Glutathione Depletion Disrupts Redox Homeostasis in an Anoxia-Tolerant Invertebrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Glutathione Depletion
2.3. Anoxia and Reoxygenation
2.4. Antioxidant Enzymes
2.5. Oxidative Stress Markers and Glutathione
2.6. Statistics
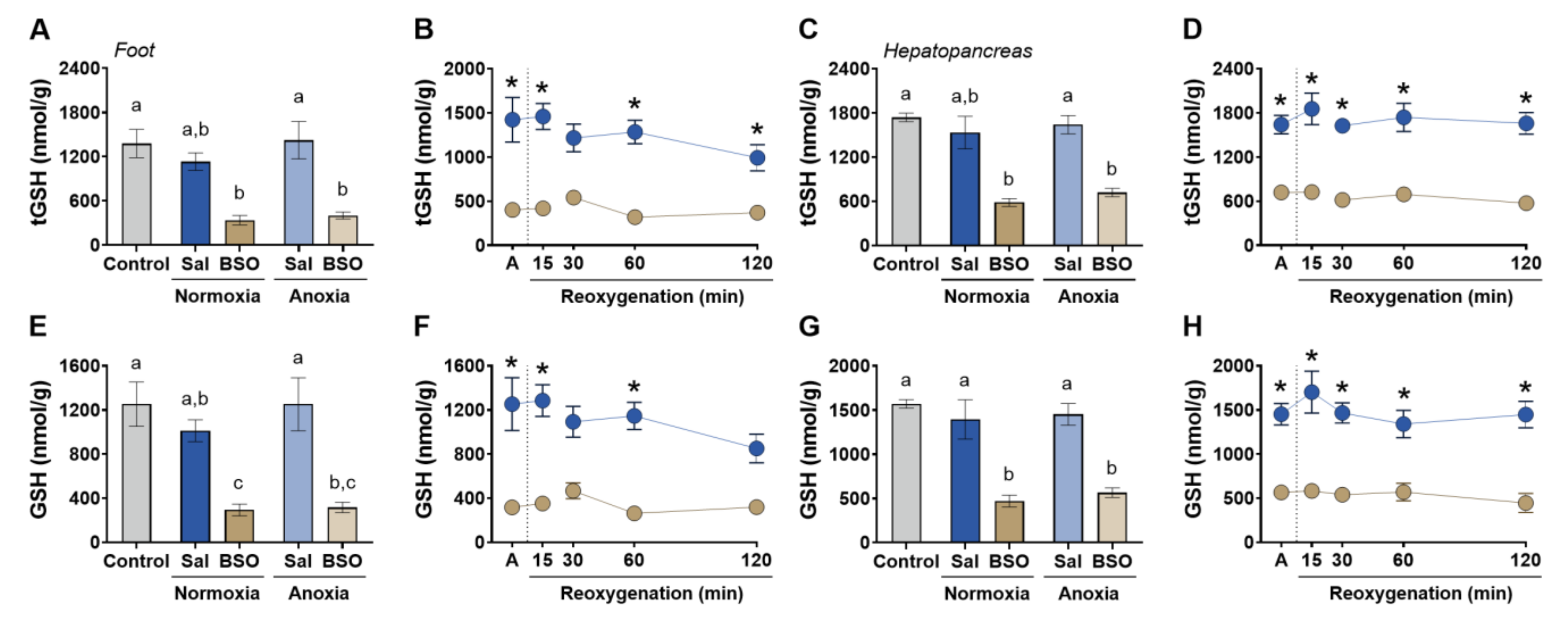
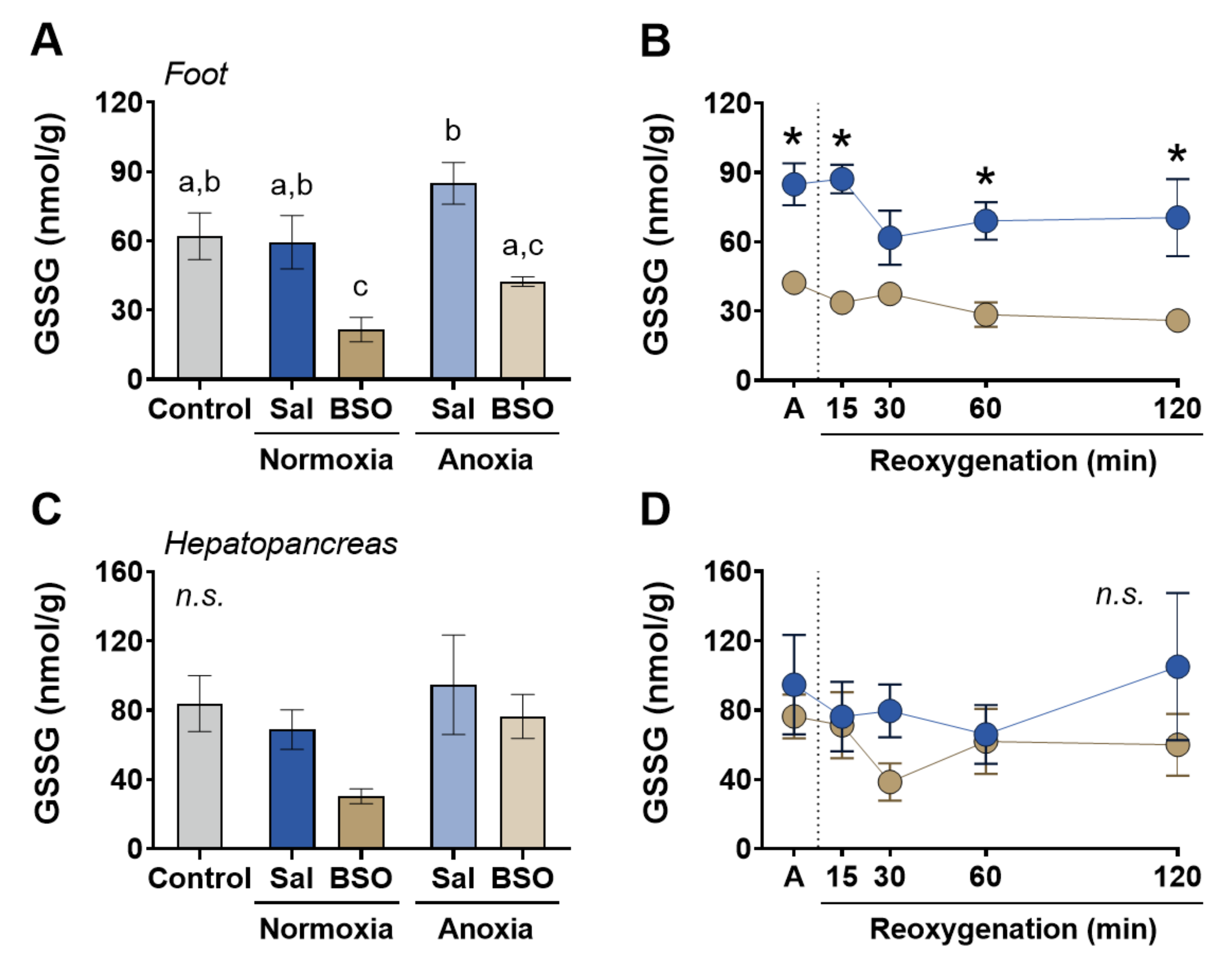
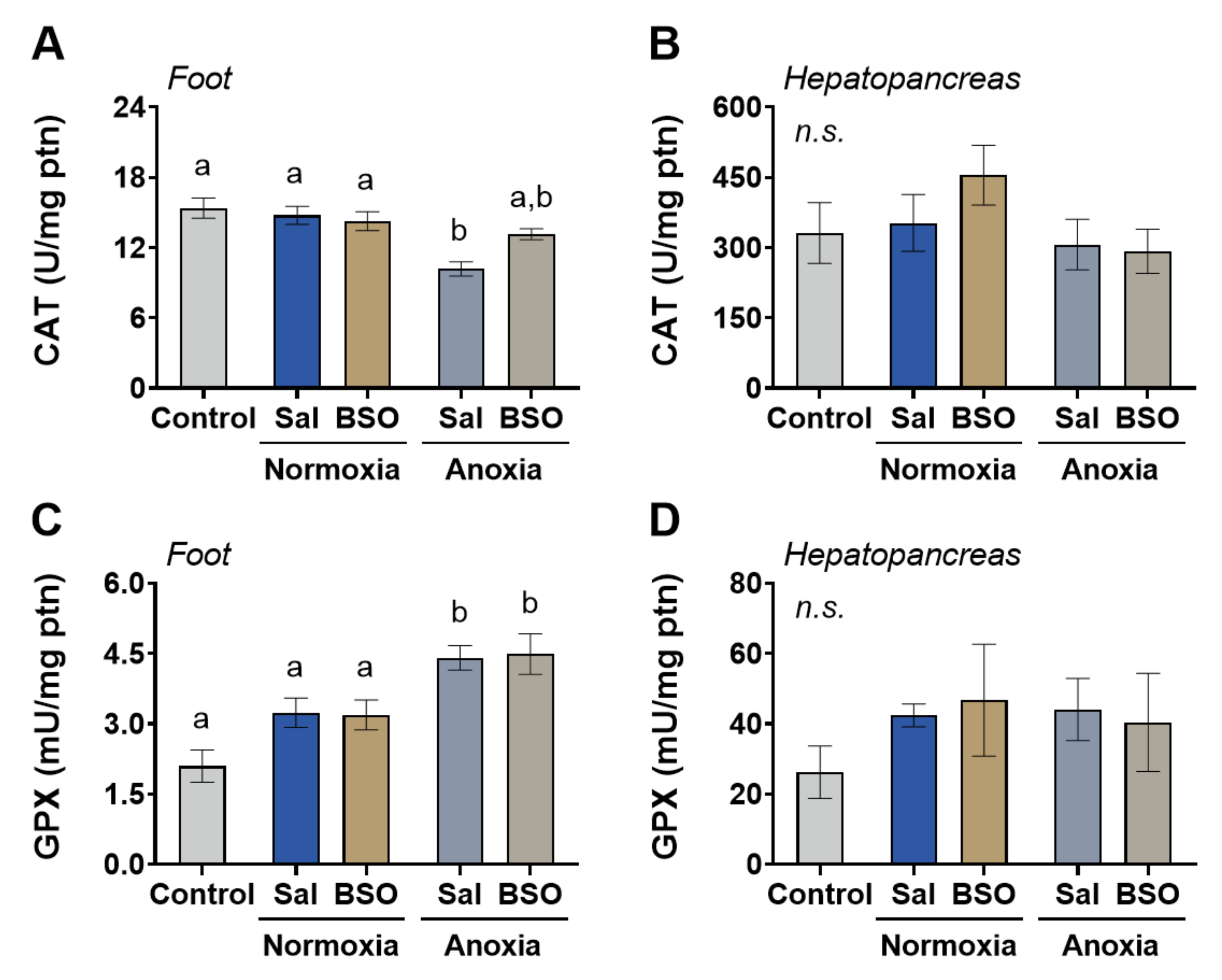
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welker, A.F.; Moreira, D.C.; Campos, É.G.; Hermes-Lima, M. Role of Redox Metabolism for Adaptation of Aquatic Animals to Drastic Changes in Oxygen Availability. Comp. Biochem. Physiol. A 2013, 165, 384–404. [Google Scholar] [CrossRef]
- Moreira, D.C.; Venancio, L.P.R.; Sabino, M.A.C.T.; Hermes-Lima, M. How Widespread Is Preparation for Oxidative Stress in the Animal Kingdom? Comp. Biochem. Physiol. A 2016, 200, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Somero, G.N.; Lockwood, B.L.; Tomanek, L. Biochemical Adaptation: Response to Environmental Challenges, from Life’s Origins to the Anthropocene; Sinauer Associates, Inc. Publishers: Sunderland, MA, USA, 2017; ISBN 978-1-60535-564-1. [Google Scholar]
- Hermes-Lima, M.; Zenteno-Savín, T. Animal Response to Drastic Changes in Oxygen Availability and Physiological Oxidative Stress. Comp. Biochem. Physiol. C 2002, 133, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.C.; Campos, É.G.; Giraud-Billoud, M.; Storey, K.B.; Hermes-Lima, M. Commentary: On the Merit of an Early Contributor of the “Preparation for Oxidative Stress” (POS) Theory. Comp. Biochem. Physiol. A 2023, 276, 111341. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.C.; Oliveira, M.F.; Liz-Guimarães, L.; Diniz-Rojas, N.; Campos, É.G.; Hermes-Lima, M. Current Trends and Research Challenges Regarding “Preparation for Oxidative Stress”. Front. Physiol. 2017, 8, 702. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Rivera-Ingraham, G.A.; Moreira, D.C.; Burmester, T.; Castro-Vazquez, A.; Carvajalino-Fernández, J.M.; Dafre, A.; Niu, C.; Tremblay, N.; Paital, B.; et al. Twenty Years of the ‘Preparation for Oxidative Stress’ (POS) Theory: Ecophysiological Advantages and Molecular Strategies. Comp. Biochem. Physiol. A 2019, 234, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Istomina, A.; Belcheva, N.; Chelomin, V. Antioxidant System of the Intertidal Mollusk Littorina kurila in Its Natural Habitat. J. Environ. Health Sci. Eng. A 2013, 2, 713–718. [Google Scholar]
