Improved Photocatalytic and Antioxidant Activity of Olive Fruit Extract-Mediated ZnO Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Olive Fruit Extract (OFE)
2.3. Biosynthesis of O. europaea-Mediated ZnO NPs
2.4. Characterization of ZnO@OFE
2.5. Adsorptive Study of ZnO@OFE
2.6. Photocatalytic Activity of ZnO@OFE NPs
2.7. Antioxidant Activity
2.7.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
2.7.2. Hydroxyl Radical Scavenging Assay
2.7.3. Hydrogen Peroxide Scavenging Activity
2.7.4. Superoxide Radical Scavenging Assay
3. Results and Discussion
3.1. Green Synthesis and Characterization of OFE-Mediated ZnO NPs (ZnO@OFE)
3.1.1. Powder X-ray Diffraction (XRD) Characterization
3.1.2. FTIR Analysis
3.1.3. UV-Vis Analysis of ZnO@OFE NPs
3.1.4. SEM-EDX Characterization
3.2. Adsorptive Assay of MB and MO Dyes
3.3. Photocatalytic Activity of ZnO@OFE NPs against MB and MO Dyes
3.3.1. Photocatalytic Degradation of Methylene Blue
3.3.2. Photocatalytic Degradation of Methyl Orange
3.3.3. Kinetics Rate of Photodegradation of MO and MB at ZnO NPs
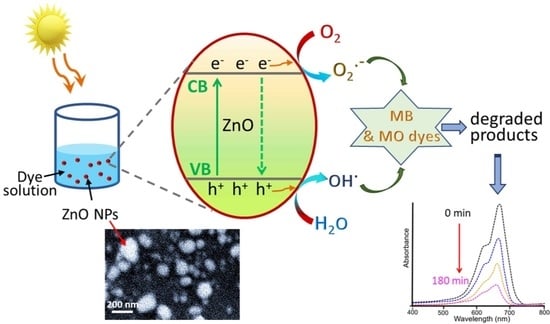
3.3.4. Mechanism of Photodegradation at ZnO
| Generation of electron–hole pairs: | ZnO + hν → ZnO + e− + h+ |
| Water photooxidation: | h+ + H2O → OH• + H+ |
| Oxygen photoreduction: | O2 + e− → O2•− |
| Dyes degradation: | OH• + O2•− + organic dye (MB/MO) → mineral acids + CO2 + H2O |
3.4. Antioxidant Activity
3.4.1. DPPH Scavenging Assay
3.4.2. Hydroxyl Free Radical Scavenging Assay
3.4.3. Hydrogen Peroxide Scavenging Assay
3.4.4. Superoxide Scavenging Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balcha, A.; Yadav, O.P.; Dey, T. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 2016, 23, 25485–25493. [Google Scholar] [CrossRef] [PubMed]
- Pirsaheb, M.; Karimi, K.; Shahmoradi, B.; Moradi, M.; Vasseghian, Y.; Niculina Dragoi, E. Photocatalyzed degradation of acid orange 7 dye under sunlight and ultraviolet irradiation using Ni-doped ZnO nanoparticles. J. Desalination Water Treat. 2019, 165, 321–332. [Google Scholar] [CrossRef]
- Ghaffar, A.; Zhang, L.; Zhu, X.; Chen, B. Porous PVdF/GO Nanofibrous Membranes for Selective Separation and Recycling of Charged Organic Dyes from Water. Environ. Sci. Technol. 2018, 52, 4265–4274. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thampi, K.R.; Tayade, R.J. Visible light driven redox-mediator-free dual semiconductor photocatalytic systems for pollutant degradation and the ambiguity in applying Z-scheme concept. Appl. Catal. B Environ. 2018, 227, 296–311. [Google Scholar] [CrossRef]
- Quek, J.-A.; Sin, J.-C.; Lam, S.-M.; Mohamed, A.R.; Zeng, H. Bioinspired green synthesis of ZnO structures with enhanced visible light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2020, 31, 1144–1158. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Ling, C.M.; Mohamed, A.R.; Bhatia, S. Performance of photocatalytic reactors using immobilized TiO2 film for the degradation of phenol and methylene blue dye present in water stream. Chemosphere 2004, 57, 547–554. [Google Scholar] [CrossRef]
- Wang, W.; Li, G.; Xia, D.; An, T.; Zhao, H.; Wong, P.K. Photocatalytic nanomaterials for solar-driven bacterial inactivation: Recent progress and challenges. Environ. Sci. Nano 2017, 4, 782–799. [Google Scholar] [CrossRef]
