Protective and Pain-Killer Effects of AMC3, a Novel N-Formyl Peptide Receptors (FPRs) Modulator, in Experimental Models of Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Description
2.2. Chondrocytes Isolation and Culture
2.3. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide Assay (MTT)
2.4. Measurement of ROS Production
2.5. Catalase Activity
2.6. RNA Isolation, Reverse Transcription and Real Time Polymerase Chain Reaction (RT-PCR)
2.7. Animals
2.8. Complete Freund’s Adjuvant-Induced Inflammatory Arthritis
2.9. FPR agonist Administration
2.10. Paw Pressure Test
2.11. Incapacitance Test
2.12. Histological Evaluation
2.13. Immunofluorescence Analysis
2.14. Statistical Analysis
3. Results
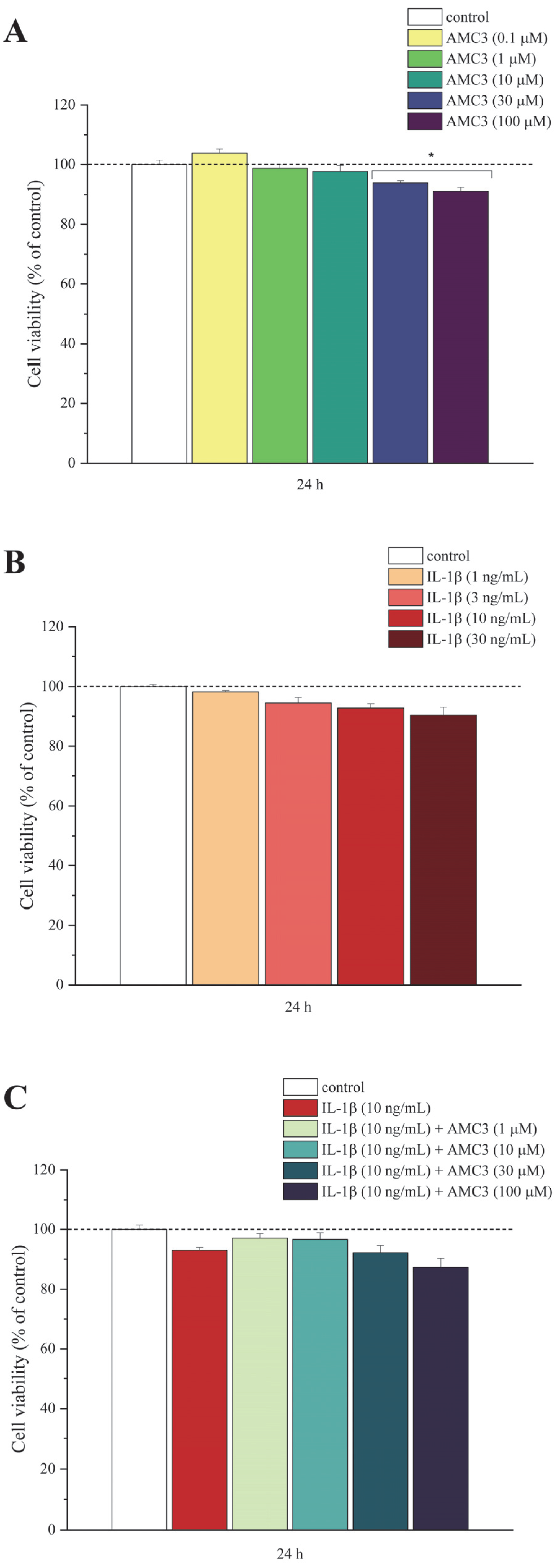
3.1. RA Modelling In Vitro and Evaluation of AMC3 Effects on Primary Rat Chondrocytes Viability
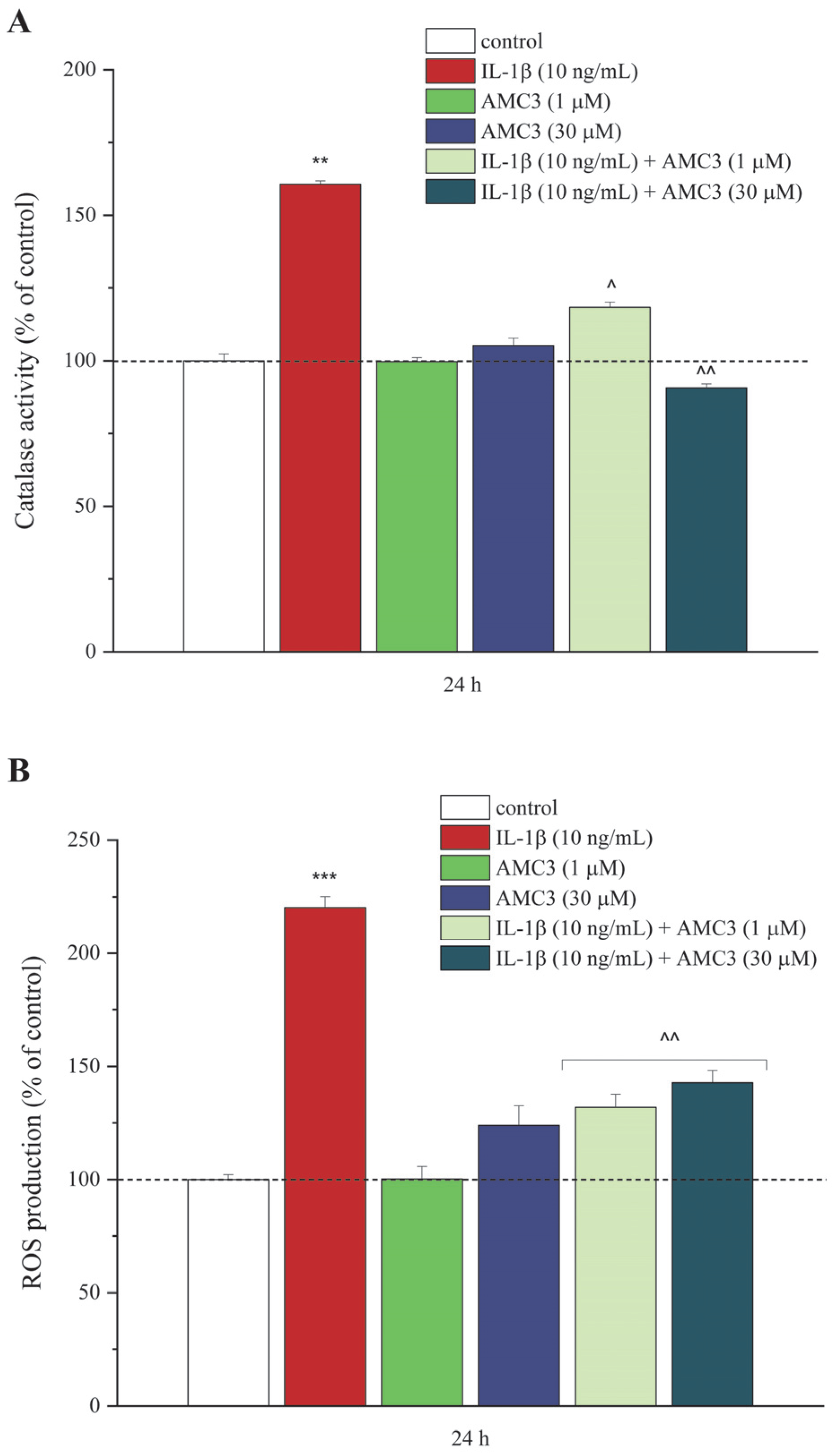
3.2. AMC3 Counteracts IL-1β-Exacerbated Oxidative Stress
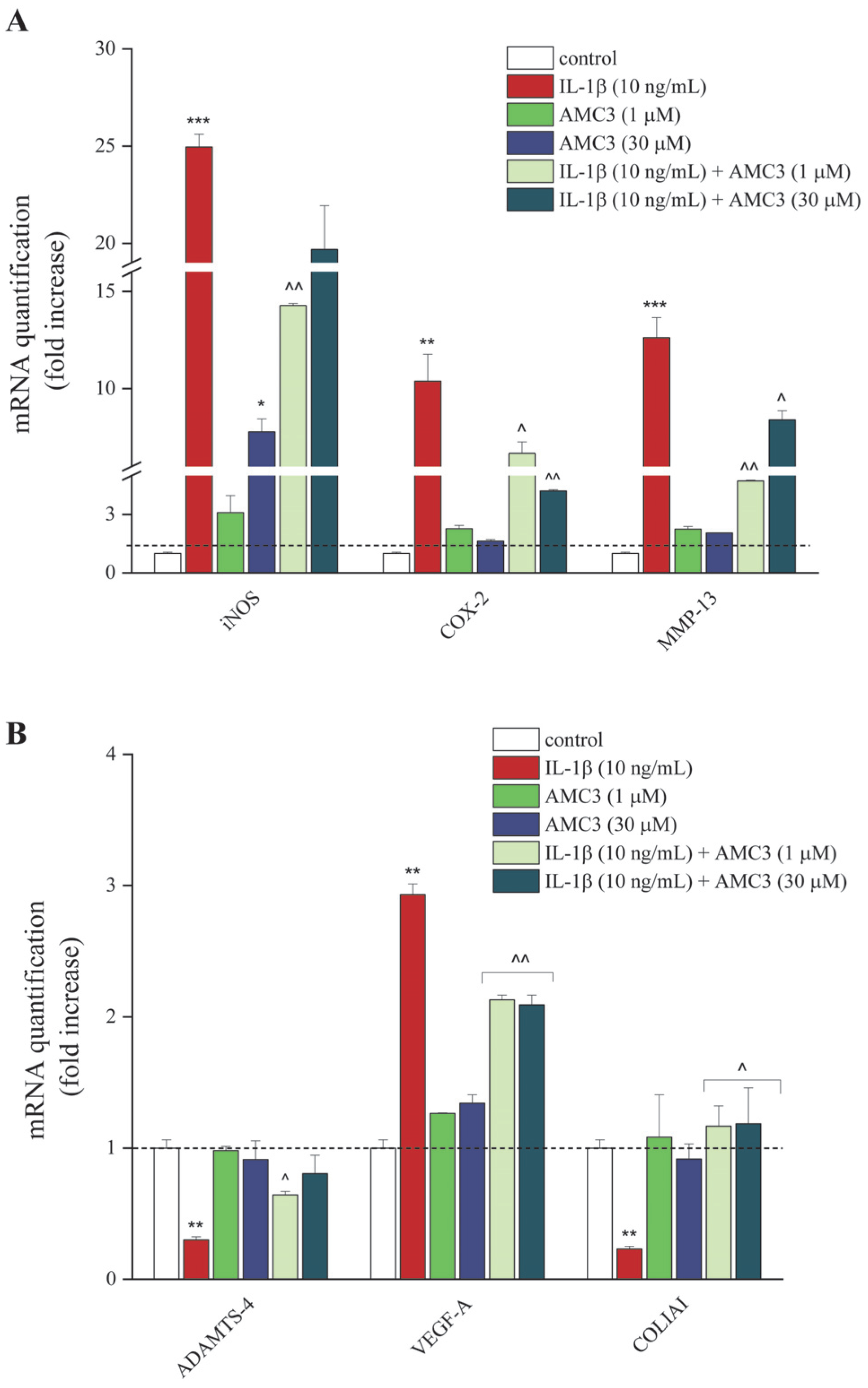
3.3. AMC3 Modulates Chondrocytic Expression Genes Involved in Inflammation and Tissue Structural Integrity Maintenance
3.4. Pain-Relieving Effect of AMC3 on CFA-Induced Rheumatoid Arthritis
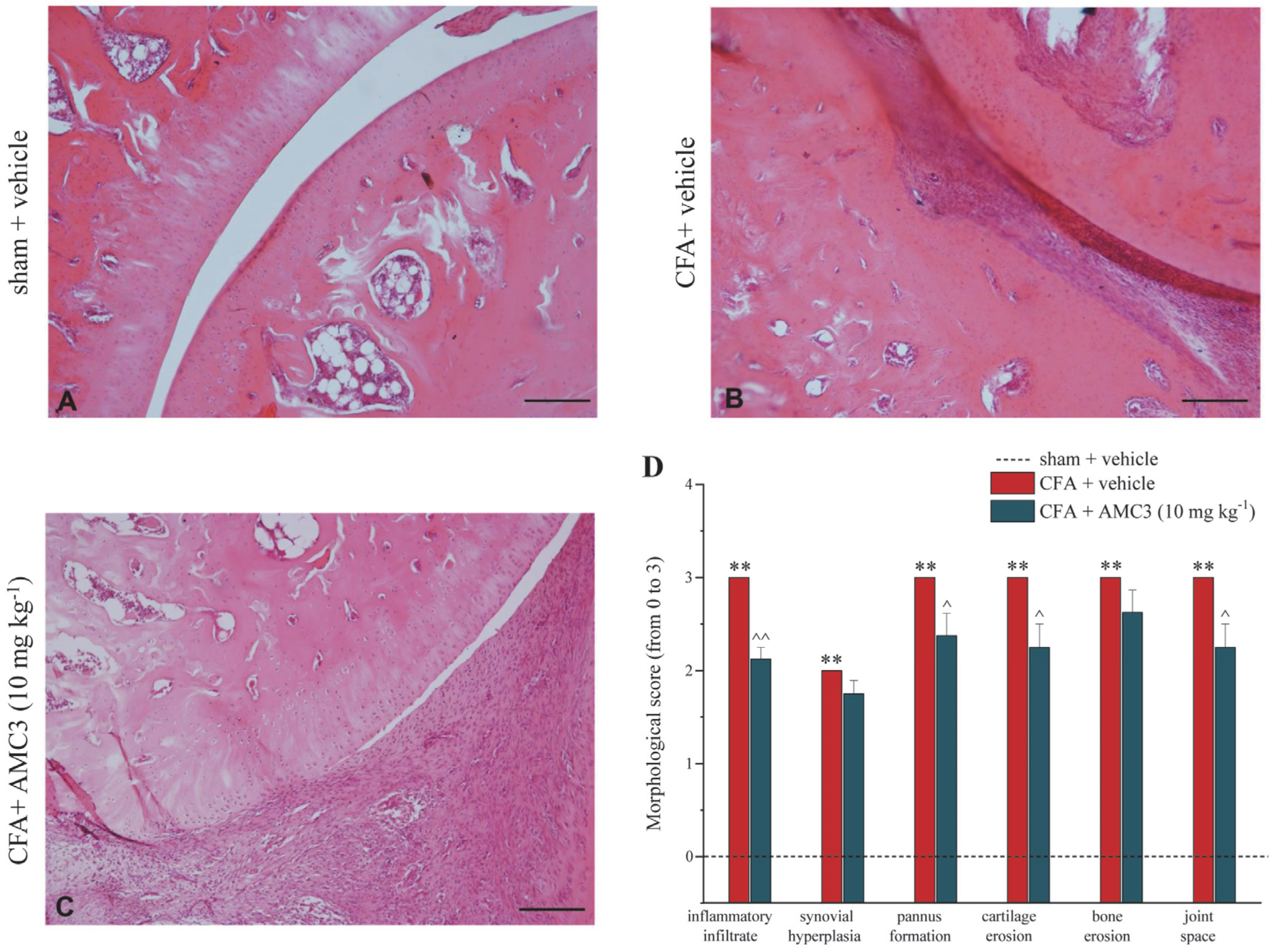