- Moreira, D.C.; Carvajalino-Fernández, J.M.; Navas, C.A.; de Carvalho, J.E.; Hermes-Lima, M. Metabolic and Redox Biomarkers in Skeletal Muscle Underlie Physiological Adaptations of Two Estivating Anuran Species in a South American Semi-Arid Environment. Front. Physiol. 2021, 12, 769833. [Google Scholar] [CrossRef]
- Patnaik, P.; Sahoo, D.D. Variations in Oxidative Stress and Antioxidant Defense Level during Different Phases of Hibernation in Common Asian Toad, Duttaphrynus melanostictus. Biol. Open. 2021, 10, bio058567. [Google Scholar] [CrossRef]
- Moreira, D.C.; Aurélio Da Costa Tavares Sabino, M.; Minari, M.; Torres Brasil Kuzniewski, F.; Angelini, R.; Hermes-Lima, M. The Role of Solar Radiation and Tidal Emersion on Oxidative Stress and Glutathione Synthesis in Mussels Exposed to Air. PeerJ 2023, 11, e15345. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Storey, J.M.; Storey, K.B. Antioxidant Defenses and Metabolic Depression. The Hypothesis of Preparation for Oxidative Stress in Land Snails. Comp. Biochem. Physiol. B 1998, 120, 437–448. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Moreira, D.C.; Rivera-Ingraham, G.A.; Giraud-Billoud, M.; Genaro-Mattos, T.C.; Campos, É.G. Preparation for Oxidative Stress under Hypoxia and Metabolic Depression: Revisiting the Proposal Two Decades Later. Free Radic. Biol. Med. 2015, 89, 1122–1143. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.F.; Geihs, M.A.; França, T.F.A.; Moreira, D.C.; Hermes-Lima, M. Is “Preparation for Oxidative Stress” a Case of Physiological Conditioning Hormesis? Front. Physiol. 2018, 9, 945. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Ingraham, G.A.; Lignot, J.-H. Osmoregulation, Bioenergetics and Oxidative Stress in Coastal Marine Invertebrates: Raising the Questions for Future Research. J. Exp. Biol. 2017, 220, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Welker, A.F.; Campos, É.G.; Cardoso, L.A.; Hermes-Lima, M. Role of Catalase on the Hypoxia/Reoxygenation Stress in the Hypoxia-Tolerant Nile Tilapia. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2012, 302, R1111–R1118. [Google Scholar] [CrossRef]
- Welker, A.F.; Moreira, D.C.; Hermes-Lima, M. Roles of Catalase and Glutathione Peroxidase in the Tolerance of a Pulmonate Gastropod to Anoxia and Reoxygenation. J. Comp. Physiol. B 2016, 186, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Hochachka, P.W.; Buck, L.T.; Doll, C.J.; Land, S.C. Unifying Theory of Hypoxia Tolerance: Molecular/Metabolic Defense and Rescue Mechanisms for Surviving Oxygen Lack. Proc. Natl. Acad. Sci. USA 1996, 93, 9493–9498. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Lutz, P.L. Mechanism, Origin, and Evolution of Anoxia Tolerance in Animals. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 130, 435–459. [Google Scholar] [CrossRef]
