Vitamin B12 Status in Recreational Users of Nitrous Oxide: A Systematic Review Focusing on the Prevalence of Laboratory Abnormalities
Abstract
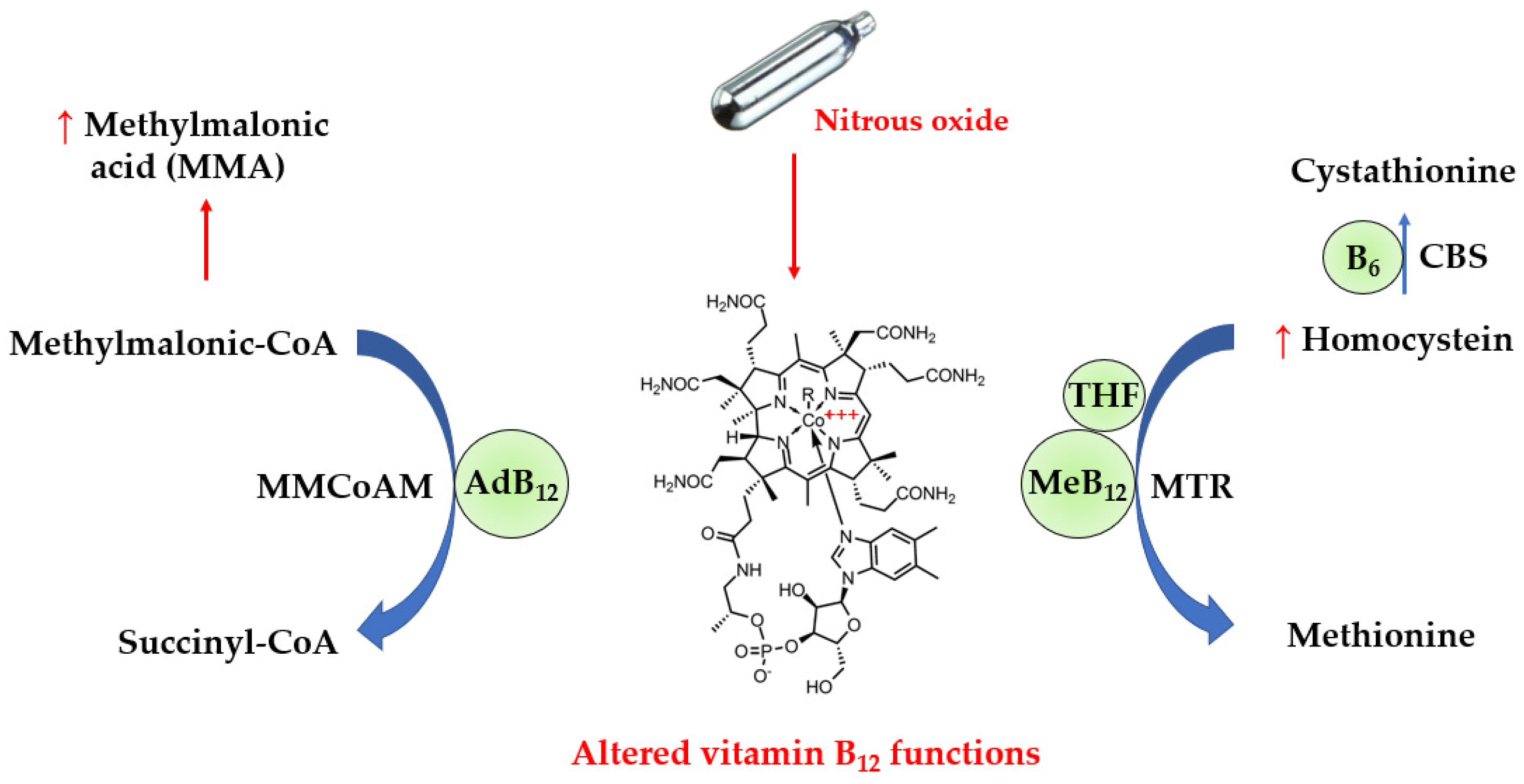
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria and Data Items
2.2. Information Sources and Search Strategy
2.3. Selection and Data Collection Process
2.4. Study Risk of Bias Assessment
2.5. Effect Measures
3. Results
3.1. Study Selection
3.2. Prevalence of Laboratory Abnormalities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drug Misuse in England and Wales—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/crimeandjustice/articles/drugmisuseinenglandandwales/yearendingjune2022 (accessed on 13 February 2023).