3.5. AMC3 Improves Morphological Alterations of Tibio-Tarsal Joint
3.6. AMC3-Modulated Expression of Inflammatory and Painful-Related Genes in Spinal Cord
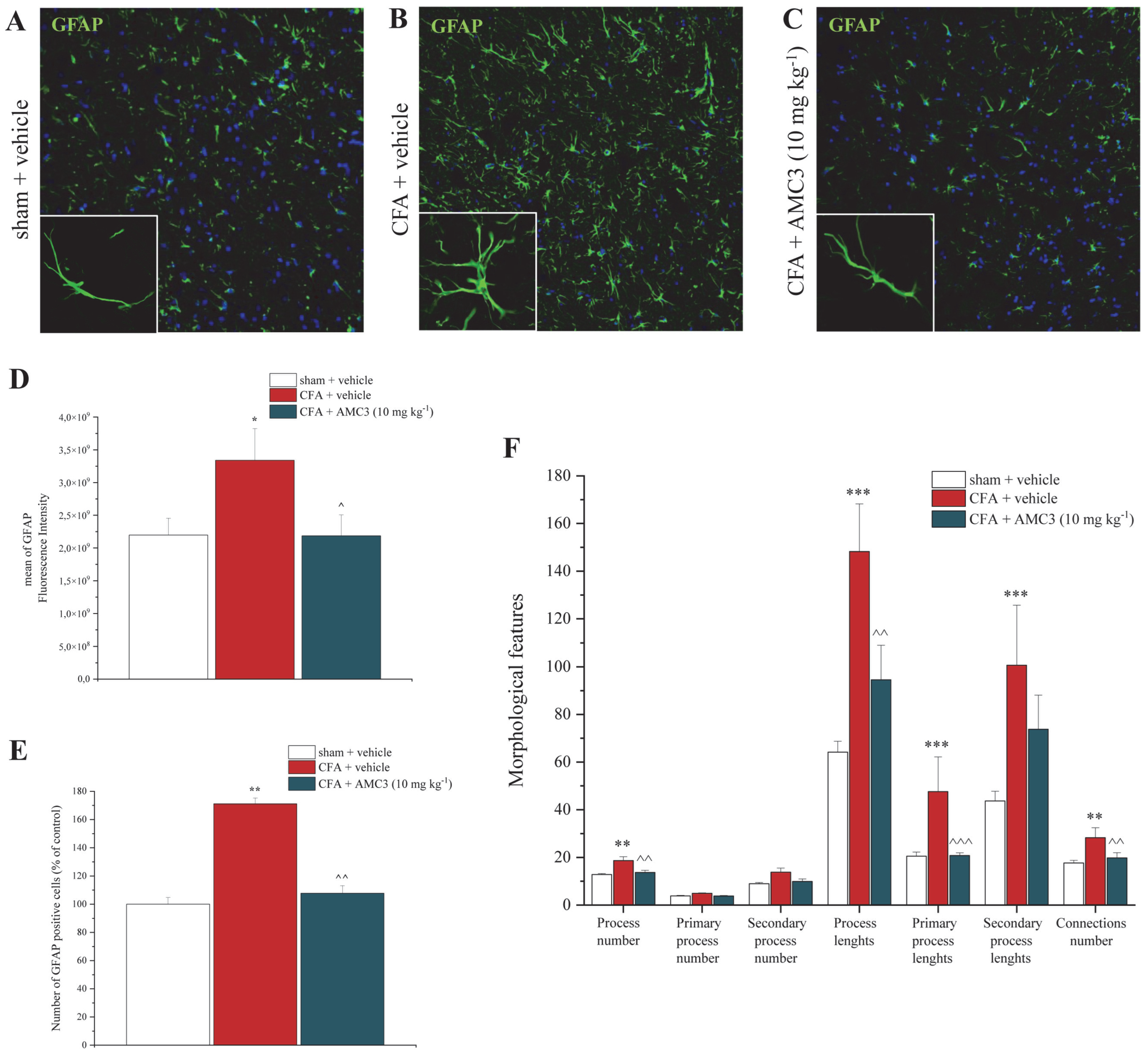
3.7. AMC3 Impairs Astroglial Activation in Spinal Cord
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The Etiology of Rheumatoid Arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L.; Pugner, K.; Kaarela, K.; Doyle, D.V.; Woolf, A.; Holmes, J.; Hieke, K. The Links between Joint Damage and Disability in Rheumatoid Arthritis. Rheumatology 2000, 39, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.A.; McWilliams, D.F. Mechanisms, Impact and Management of Pain in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2014, 10, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Tatangelo, M.; Tomlinson, G.; Paterson, J.M.; Keystone, E.; Bansback, N.; Bombardier, C. Health Care Costs of Rheumatoid Arthritis: A Longitudinal Population Study. PLoS ONE 2021, 16, e0251334. [Google Scholar] [CrossRef]
- Burmester, G.R.; Pope, J.E. Novel Treatment Strategies in Rheumatoid Arthritis. Lancet 2017, 339, 2338–2348. [Google Scholar] [CrossRef]