- Larson, J.; Drew, K.L.; Folkow, L.P.; Milton, S.L.; Park, T.J. No Oxygen? No Problem! Intrinsic Brain Tolerance to Hypoxia in Vertebrates. J. Exp. Biol. 2014, 217, 1024–1039. [Google Scholar] [CrossRef]
- Country, M.W.; Jonz, M.G. Goldfish and Crucian Carp Are Natural Models of Anoxia Tolerance in the Retina. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 270, 111244. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, A.; Ruhr, I.M.; Fago, A.; Galli, G.L.J. Metabolic Adaptations to Anoxia and Reoxygenation: New Lessons from Freshwater Turtles and Crucian Carp. Curr. Opin. Endocr. Metab. Res. 2020, 11, 55–64. [Google Scholar] [CrossRef]
- Kluytmans, J.H.; Zandee, D.I. Comparative Study of the Formation and Excretion of Anaerobic Fermentation Products in Bivalves and Gastropods. Comp. Biochem. Physiol. Part B Comp. Biochem. 1983, 75, 729–732. [Google Scholar] [CrossRef]
- Pedrini-Martha, V.; Niederwanger, M.; Kopp, R.; Schnegg, R.; Dallinger, R. Physiological, Diurnal and Stress-Related Variability of Cadmium-Metallothionein Gene Expression in Land Snails. PLoS ONE 2016, 11, e0150442. [Google Scholar] [CrossRef] [PubMed]
- Vorhaben, J.E.; Klotz, A.V.; Campbell, J.W. Activity and Oxidative Metabolism of the Land Snail Helix Aspersa. Physiol. Zool. 1984, 57, 357–365. [Google Scholar] [CrossRef]
- Ramos-Vasconcelos, G.R. Metabolismo de Radicais Livres Em Gastrópodes Terrestres. Ph.D. Thesis, University of Brasilia, Brasilia, Brazil, 2005. [Google Scholar]
- Griffith, O.W. Biologic and Pharmacologic Regulation of Mammalian Glutathione Synthesis. Free Radic. Biol. Med. 1999, 27, 922–935. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and Its Role in Cellular Functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Betti, M.; Gallo, G. Growth Factor-Mediated Signal Transduction and Redox Balance in Isolated Digestive Gland Cells from Mytilus Galloprovincialis Lam. Comp. Biochem. Physiol. C 2000, 125, 355–363. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; Ringwood, A.H.; Weis, V.M. Differential Accumulation of Cadmium and Changes in Glutathione Levels as a Function of Symbiotic State in the Sea Anemone Anthopleura elegantissima. J. Exp. Mar. Biol. Ecol. 2003, 284, 71–85. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Hermes-Lima, M. The Effect of Buthionine Sulfoximine on the Glutathione Level in Goldfish Tissues. Ukr. Biokhim. Zh. 2005, 77, 35–38. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Racker, E. Glutathione Reductase from Bakers’ Yeast and Beef Liver. J. Biol. Chem. 1955, 217, 855–865. [Google Scholar] [CrossRef]
