Zou, and Jun Lu. 2023. "Disruption of Bacterial Thiol-Dependent Redox Homeostasis by Magnolol and Honokiol as an Antibacterial Strategy" Antioxidants 12, no. 6: 1180. https://doi.org/10.3390/antiox12061180
APA StyleOuyang, Y., Tang, X., Zhao, Y., Zuo, X., Ren, X., Wang, J., Zou, L., & Lu, J. (2023). Disruption of Bacterial Thiol-Dependent Redox Homeostasis by Magnolol and Honokiol as an Antibacterial Strategy. Antioxidants, 12(6), 1180. https://doi.org/10.3390/antiox12061180