- Bartok, B.; Firestein, G.S. Fibroblast-like Synoviocytes: Key Effector Cells in Rheumatoid Arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Chen, Y.-J.; Chang, W.-A.; Tsai, W.-C.; Ou, T.-T.; Wu, C.-C.; Sung, W.-Y.; Yen, J.-H.; Kuo, P.-L. Dual Role of Chondrocytes in Rheumatoid Arthritis: The Chicken and the Egg. Int. J. Mol. Sci. 2020, 21, 1071. [Google Scholar] [CrossRef]
- Ainola, M.M.; Mandelin, J.A.; Liljeström, M.P.; Li, T.F.; Hukkanen, M.V.J.; Konttinen, Y.T. Pannus Invasion and Cartilage Degradation in Rheumatoid Arthritis: Involvement of MMP-3 and Interleukin-1beta. Clin. Exp. Rheumatol. 2005, 23, 644–650. [Google Scholar]
- Inglis, J.J.; Nissim, A.; Lees, D.M.; Hunt, S.P.; Chernajovsky, Y.; Kidd, B.L. The Differential Contribution of Tumour Necrosis Factor to Thermal and Mechanical Hyperalgesia during Chronic Inflammation. Arthritis Res. Ther. 2005, 7, R807–R816. [Google Scholar] [CrossRef]
- Goldring, M.B.; Marcu, K.B. Cartilage Homeostasis in Health and Rheumatic Diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Moin, S.; Shahzad, S.; Khan, A.Q. Level of Inflammatory Cytokines in Rheumatoid Arthritis Patients: Correlation with 25-Hydroxy Vitamin D and Reactive Oxygen Species. PLoS ONE 2017, 21, e0178879. [Google Scholar] [CrossRef] [PubMed]