- Inquimbert, C.; Maitre, Y.; Moulis, E.; Gremillet, V.; Tramini, P.; Valcarcel, J.; Carayon, D. Recreational Nitrous Oxide Use and Associated Factors among Health Profession Students in France. Int. J. Environ. Res. Public Health 2022, 19, 5237. [Google Scholar] [CrossRef]
- Alam, A.; Woo, J.-S.; Schmitz, J.; Prinz, B.; Root, K.; Chen, F.; Bloch, J.S.; Zenobi, R.; Locher, K.P. Structural Basis of Transcobalamin Recognition by Human CD320 Receptor. Nat. Commun. 2016, 7, 12100. [Google Scholar] [CrossRef]
- Jarquin Campos, A.; Risch, L.; Nydegger, U.; Wiesner, J.; Vazquez Van Dyck, M.; Renz, H.; Stanga, Z.; Risch, M. Diagnostic Accuracy of Holotranscobalamin, Vitamin B12, Methylmalonic Acid, and Homocysteine in Detecting B12 Deficiency in a Large, Mixed Patient Population. Dis. Markers 2020, 2020, 7468506. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological Quality and Synthesis of Case Series and Case Reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Caris, M.G.; Kuipers, R.S.; Kiestra, B.E.; Ruijter, B.J.; Riezebos, R.K.; Coppens, M.; Mooij, H.L. Nitrous Oxide Abuse Leading to Extreme Homocysteine Levels and Thrombosis in Young Adults: A Case Series. J. Thromb. Haemost. 2023, 21, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Grzych, G.; Deheul, S.; Gernez, E.; Davion, J.-B.; Dobbelaere, D.; Carton, L.; Kim, I.; Guichard, J.C.; Girot, M.; Humbert, L.; et al. Comparison of Biomarker for Diagnosis of Nitrous Oxide Abuse: Challenge of Cobalamin Metabolic Parameters, a Retrospective Study. J. Neurol. 2023, 270, 2237–2245. [Google Scholar] [CrossRef]
- Wu, G.; Wang, S.; Wang, T.; Han, J.; Yu, A.; Feng, C.; Wang, Y.; Liu, S. Neurological and Psychological Characteristics of Young Nitrous Oxide Abusers and Its Underlying Causes During the COVID-19 Lockdown. Front. Public Health 2022, 10, 854977. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Kang, L.; Liu, X.; Jin, J.; Hu, F.; Lu, W.; Deng, Y.; Chen, Q.Y.; Dang, J. Acute Nitrous Oxide-Induced Neuropathy Mimicking Guillain-Barré Syndrome. J. Peripher. Nerv. Syst. 2022, 27, 189–196. [Google Scholar] [CrossRef]
- Largeau, B.; Karam, A.; Potey, C.; Caous, A.-S.; Tard, C.; Carton, L.; Kuchcinski, G.; Gautier, S.; Deheul, S.; Bordet, R. Myeloneuropathy Induced by Recreational Nitrous Oxide Use with Variable Exposure Levels. Eur. J. Neurol. 2022, 29, 2173–2180. [Google Scholar] [CrossRef]
- Yu, M.; Qiao, Y.; Li, W.; Fang, X.; Gao, H.; Zheng, D.; Ma, Y. Analysis of Clinical Characteristics and Prognostic Factors in 110 Patients with Nitrous Oxide Abuse. Brain Behav. 2022, 12, e2533. [Google Scholar] [CrossRef]