- Lam, S.-M.; Chew, K.-C.; Sin, J.-C.; Zeng, H.; Lin, H.; Li, H.; Lim, J.W.; Mohamed, A.R. Ameliorated photodegradation performance of polyethylene and polystyrene films incorporated with ZnO-PVP catalyst. J. Environ. Chem. Eng. 2022, 10, 107594. [Google Scholar] [CrossRef]
- Yasin, M.; Saeed, M.; Muneer, M.; Usman, M.; ul Haq, A.; Sadia, M.; Altaf, M. Development of Bi2O3-ZnO heterostructure for enhanced photodegradation of rhodamine B and reactive yellow dyes. Surf. Interfaces 2022, 30, 101846. [Google Scholar] [CrossRef]
- Atta, N.; Galal, A.; Amin, H.M. Synthesis and photoelectrochemical behavior of a hybrid electrode composed of polyaniline encapsulated in highly ordered TiO2 nanotubes array. Int. J. Electrochem. Sci. 2012, 7, 3610–3626. [Google Scholar]
- Atta, N.; Amin, H.M.; Khalil, M.W.; Galal, A. Nanotube arrays as photoanodes for dye sensitized solar cells using metal phthalocyanine dyes. Int. J. Electrochem. Sci. 2011, 6, 3316–3332. [Google Scholar]
- Daneshvar, N.; Salari, D.; Khataee, A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Bechambi, O.; Chalbi, M.; Najjar, W.; Sayadi, S. Photocatalytic activity of ZnO doped with Ag on the degradation of endocrine disrupting under UV irradiation and the investigation of its antibacterial activity. Appl. Surf. Sci. 2015, 347, 414–420. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Alallam, B.; Yong, Y.K.; Lim, V. Synthesis of Zinc Oxide Nanoparticles with Bioflavonoid Rutin: Characterisation, Antioxidant and Antimicrobial Activities and In Vivo Cytotoxic Effects on Artemia Nauplii. Antioxidants 2022, 11, 1853. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M.H.A. Green Synthesis and Characterization of ZnO Nanoparticles Using Pelargonium odoratissimum (L.) Aqueous Leaf Extract and Their Antioxidant, Antibacterial and Anti-inflammatory Activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; El-Din Hassan, S.; Salem, S.S.; Shaheen, T.I. In-Vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized Zinc oxide nanoparticles for medical textile applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef]
- Suchea, M.P.; Petromichelaki, E.; Romanitan, C.; Androulidaki, M.; Manousaki, A.; Viskadourakis, Z.; Ikram, R.; Pascariu, P.; Kenanakis, G. Obtaining Nanostructured ZnO onto Si Coatings for Optoelectronic Applications via Eco-Friendly Chemical Preparation Routes. Nanomaterials 2021, 11, 2490. [Google Scholar] [CrossRef]
- Kiriarachchi, H.D.; Abouzeid, K.M.; Bo, L.; El-Shall, M.S. Growth Mechanism of Sea Urchin ZnO Nanostructures in Aqueous Solutions and Their Photocatalytic Activity for the Degradation of Organic Dyes. ACS Omega 2019, 4, 14013–14020. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Facile Synthesis of Quasi Spherical ZnO Nanoparticles with Excellent Photocatalytic Activity. J. Clust. Sci. 2015, 26, 1187–1201. [Google Scholar] [CrossRef]
- Meena, P.L.; Poswal, K.; Surela, A.K. Facile synthesis of ZnO nanoparticles for the effective photodegradation of malachite green dye in aqueous solution. Water Environ. J. 2022, 36, 513–524. [Google Scholar] [CrossRef]