- Glock, G.E.; McLean, P. Further Studies on the Properties and Assay of Glucose 6-Phosphate Dehydrogenase and 6-Phosphogluconate Dehydrogenase of Rat Liver. Biochem. J. 1953, 55, 400–408. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic Method for Quantitative Determination of Nanogram Amounts of Total and Oxidized Glutathione: Applications to Mammalian Blood and Other Tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for Quantitative Determination of Glutathione and Glutathione Disulfide Levels Using Enzymatic Recycling Method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of Glutathione and Glutathione Disulfide Using Glutathione Reductase and 2-Vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Negroni, M.; Altiero, T.; Montorfano, G.; Corsetto, P.; Berselli, P.; Berra, B.; Guidetti, R.; Rebecchi, L. Antioxidant Defences in Hydrated and Desiccated States of the Tardigrade Paramacrobiotus richtersi. Comp. Biochem. Physiol. B 2010, 156, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, S.; He, J.; Bai, Y.; Niu, Y.; Tang, X.; Li, D.; Chen, Q. Oxidative Stress and Antioxidant Status in a Lizard Phrynocephalus Vlangalii at Different Altitudes or Acclimated to Hypoxia. Comp. Biochem. Physiol. A 2015, 190, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Pannunzio, T.M.; Storey, K.B. Antioxidant Defenses and Lipid Peroxidation during Anoxia Stress and Aerobic Recovery in the Marine Gastropod Littorina littorea. J. Exp. Mar. Biol. Ecol. 1998, 221, 277–292. [Google Scholar] [CrossRef]
- Moreira, D.C.; Sabino, M.A.C.T.; Kuzniewski, F.T.B.; Furtado-Filho, O.V.; Carvajalino-Fernández, J.M.; Angelini, R.; Freire, C.A.; Hermes-Lima, M. Redox Metabolism in Mussels (Brachidontes solisianus) under the Influence of Tides in a Rocky Beach in Southern Brazil. Estuar. Coast. Shelf Sci. 2021, 258, 107424. [Google Scholar] [CrossRef]
- Ramos-Vasconcelos, G.R.; Hermes-Lima, M. Hypometabolism, Antioxidant Defenses and Free Radical Metabolism in the Pulmonate Land Snail Helix aspersa. J. Exp. Biol. 2003, 206, 675–685. [Google Scholar] [CrossRef]
- Ramos-Vasconcelos, G.R.; Cardoso, L.A.; Hermes-Lima, M. Seasonal Modulation of Free Radical Metabolism in Estivating Land Snails Helix Aspersa. Comp. Biochem. Physiol. C 2005, 140, 165–174. [Google Scholar] [CrossRef]
- Windrem, D.A.; Plachy, W.Z. The Diffusion-Solubility of Oxygen in Lipid Bilayers. Biochim. Biophys. Acta 1980, 600, 655–665. [Google Scholar] [CrossRef]