- Sluyts, Y.; Vanherpe, P.; Amir, R.; Vanhoenacker, F.; Vermeersch, P. Vitamin B12 Deficiency in the Setting of Nitrous Oxide Abuse: Diagnostic Challenges and Treatment Options in Patients Presenting with Subacute Neurological Complications. Acta Clin. Belg. 2022, 77, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, J.; Xu, R.; Feng, F.; Kan, W.; Ding, H.; Wang, X.; Chen, Y.; Wang, X.; Zhu, S.; et al. Clinical Epidemiological Characteristics of Nitrous Oxide Abusers: A Single-Center Experience in a Hospital in China. Brain Behav. 2021, 11, e2416. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shang, X.; Wang, X.; Chen, H.; Li, W.; Wang, Y.; Xu, J. Nitrous Oxide-Related Neurological Disorders: Clinical, Laboratory, Neuroimaging, and Electrophysiological Findings. Brain Behav. 2021, 11, e2402. [Google Scholar] [CrossRef] [PubMed]
- Berling, E.; Fargeot, G.; Aure, K.; Tran, T.H.; Kubis, N.; Lozeron, P.; Zanin, A. Nitrous Oxide-Induced Predominantly Motor Neuropathies: A Follow-up Study. J. Neurol. 2022, 269, 2720–2726. [Google Scholar] [CrossRef]
- Swart, G.; Blair, C.; Lu, Z.; Yogendran, S.; Offord, J.; Sutherland, E.; Barnes, S.; Palavra, N.; Cremer, P.; Bolitho, S.; et al. Nitrous Oxide-Induced Myeloneuropathy. Eur. J. Neurol. 2021, 28, 3938–3944. [Google Scholar] [CrossRef]
- Vollhardt, R.; Mazoyer, J.; Bernardaud, L.; Haddad, A.; Jaubert, P.; Coman, I.; Manceau, P.; Mongin, M.; Degos, B. Neurological Consequences of Recreational Nitrous Oxide Abuse during SARS-CoV-2 Pandemic. J. Neurol. 2022, 269, 1921–1926. [Google Scholar] [CrossRef]
- Einsiedler, M.; Voulleminot, P.; Demuth, S.; Kalaaji, P.; Bogdan, T.; Gauer, L.; Reschwein, C.; Nadaj-Pakleza, A.; de Sèze, J.; Kremer, L.; et al. A Rise in Cases of Nitrous Oxide Abuse: Neurological Complications and Biological Findings. J. Neurol. 2022, 269, 577–582. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, D.; Zou, Y.; Yu, X.; Ji, Y.; Wang, C.; Lv, X.; Zhou, N.; Jiang, X.; Wang, K.; et al. Key Characteristics of Nitrous Oxide-Induced Neurological Disorders and Differences Between Populations. Front. Neurol. 2021, 12, 627183. [Google Scholar] [CrossRef]
- Lee, M.; Abbas, A.; Lee, O.; Record, C.J.; Moodley, K.K.; Nirmalananthan, N. Nitrous Oxide-Induced Motor-Predominant Axonal Peripheral Neuropathy: A Phenotype Distinct from Isolated Vitamin B12 Deficiency. J. Neurol. Sci. 2021, 424, 117390. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, Q.; Li, M.; Liu, F.; Zhang, Y.; Zhao, B.; Sun, Y.; Zhang, D.; Yan, C.; Zhao, Y.; et al. Reversible Neuropsychiatric Disturbances Caused by Nitrous Oxide Toxicity: Clinical, Imaging and Electrophysiological Profiles of 21 Patients with 6–12 Months Follow-Up. Neuropsychiatr. Dis. Treat. 2020, 16, 2817–2825. [Google Scholar] [CrossRef]
- Tuan, T.A.; Minh Duc, N.; Sy, T.V.; Hung, T.M.; Cuong, T.; Anh, N.Q.; Luu, V.D.; Thong, P.M. The Clinical and Subclinical Features of Spinal Cord Injury on Magnetic Resonance Imaging of Patients with N2O Intoxication. Neurol. Int. 2020, 12, 8652. [Google Scholar] [CrossRef]