- Hashemi, S.; Asrar, Z.; Pourseyedi, S.; Nadernejad, N. Green synthesis of ZnO nanoparticles by Olive (Olea europaea). IET Nanobiotechnol. 2016, 10, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Sreelekshmi, P.B.; Pillai, R.R.; Unnimaya, S.; Anju, A.L.; Meera, A.P. Biofabrication of novel ZnO nanoparticles for efficient photodegradation of industrial dyes. Clean Technol. Environ. Policy 2023, 1–14. [Google Scholar] [CrossRef]
- Nilavukkarasi, M.; Vijayakumar, S.; Prathipkumar, S. Capparis zeylanica mediated bio-synthesized ZnO nanoparticles as antimicrobial, photocatalytic and anti-cancer applications. Mater. Sci. Energy Technol. 2020, 3, 335–343. [Google Scholar] [CrossRef]
- Mutukwa, D.; Taziwa, R.T.; Khotseng, L. Antibacterial and Photodegradation of Organic Dyes Using Lamiaceae-Mediated ZnO Nanoparticles: A Review. Nanomaterials 2022, 12, 4469. [Google Scholar] [CrossRef]
- Alharthi, M.N.; Ismail, I.; Bellucci, S.; Khdary, N.H.; Abdel Salam, M. Biosynthesis Microwave-Assisted of Zinc Oxide Nanoparticles with Ziziphus jujuba Leaves Extract: Characterization and Photocatalytic Application. Nanomaterials 2021, 11, 1682. [Google Scholar] [CrossRef]
- Meer, B.; Andleeb, A.; Iqbal, J.; Ashraf, H.; Meer, K.; Ali, J.S.; Drouet, S.; Anjum, S.; Mehmood, A.; Khan, T.; et al. Bio-Assisted Synthesis and Characterization of Zinc Oxide Nanoparticles from Lepidium sativum and Their Potent Antioxidant, Antibacterial and Anticancer Activities. Biomolecules 2022, 12, 855. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S. Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Appl. Surf. Sci. 2015, 345, 329–336. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Karthik, K.V.; Raghu, A.V.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Effect of chemically synthesis compared to biosynthesized ZnO-NPs using Solanum nigrum leaf extract and their photocatalytic, antibacterial and in-vitro antioxidant activity. J. Environ. Chem. Eng. 2020, 8, 103705. [Google Scholar] [CrossRef]
- Osuntokun, J.; Onwudiwe, D.C.; Ebenso, E.E. Green synthesis of ZnO nanoparticles using aqueous Brassica oleracea L. var. italica and the photocatalytic activity. Green Chem. Lett. Rev. 2019, 12, 444–457. [Google Scholar] [CrossRef]
- BLOIS, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Kunchandy, E.; Rao, M. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Cetinkaya, Y.; Göçer, H.; Menzek, A.; Gülçin, I. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch. Pharm. 2012, 345, 323–334. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- AbuDalo, M.A.; Al-Mheidat, I.R.; Al-Shurafat, A.W.; Grinham, C.; Oyanedel-Craver, V. Synthesis of silver nanoparticles using a modified Tollens’ method in conjunction with phytochemicals and assessment of their antimicrobial activity. PeerJ 2019, 7, e6413. [Google Scholar] [CrossRef]