- Polasik, K.; Piotrowska, E.; Lipińska, B.; Witkowski, J.M.; Bryl, E.; Tukaj, S. Vitamin D Status in Patients with Rheumatoid Arthritis: Correlation Analysis with Disease Activity and Progression, as Well as Serum IL-6 Levels. Acta Biochim. Pol. 2017, 64, 667–670. [Google Scholar] [CrossRef]
- Bas, D.B.; Su, J.; Wigerblad, G.; Svensson, C.I. Pain in Rheumatoid Arthritis: Models and Mechanisms. Pain Manag. 2016, 6, 265–284. [Google Scholar] [CrossRef] [PubMed]
- Önnheim, K.; Huang, S.; Holmertz, A.S.; Andersson, S.; Lönnblom, E.; Jonsson, C.; Holmdahl, R.; Gjertsson, I. Rheumatoid Arthritis Chondrocytes Produce Increased Levels of Pro-Inflammatory Proteins. Osteoarthr. Cartil. Open 2022, 6, 100235. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Resolution Phase Lipid Mediators of Inflammation: Agonists of Resolution. Curr. Opin. Pharmacol. 2013, 13, 632–640. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in Inflammation: Emergence of the pro-Resolving Superfamily of Mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Tsai, Y.-F.; Yang, S.-C.; Hwang, T.-L. Formyl Peptide Receptor Modulators: A Patent Review and Potential Applications for Inflammatory Diseases (2012–2015). Expert Opin. Ther. Pat. 2016, 26, 1139–1156. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.; Gong, W.; Tang, P.; Yoshimura, T.; Wang, J.M. Regulation of Inflammation by Members of the Formyl-Peptide Receptor Family. J. Autoimmun. 2017, 85, 64–77. [Google Scholar] [CrossRef]
- Migeotte, I.; Communi, D.; Parmentier, M. Formyl Peptide Receptors: A Promiscuous Subfamily of G Protein-Coupled Receptors Controlling Immune Responses. Cytokine Growth Factor Rev. 2006, 17, 501–519. [Google Scholar] [CrossRef]
- Ye, R.D.; BOULAY, F.; WANG, J.M.; DAHLGREN, C.; GERARD, C.; PARMENTIER, M.; SERHAN, C.N.; MURPHY, P.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the Formyl Peptide Receptor (FPR) Family. Pharmacol. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef] [PubMed]
- Prevete, N.; Liotti, F.; Illiano, A.; Amoresano, A.; Pucci, P.; de Paulis, A.; Melillo, R.M. Formyl Peptide Receptor 1 Suppresses Gastric Cancer Angiogenesis and Growth by Exploiting Inflammation Resolution Pathways. OncoImmunology 2017, 6, e1293213. [Google Scholar] [CrossRef] [PubMed]
- Dufton, N.; Hannon, R.; Brancaleone, V.; Dalli, J.; Patel, H.B.; Gray, M.; D’Acquisto, F.; Buckingham, J.C.; Perretti, M.; Flower, R.J. Anti-Inflammatory Role of the Murine Formyl-Peptide Receptor 2: Ligand-Specific Effects on Leukocyte Responses and Experimental Inflammation. J. Immunol. 