- Dzikovski, B.G.; Livshits, V.A.; Marsh, D. Oxygen Permeation Profile in Lipid Membranes: Comparison with Transmembrane Polarity Profile. Biophys. J. 2003, 85, 1005–1012. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox Environment of the Cell as Viewed through the Redox State of the Glutathione Disulfide/Glutathione Couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Yoshida, M.; Saegusa, Y.; Fukuda, A.; Akama, Y.; Owada, S. Measurement of Radical-Scavenging Ability in Hepatic Metallothionein of Rat Using in vivo Electron Spin Resonance Spectroscopy. Toxicology 2005, 213, 74–80. [Google Scholar] [CrossRef]
- Denniss, S.G.; Levy, A.S.; Rush, J.W.E. Effects of Glutathione-Depleting Drug Buthionine Sulfoximine and Aging on Activity of Endothelium-Derived Relaxing and Contracting Factors in Carotid Artery of Sprague–Dawley Rats. J. Cardiovasc. Pharmacol. 2011, 58, 272–283. [Google Scholar] [CrossRef]
- Abalenikhina, Y.V.; Myl’nikov, P.Y.; Shchul’kin, A.V.; Chernykh, I.V.; Yakusheva, E.N. Regulation and Role of Hypoxia-Induced Factor 1α (HIF-1α) under Conditions of Endogenous Oxidative Stress in vitro. Bull. Exp. Biol. Med. 2022, 173, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Merad-Boudia, M.; Nicole, A.; Santiard-Baron, D.; Saille, C.; Ceballos-Picot, I. Mitochondrial Impairment as an Early Event in the Process of Apoptosis Induced by Glutathione Depletion in Neuronal Cells: Relevance to Parkinson’s Disease. Biochem. Pharmacol. 1998, 56, 645–655. [Google Scholar] [CrossRef]
- Abdelhamid, G.; El-Kadi, A.O.S. Buthionine Sulfoximine, an Inhibitor of Glutathione Biosynthesis, Induces Expression of Soluble Epoxide Hydrolase and Markers of Cellular Hypertrophy in a Rat Cardiomyoblast Cell Line: Roles of the NF-ΚB and MAPK Signaling Pathways. Free Radic. Biol. Med. 2015, 82, 1–12. [Google Scholar] [CrossRef]
- Morales, N.P.; Yamaguchi, Y.; Murakami, K.; Kosem, N.; Utsumi, H. Hepatic Reduction of Carbamoyl-PROXYL in Ferric Nitrilotriacetate Induced Iron Overloaded Mice: An in vivo ESR Study. Biol. Pharm. Bull. 2012, 35, 1035–1040. [Google Scholar] [CrossRef]
- Li, Y.; Liang, K.; Yuan, L.; Gao, J.; Wei, L.; Zhao, L. The Role of Thioredoxin and Glutathione Systems in Arsenic-Induced Liver Injury in Rats under Glutathione Depletion. Int. J. Environ. Health Res. 2022, 1–17. [Google Scholar] [CrossRef]
- Ono, K.; Komuro, C.; Nishidai, T.; Shibamoto, Y.; Tsutsui, K.; Takahashi, M.; Abe, M. Radiosensitizing Effect of Misonidazole in Combination with an Inhibitor of Glutathione Synthesis in Murine Tumors. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1661–1666. [Google Scholar] [CrossRef]