- Bao, L.; Li, Q.; Li, Q.; Chen, H.; Zhang, R.; Shi, H.; Cui, G. Clinical, Electrophysiological and Radiological Features of Nitrous Oxide-Induced Neurological Disorders. Neuropsychiatr. Dis. Treat. 2020, 16, 977–984. [Google Scholar] [CrossRef]
- McArdle, D.J.T.; Gaillard, F. Pernicious Azotaemia? A Case Series of Subacute Combined Degeneration of the Cord Secondary to Nitrous Oxide Abuse. J. Clin. Neurosci. 2020, 72, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, W.; Gao, H.; Ma, Y.; Dong, X.; Zheng, D. Skin Hyperpigmentation: A Rare Presenting Symptom of Nitrous Oxide Abuse. Clin. Toxicol. 2020, 58, 476–481. [Google Scholar] [CrossRef]
- Keddie, S.; Adams, A.; Kelso, A.R.C.; Turner, B.; Schmierer, K.; Gnanapavan, S.; Malaspina, A.; Giovannoni, G.; Basnett, I.; Noyce, A.J. No Laughing Matter: Subacute Degeneration of the Spinal Cord Due to Nitrous Oxide Inhalation. J. Neurol. 2018, 265, 1089–1095. [Google Scholar] [CrossRef]
- Li, H.-T.; Chu, C.-C.; Chang, K.-H.; Liao, M.-F.; Chang, H.-S.; Kuo, H.-C.; Lyu, R.-K. Clinical and Electrodiagnostic Characteristics of Nitrous Oxide-Induced Neuropathy in Taiwan. Clin. Neurophysiol. 2016, 127, 3288–3293. [Google Scholar] [CrossRef]
- Lin, R.-J.; Chen, H.-F.; Chang, Y.-C.; Su, J.-J. Subacute Combined Degeneration Caused by Nitrous Oxide Intoxication: Case Reports. Acta Neurol. Taiwanica 2011, 20, 129–137. [Google Scholar]
- Joncquel Chevalier-Curt, M.; Grzych, G.; Tard, C.; Lannoy, J.; Deheul, S.; Hanafi, R.; Douillard, C.; Vamecq, J. Nitrous Oxide Abuse in the Emergency Practice, and Review of Toxicity Mechanisms and Potential Markers. Food Chem. Toxicol. 2022, 162, 112894. [Google Scholar] [CrossRef]
- Fang, X.; Yu, M.; Zheng, D.; Gao, H.; Li, W.; Ma, Y. Electrophysiologic Characteristics of Nitrous-Oxide-Associated Peripheral Neuropathy: A Retrospective Study of 76 Patients. J. Clin. Neurol. 2023, 19, 44–51. [Google Scholar] [CrossRef]
- Gao, H.; Li, W.; Ren, J.; Dong, X.; Ma, Y.; Zheng, D. Clinical and MRI Differences Between Patients With Subacute Combined Degeneration of the Spinal Cord Related vs. Unrelated to Recreational Nitrous Oxide Use: A Retrospective Study. Front. Neurol. 2021, 12, 626174. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Ba, F.; Bi, G.; Guo, Y.; Gao, Y.; Li, W. The Sharp Rise of Neurological Disorders Associated with Recreational Nitrous Oxide Use in China: A Single-Center Experience and a Brief Review of Chinese Literature. J. Neurol. 2020, 267, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Temple, C.; Horowitz, B.Z. Nitrous Oxide Abuse Induced Subacute Combined Degeneration despite Patient Initiated B12 Supplementation. Clin. Toxicol. 2022, 60, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Cogswell, M.E.; Hamner, H.C.; Carriquiry, A.; Bailey, L.B.; Pfeiffer, C.M.; Berry, R.J. Folic Acid Source, Usual Intake, and Folate and Vitamin B-12 Status in US Adults: National Health and Nutrition Examination Survey (NHANES) 2003-2006. Am. J. Clin. Nutr. 2010, 91, 64–72. [Google Scholar] [CrossRef]