2010, 184, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Stama, M.L.; Ślusarczyk, J.; Lacivita, E.; Kirpotina, L.N.; Schepetkin, I.A.; Chamera, K.; Riganti, C.; Perrone, R.; Quinn, M.T.; Basta-Kaim, A.; et al. Novel Ureidopropanamide Based N-Formyl Peptide Receptor 2 (FPR2) Agonists with Potential Application for Central Nervous System Disorders Characterized by Neuroinflammation. Eur. J. Med. Chem. 2017, 141, 703–720. [Google Scholar] [CrossRef]
- Perretti, M.; Leroy, X.; Bland, E.J. Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation: Trends Pharmacol. Sci. 2015, 36, 737–755. [Google Scholar] [CrossRef]
- Mastromarino, M.; Favia, M.; Schepetkin, I.A.; Kirpotina, L.N.; Trojan, E.; Niso, M.; Carrieri, A.; Leśkiewicz, M.; Regulska, M.; Darida, M.; et al. Design, Synthesis, Biological Evaluation, and Computational Studies of Novel Ureidopropanamides as Formyl Peptide Receptor 2 (FPR2) Agonists to Target the Resolution of Inflammation in Central Nervous System Disorders. J. Med. Chem. 2022, 65, 5004–5028. [Google Scholar] [CrossRef]