- Nakagawa, I.; Suzuki, M.; Imura, N.; Naganuma, A. Enhancement of Paraquat Toxicity by Glutathione Depletion in Mice in vivo and in vitro. J. Toxicol. Sci. 1995, 20, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.; Xie, P.; Guo, X.; Fan, H.; Yu, D.; Zeng, C.; Chen, L. The Role of Glutathione Detoxification Pathway in MCLR-Induced Hepatotoxicity in SD Rats: Glutathione Detoxification Pathway of Microcystin-LR. Environ. Toxicol. 2015, 30, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Mello, D.F.; Trevisan, R.; Danielli, N.M.; Dafre, A.L. Vulnerability of Glutathione-Depleted Crassostrea gigas Oysters to Vibrio Species. Mar. Environ. Res. 2020, 154, 104870. [Google Scholar] [CrossRef]
- Standeven, A.M.; Wetterhahn, K.E. Tissue-Specific Changes in Glutathione and Cysteine after Buthionine Sulfoximine Treatment of Rats and the Potential for Artifacts in Thiol Levels Resulting from Tissue Preparation. Toxicol. Appl. Pharmacol. 1991, 107, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Sagisaka, H.; Arakawa, S.; Shibaya, Y.; Watanabe, M.; Igarashi, I.; Tanaka, K.; Totsuka, S.; Takasaki, W.; Manabe, S. A Novel Model of Continuous Depletion of Glutathione in Mice Treated with L-Buthionine(S,R)-Sulfoximine. J. Toxicol. Sci. 2003, 28, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Mizui, T.; Kinouchi, H.; Chan, P.H. Depletion of Brain Glutathione by Buthionine Sulfoximine Enhances Cerebral Ischemic Injury in Rats. Am. J. Physiol.-Heart Circ. Physiol. 1992, 262, H313–H317. [Google Scholar] [CrossRef] [PubMed]
- Lluis, J.M.; Morales, A.; Blasco, C.; Colell, A.; Mari, M.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Critical Role of Mitochondrial Glutathione in the Survival of Hepatocytes during Hypoxia. J. Biol. Chem. 2005, 280, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.; Ascensão, A.; Soares, J.M.C.; Neuparth, M.J.; Ferreira, R.; Oliveira, J.; Amado, F.; Duarte, J.A. Acute and Severe Hypobaric Hypoxia-Induced Muscle Oxidative Stress in Mice: The Role of Glutathione against Oxidative Damage. Eur. J. Appl. Physiol. 2004, 91, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Lushchak, V.I. Time-Course and Intensity-Based Classifications of Oxidative Stresses and Their Potential Application in Biomedical, Comparative and Environmental Research. Redox Rep. 2016, 21, 262–270. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Storey, K.B. Antioxidant Defenses in the Tolerance of Freezing and Anoxia by Garter Snakes. Am. J. Physiol. Integr. Comp. Physiol. 1993, 265, 646–652. [Google Scholar] [CrossRef]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial Reactive Oxygen Species Trigger Hypoxia-Induced Transcription. Proc. Natl. Acad. Sci. USA 1998, 95, 11715–11720. [Google Scholar] [CrossRef]