- Marsden, P.; Sharma, A.A.; Rotella, J.-A. Review Article: Clinical Manifestations and Outcomes of Chronic Nitrous Oxide Misuse: A Systematic Review. Emerg. Med. Australas. 2022, 34, 492–503. [Google Scholar] [CrossRef]
- Oussalah, A.; Julien, M.; Levy, J.; Hajjar, O.; Franczak, C.; Stephan, C.; Laugel, E.; Wandzel, M.; Filhine-Tresarrieu, P.; Green, R.; et al. Global Burden Related to Nitrous Oxide Exposure in Medical and Recreational Settings: A Systematic Review and Individual Patient Data Meta-Analysis. J. Clin. Med. 2019, 8, 551. [Google Scholar] [CrossRef]
- Garakani, A.; Jaffe, R.J.; Savla, D.; Welch, A.K.; Protin, C.A.; Bryson, E.O.; McDowell, D.M. Neurologic, Psychiatric, and Other Medical Manifestations of Nitrous Oxide Abuse: A Systematic Review of the Case Literature. Am. J. Addict. 2016, 25, 358–369. [Google Scholar] [CrossRef]


| PICOS Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | N2O users for recreational purposes with clinical symptoms attributable to N2O-related toxicity. Any age, gender or ethnicity. | N2O use in the context of anesthesia or other medical situations. |
| Intervention | Blood testing including at least one of the following biological parameters: total vitamin B12, tHcy, MMA or holoTC. | Vitamin B12 supplementation before blood collection. |
| Comparison | Comparison group not required. | |
| Outcomes | The primary outcomes were the proportion of N2O users with abnormal values of:
| Reports were excluded if the expression of results did not allow us to calculate the percentage of abnormal values. |
| Study design | Case series including at least 3 N2O users. Articles published in peer-reviewed journals in English. | Articles not published in English, case reports of one or two cases, meta-analyses, reviews, expert opinions, conference reports and studies in animal models. |
| Study | Selection | Ascertainment | Causality | Reporting | Overall Judgement |
|---|---|---|---|---|---|
| [7] | |||||
| [8] | |||||
| [9] | |||||
| [10] | |||||
| [11] | |||||
| [12] | |||||
| [13] | |||||
| [14] | |||||
| [15] | |||||
| [16] | |||||
| [17] | |||||
| [18] | |||||
| [19] | |||||
| [20] | |||||
| [21] | |||||
| [22] | |||||
| [23] | |||||
| [24] | |||||
| [25] | |||||
| [26] | |||||
| [27] | |||||
| [28] | |||||
| [29] |
| Study | Location 1 | Time | Recruited N2O Users | Age (y) | Gender (F/M) |
|---|---|---|---|---|---|
| [7] | Amsterdam, Netherlands (monocentric) | January 2015 to May 2021 | 17 users with thrombotic events | 26 [range 18–53] | 5/12 |
| [8] | Lille, France (monocentric) | March 2020 to March 2022 | 52 users admitted to hospital | 22 | 14/38 |
| [9] | Linhai, China | May 2020 to June 2020 | 6 users | 22 ± 4 | 2/4 |