- Masum, M.M.I.; Siddiqa, M.M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus emblica Fruit Extract and Its Inhibitory Action Against the Pathogen Acidovorax oryzae Strain RS-2 of Rice Bacterial Brown Stripe. Front. Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Amin, S.; Sher, M.; Ali, A.; Rehman, M.F.; Hayat, A.; Ikram, M.; Abbas, A.; Amin, H.M. Sulfonamide-functionalized silver nanoparticles as an analytical nanoprobe for selective Ni(II) sensing with synergistic antimicrobial activity. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100735. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)-a review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-H.; Dong, S.-Y.; Feng, J.-L.; Yin, X.-J.; Zhao, X.-C. Enhanced sunlight photocatalytic performance of Sn-doped ZnO for Methylene Blue degradation. J. Mol. Cat. A Chem. 2011, 335, 145–150. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q. Organic Nanoparticles in Foods: Fabrication, Characterization, and Utilization. Annu. Rev. Food Sci. Technol. 2016, 7, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Ambika, S.; Bharathi, K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015, 26, 1294–1299. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.-W.; Jung, S.-H.; Lee, B.R.; Oh, E.; Lee, K.-H. Precursor effects of citric acid and citrates on ZnO crystal formation. Langmuir 2009, 25, 3825–3831. [Google Scholar] [CrossRef]
- Siripireddy, B.; Mandal, B.K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol. 2017, 28, 785–797. [Google Scholar] [CrossRef]
- Lu, J.; Ali, H.; Hurh, J.; Han, Y.; Batjikh, I.; Rupa, E.J.; Anandapadmanaban, G.; Park, J.K.; Yang, D.-C. The assessment of photocatalytic activity of zinc oxide nanoparticles from the roots of Codonopsis lanceolata synthesized by one-pot green synthesis method. Optik 2019, 184, 82–89. [Google Scholar] [CrossRef]
- Karnan, T.; Selvakumar, S.A.S. Biosynthesis of ZnO nanoparticles using rambutan (Nephelium lappaceum L.) peel extract and their photocatalytic activity on methyl orange dye. J. Mol. Struct. 2016, 1125, 358–365. [Google Scholar] [CrossRef]
- Gawade, V.V.; Sabale, S.R.; Dhabbe, R.S.; Kite, S.V.; Garadkar, K.M. Bio-mediated synthesis of ZnO nanostructures for efficient photodegradation of methyl orange and methylene blue. J. Mater. Sci. Mater. Electron. 2021, 32, 28573–28586. [Google Scholar] [CrossRef]
- Nava, O.J.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Vilchis-Nestor, A.R.; Castro-Beltrán, A.; Mota-González, M.L.; Olivas, A. Influence of Camellia sinensis extract on Zinc Oxide nanoparticle green synthesis. J. Mol. Struct. 2017, 1134, 121–125. [Google Scholar] [CrossRef]
- Algarni, T.S.; Abduh, N.A.Y.; Aouissi, A.; Al Kahtani, A. Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract. Green Process. Synth. 2022, 11, 895–906. [Google Scholar] [CrossRef]
- Azarang, M.; Shuhaimi, A.; Yousefi, R.; Jahromi, S.P. One-pot sol–gel synthesis of reduced graphene oxide uniformly decorated zinc oxide nanoparticles in starch environment for highly efficient photodegradation of Methylene Blue. RSC Adv. 2015, 5, 21888–21896. [Google Scholar] [CrossRef]
- Rodríguez-Cabo, B.; Rodríguez-Palmeiro, I.; Corchero, R.; Rodil, R.; Rodil, E.; Arce, A.; Soto, A. Photocatalytic degradation of methyl orange, methylene blue and rhodamine B with AgCl nanocatalyst synthesised from its bulk material in the ionic liquid P6 6 6 14Cl. Water Sci. Technol. 2017, 75, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Minero, C.; Pellizzari, P.; Maurino, V.; Pelizzetti, E.; Vione, D. Enhancement of dye sonochemical degradation by some inorganic anions present in natural waters. Appl. Catal. B Environ. 2008, 77, 308–316. [Google Scholar] [CrossRef]