- Chandel, N.S.; McClintock, D.S.; Feliciano, C.E.; Wood, T.M.; Melendez, J.A.; Rodriguez, A.M.; Schumacker, P.T. Reactive Oxygen Species Generated at Mitochondrial Complex III Stabilize Hypoxia-Inducible Factor-1α during Hypoxia. J. Biol. Chem. 2000, 275, 25130–25138. [Google Scholar] [CrossRef]
- Clanton, T. Yet Another Oxygen Paradox. J. Appl. Physiol. 2005, 99, 1245–1246. [Google Scholar] [CrossRef] [PubMed]
- Hernansanz-Agustín, P.; Izquierdo-Álvarez, A.; Sánchez-Gómez, F.J.; Ramos, E.; Villa-Piña, T.; Lamas, S.; Bogdanova, A.; Martínez-Ruiz, A. Acute Hypoxia Produces a Superoxide Burst in Cells. Free Radic. Biol. Med. 2014, 71, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Sollitto, M.; Pallavicini, A.; Castellano, I. The Complex Evolutionary History of Sulfoxide Synthase in Ovothiol Biosynthesis. Proc. R. Soc. B 2019, 286, 20191812. [Google Scholar] [CrossRef]
- Castellano, I.; Seebeck, F.P. On Ovothiol Biosynthesis and Biological Roles: From Life in the Ocean to Therapeutic Potential. Nat. Prod. Rep. 2018, 35, 1241–1250. [Google Scholar] [CrossRef]
- Giraud-Billoud, M.; Vega, I.A.; Rinaldi Tosi, M.E.; Abud, M.A.; Calderón, M.L.; Castro-Vazquez, A. Antioxidant and Molecular Chaperone Defenses during Estivation and Arousal in the South American Apple-Snail Pomacea canaliculata. J. Exp. Biol. 2013, 216, 614–622. [Google Scholar] [CrossRef]
- Rodriguez, C.; Campoy-Diaz, A.D.; Giraud-Billoud, M. Short-Term Estivation and Hibernation Induce Changes in the Blood and Circulating Hemocytes of the Apple Snail Pomacea Canaliculata. Metabolites 2023, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Castro-Vazquez, A.; Campoy-Diaz, A.D.; Giuffrida, P.M.; Vega, I.A. Tolerance to Hypometabolism and Arousal Induced by Hibernation in the Apple Snail Pomacea Canaliculata (Caenogastropoda, Ampullariidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 224, 129–137. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.V. Hepatopancreatic Unsaturated Fatty Acids during Aestivation of the Snail, Pila Globosa. Comp. Biochem. Physiol. 1968, 24, 279–282. [Google Scholar] [CrossRef]
- Murano, C.; Zuccarotto, A.; Leone, S.; Sollitto, M.; Gerdol, M.; Castellano, I.; Palumbo, A. A Survey on the Distribution of Ovothiol and OvoA Gene Expression in Different Tissues and Cells: A Comparative Analysis in Sea Urchins and Mussels. Mar. Drugs 2022, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Castellano, I.; Migliaccio, O.; D’Aniello, S.; Merlino, A.; Napolitano, A.; Palumbo, A. Shedding Light on Ovothiol Biosynthesis in Marine Metazoans. Sci. Rep. 2016, 6, 21506. [Google Scholar] [CrossRef]