| [10] | Xi’an, China | May 2020 to November 2020 | 15 users with peripheral neuropathy | 22 ± 5 | 8/7 |
| [11] | Lille, France (multicentric) | January 2019 to August 2020 | 20 users with neuropathy | 19 [range 16–34] | 17/3 |
| [12] | Shenyang, China (monocentric) | January 2018 to December 2020 | 110 users with neuropathy | 21 ± 4 | 53/57 |
| [13] | Edegem, Belgium (monocentric) | N.P. | 8 users with neuropathy in limbs | 22 ± 4 | 2/6 |
| [14] | Xuzhou, China (monocentric) | January 2017 to December 2020 | 61 users with neuropathy | 22 ± 3 | 19/42 |
| [15] | Shenyang, China (monocentric) | February 2017 to July 2020 | 63 users with neuropathy | 23 ± 4 | 25/38 |
| [16] | Paris, France | July 2020 to April 2021 | 7 users referred for electroneuromyography | 21 ± 4 | 1/6 |
| [17] | Sydney, Australia (multicentric) | 2016 to 2020 | 20 users with myeloneuropathy | 24 (range 18–40) | 11/9 |
| [18] | Bobigny, France (monocentric) | August 2020 to April 2021 | 12 users with spinal cord injury and/or peripheral neuropathies | 22 ± 3 | 6/6 |
| [19] | Strasbourg, France (monocentric) | April 2020 to February 2021 | 5 users with neuropathy | 24 ± 4 | 2/3 |
| [20] | Hefei, China (multicentric) | October 2018 to May 2020 | 20 users with neuropathy | 23 (IQR 20–28) | 9/11 |
| [21] | London, UK (monocentric) | N.P. | 3 users with peripheral neuropathy | 21 ± 2 | 2/1 |
| [22] | Qingdao, China (monocentric) | January 2016 to August 2019 | 21 users with neuropathy | 22 ± 5 | 7/14 |
| [23] | Hanoi, Vietnam | May 2018 to July 2019 | 47 users admitted to hospital | 24 ± 6 | 24/23 |
| [24] | Xuzhou, China | 2015 to 2019 | 33 users with neuropathy | 22 ± 3 | 4/29 |
| [25] | Melbourne, Australia (monocentric) | N.P. | 4 users with neuropathy | 20 ± 3 | 4/0 |
| [26] | Shenyang, China (monocentric) | January 2014 to June 2019 | 4 users with neuropathy and skin hyperpigmentation | 20 ± 3 | 3/1 |
| [27] | London, UK (monocentric) | November 2016 to May 2017 | 10 users with symptoms of subacute degeneration of the spinal cord | 22 (range 17–26) | 3/7 |
| [28] | Taoyuan, Taiwan (monocentric) | 2005 to 2015 | 33 users with myeloneuropathy | 23 ± 3 | 14/19 |
| [29] | Taiwan | N.P. | 3 users with myeloneuropathy and peripheral neuropathy | 21 ± 3 | 2/1 |
| Study | Participants | ↘ Total B12 | ↗ tHcy | ↗ MMA | ↘ HoloTC |
|---|---|---|---|---|---|
| [7] | 17 | 46% (6/13) | 89% (8/9) | N.P. | N.P. |
| [8] | 52 | 56% (29/52) | 98% (51/52) | 75% (39/52) | N.P. |
| [9] | 6 | 67% (4/6) | 50% (3/6) | N.P. | N.P. |
| [10] | 15 | 33% (3/9) | N.C. | N.P. | N.P. |
| [11] | 20 | 64% (9/14) | 100% (13/13) | 100% (7/7) | N.P. |
| [12] | 110 | 60% (34/57) | 69% (31/45) | N.P. | N.P. |
| [13] | 8 | 13% (1/8) | 100% (8/8) | U: 88% (7/8) | 0% (0/4) |
| [14] | 61 | 44% (20/45) | 68% (27/40) | N.P. | N.P. |
| [15] | 63 | 35% (22/63) | 87% (55/63) | N.P. | N.P. |
| [16] | 7 | 14% (1/7) | 100% (6/6) | N.P. | N.P. |