- Barbero, N.; Vione, D. Why Dyes Should Not Be Used to Test the Photocatalytic Activity of Semiconductor Oxides. Environ. Sci. Technol. 2016, 50, 2130–2131. [Google Scholar] [CrossRef]
- Kadam, V.V.; Shanmugam, S.D.; Ettiyappan, J.P.; Balakrishnan, R.M. Photocatalytic degradation of p-nitrophenol using biologically synthesized ZnO nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 12119–12130. [Google Scholar] [CrossRef]
- Houas, A. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M.D. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO:Eu nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Jacob, V.; Rajiiv, P. In vitro analysis: The antimicrobial and antioxidant activity of zinc oxide nanoparticles from curcuma longa. Asian J. Pharm. Clin. Res. 2019, 12, 200. [Google Scholar] [CrossRef]
- Singh, B.N.; Rawat, A.K.S.; Khan, W.; Naqvi, A.H.; Singh, B.R. Biosynthesis of stable antioxidant ZnO nanoparticles by Pseudomonas aeruginosa rhamnolipids. PLoS ONE 2014, 9, e106937. [Google Scholar] [CrossRef]











| Dye | Adsorption Efficiency/% | Degradation Efficiency/% | k × 103 min−1 | R2 |
|---|---|---|---|---|
| MB | 12 | 75 | 8 | 0.991 |
| MO | 10 | 87 | 13 | 0.978 |
| 4-NP | -- | 77 | 2 | 0.938 |
| Catalyst | Preparation Method/ Plant | Dye | Irradiation | Irradiation Time/ min | Catalyst Dose/ g L−1 | Dye Conc./ mg L−1 | Degradation Efficiency/% | k × 103 min−1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ZnO | Biosynthesis/ Cassia fistula | MO | UV (364 nm) | 120 | 1 | 10 | 84 | -- | [49] |
| ZnO | Biosynthesis/ Syzygium cumini | MB MO | UV (365 nm) | 60 150 | 2 1.5 | 1–2 | 84 88 | 35 12 | [50] |
| ZnO | Biosynthesis/ Camellia sinensis | MB | UV (10 W) | 10 | 1 | 15 | 75 | -- | [51] |
| Fe-doped ZnO | Biosynthesis/ wild olive | MO | Sunlight | 90 | 5 | 10 | 92 | 25 | [52] |
| ZnO | Sol gel | MB | UV (Hg lamp 365 nm) | 120 | 0.33 | 10 | 37 | 11 | [53] |
| P25 Degussa | -- | MB MO | UV lamp | 60 | 1 | 10 | 41 43 | -- | [54] |
| ZnO@OFE | Biosynthesis/sunlight/ O. europaea | MB MO | Sunlight | 180 | 1 | 10 | 75 87 | 8 13 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffar, S.; Abbas, A.; Naeem-ul-Hassan, M.; Assad, N.; Sher, M.; Ullah, S.; Alhazmi, H.A.; Najmi, A.; Zoghebi, K.; Al Bratty, M.; et al. Improved Photocatalytic and Antioxidant Activity of Olive Fruit Extract-Mediated ZnO Nanoparticles. Antioxidants 2023, 12, 1201. https://doi.org/10.3390/antiox12061201
Ghaffar S, Abbas A, Naeem-ul-Hassan M, Assad N, Sher M, Ullah S, Alhazmi HA, Najmi A, Zoghebi K, Al Bratty M, et al. Improved Photocatalytic and Antioxidant Activity of Olive Fruit Extract-Mediated ZnO Nanoparticles. Antioxidants. 2023; 12(6):1201. https://doi.org/10.3390/antiox12061201
Chicago/Turabian StyleGhaffar, Sadia, Azhar Abbas, Muhammad Naeem-ul-Hassan, Nasir Assad, Muhammad Sher, Sami Ullah, Hassan A. Alhazmi, Asim Najmi, Khalid Zoghebi, Mohammed Al Bratty, and et al. 2023. "Improved Photocatalytic and Antioxidant Activity of Olive Fruit Extract-Mediated ZnO Nanoparticles" Antioxidants 12, no. 6: 1201. https://doi.org/10.3390/antiox12061201