| Group | Foot Muscle | Hepatopancreas | ||||||
|---|---|---|---|---|---|---|---|---|
| TBARS (nmol/gww) | Carbonyl (nmol/mg prot.) | TBARS (nmol/gww) | Carbonyl (nmol/mg prot.) | |||||
| Control | 14.85 ± 2.73 | 5 | 10.41 ± 1.51 | 5 | 23.27 ± 9.41 | 8 | 11.98 ± 5.04 | 8 |
| Normoxia | ||||||||
| Saline | 16.27 ± 2.98 | 6 | 12.24 ± 2.53 | 5 | 20.13 ± 5.09 | 6 | 15.40 ± 8.64 | 6 |
| BSO | 15.06 ± 3.64 | 6 | 11.80 ± 1.41 | 6 | 19.87 ± 7.05 | 6 | 11.72 ± 4.07 | 6 |
| Anoxia | ||||||||
| Saline | 17.82 ± 2.94 | 6 | 9.65 ± 5.76 | 6 | 20.58 ± 6.92 | 8 | 10.78 ± 1.47 | 8 |
| BSO | 22.09 ± 12.37 | 7 | 10.09 ± 2.77 | 7 | 24.23 ± 6.00 | 7 | 12.97 ± 8.62 | 7 |
| Reoxygenation (15 min) | ||||||||
| Saline | 18.57 ± 3.50 | 7 | 9.82 ± 2.45 | 7 | 20.77 ± 6.45 | 7 | 10.19 ± 4.66 | 7 |
| BSO | 18.24 ± 5.67 | 7 | 11.16 ± 2.47 | 7 | 25.08 ± 3.71 | 5 | 8.32 ± 3.33 | 5 |
| Reoxygenation (30 min) | ||||||||
| Saline | 17.18 ± 2.91 | 6 | 10.86 ± 1.59 | 6 | 20.64 ± 11.21 | 6 | 9.87 ± 3.44 | 7 |
| BSO | 16.35 ± 2.19 | 4 | 8.35 ± 2.44 | 4 | 24.00 ± 10.23 | 5 | 9.26 ± 2.64 | 5 |
| Reoxygenation (60 min) | ||||||||
| Saline | 17.80 ± 4.40 | 7 | 12.08 ± 5.31 | 6 | 20.69 ± 7.17 | 5 | 8.23 ± 2.88 | 6 |
| BSO | 16.43 ± 2.80 | 7 | 11.58 ± 3.20 | 7 | 20.29 ± 10.69 | 4 | 9.29 ± 1.69 | 4 |
| Reoxygenation (120 min) | ||||||||
| Saline | 16.00 ± 2.74 | 5 | 11.48 ± 3.44 | 5 | 25.77 ± 6.48 | 5 | 8.24 ± 1.16 | 5 |
| BSO | 17.58 ± 3.66 | 7 | 10.35 ± 1.82 | 7 | 26.10 ± 12.39 | 7 | 10.33 ± 3.23 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira-Cravo, M.; Moreira, D.C.; Hermes-Lima, M. Glutathione Depletion Disrupts Redox Homeostasis in an Anoxia-Tolerant Invertebrate. Antioxidants 2023, 12, 1197. https://doi.org/10.3390/antiox12061197
Ferreira-Cravo M, Moreira DC, Hermes-Lima M. Glutathione Depletion Disrupts Redox Homeostasis in an Anoxia-Tolerant Invertebrate. Antioxidants. 2023; 12(6):1197. https://doi.org/10.3390/antiox12061197
Chicago/Turabian StyleFerreira-Cravo, Marlize, Daniel C. Moreira, and Marcelo Hermes-Lima. 2023. "Glutathione Depletion Disrupts Redox Homeostasis in an Anoxia-Tolerant Invertebrate" Antioxidants 12, no. 6: 1197. https://doi.org/10.3390/antiox12061197
APA StyleFerreira-Cravo, M., Moreira, D. C., & Hermes-Lima, M. (2023). Glutathione Depletion Disrupts Redox Homeostasis in an Anoxia-Tolerant Invertebrate. Antioxidants, 12(6), 1197. https://doi.org/10.3390/antiox12061197