| [17] | 20 | 50% (10/20) | 83% (10/12) | N.P. | 35% (6/17) |
| [18] | 12 | 33% (4/12) | 100% (11/11) | 100% (11/11) | N.P. |
| [19] | 5 | 0% (0/5) | 100% (5/5) | 100% (4/4) | N.P. |
| [20] | 20 | 25% (5/20) | 70% (14/20) | N.P. | N.P. |
| [21] | 3 | 66% (2/3) | 100% (2/2) | 100% (1/1) | N.P. |
| [22] | 21 | 17% (3/18) | 78% (14/18) | U: 29% (2/7) | N.P. |
| [23] | 47 | 57% (27/47) | 87% (41/47) | N.P. | N.P. |
| [24] | 33 | 27% (9/33) | 82% (27/33) | N.P. | N.P. |
| [25] | 4 | 100% (4/4) | 100% (1/1) | N.P. | N.P. |
| [26] | 4 | 100% (4/4) | 100% (4/4) | N.P. | N.P. |
| [27] | 10 | 40% (4/10) | N.P. | 88% (7/8) | N.P. |
| [28] | 33 | 9% (3/33) | 30% (10/33) | N.P. | N.P. |
| [29] | 3 | 33% (1/3) | 100% (1/1) | N.P. | N.P. |
| Total | 574 | 42.2% (205/486) | 79.7% (342/429) | 79.6% (78/98) | 28.6% (6/21) |
| Study | Participants | ↘ Total B12 + ↗ tHcy | ↘ Total B12 + ↗ MMA | ↗ tHcy + ↗ MMA | ↘ Total B12 + ↗ tHcy + ↗ MMA |
|---|---|---|---|---|---|
| [7] | 17 | 33% (3/9) | N.P. | N.P. | N.P. |
| [8] | 52 | N.P. | N.P. | 75% (39/52) | N.P. |
| [9] | 6 | 50% (3/6) | N.P. | N.P. | N.P. |
| [11] | 20 | N.P. | N.P. | 100% (7/7) | N.P. |
| [34] | 4 | 0% (0/4) | 0% (0/4) | 100% (4/4) | 0% (0/4) |
| [13] | 8 | 13% (1/8) | 13% (1/8) | U: 88% (7/8) | 13% (1/8) |
| [16] | 7 | 14% (1/7) | N.P. | N.P. | N.P. |
| [18] | 12 | 36% (4/11) | 36% (4/11) | 100% (11/11) | 36% (4/11) |
| [19] | 5 | 0% (0/5) | 0% (0/4) | 100% (4/4) | 0% (0/4) |
| [20] | 20 | 40% (4/10) | N.P. | N.P. | N.P. |
| [21] | 3 | 50% (1/2) | 100% (1/1) | 100% (1/1) | N.P. |
| [25] | 4 | 100% (1/1) | N.P. | N.P. | N.P. |
| [26] | 4 | 100% (4/4) | N.P. | N.P. | N.P. |
| [27] | 10 | N.P. | 50% (4/8) | N.P. | N.P. |
| [28] | 33 | 24% (8/33) | N.P. | N.P. | N.P. |
| Total | 205 | 30% (30/100) | 28% (10/36) | 85% (73/86) | 19% (5/27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ménétrier, T.; Denimal, D. Vitamin B12 Status in Recreational Users of Nitrous Oxide: A Systematic Review Focusing on the Prevalence of Laboratory Abnormalities. Antioxidants 2023, 12, 1191. https://doi.org/10.3390/antiox12061191
Ménétrier T, Denimal D. Vitamin B12 Status in Recreational Users of Nitrous Oxide: A Systematic Review Focusing on the Prevalence of Laboratory Abnormalities. Antioxidants. 2023; 12(6):1191. https://doi.org/10.3390/antiox12061191
Chicago/Turabian StyleMénétrier, Tanguy, and Damien Denimal. 2023. "Vitamin B12 Status in Recreational Users of Nitrous Oxide: A Systematic Review Focusing on the Prevalence of Laboratory Abnormalities" Antioxidants 12, no. 6: 1191. https://doi.org/10.3390/antiox12061191
APA StyleMénétrier, T., & Denimal, D. (2023). Vitamin B12 Status in Recreational Users of Nitrous Oxide: A Systematic Review Focusing on the Prevalence of Laboratory Abnormalities. Antioxidants, 12(6), 1191. https://doi.org/10.3390/antiox12061191