- Crocetti, L.; Vergelli, C.; Guerrini, G.; Cantini, N.; Kirpotina, L.N.; Schepetkin, I.A.; Quinn, M.T.; Parisio, C.; Di Cesare Mannelli, L.; Ghelardini, C.; et al. Novel Formyl Peptide Receptor (FPR) Agonists with Pyridinone and Pyrimidindione Scaffolds That Are Potentially Useful for the Treatment of Rheumatoid Arthritis. Bioorganic Chem. 2020, 100, 103880. [Google Scholar] [CrossRef]
- Crocetti, L.; Vergelli, C.; Guerrini, G.; Giovannoni, M.P.; Kirpotina, L.N.; Khlebnikov, A.I.; Ghelardini, C.; Di Cesare Mannelli, L.; Lucarini, E.; Schepetkin, I.A.; et al. Pyridinone Derivatives as Interesting Formyl Peptide Receptor (FPR) Agonists for the Treatment of Rheumatoid Arthritis. Molecules 2021, 26, 6583. [Google Scholar] [CrossRef]
- Butler, S.H.; Godefroy, F.; Besson, J.-M.; Weil-Fugazza, J. A Limited Arthritic Model for Chronic Pain Studies in the Rat. Pain 1992, 48, 73. [Google Scholar] [CrossRef]
- Micheli, L.; Ghelardini, C.; Lucarini, E.; Parisio, C.; Trallori, E.; Cinci, L.; Di Cesare Mannelli, L. Intra-Articular Mucilages: Behavioural and Histological Evaluations for a New Model of Articular Pain. J. Pharm. Pharmacol. 2019, 71, 971–981. [Google Scholar] [CrossRef]
- Bird, G.; Braithwaite, I.; Harper, J.; McKinstry, S.; Koorevaar, I.; Fingleton, J.; Semprini, A.; Dilcher, M.; Jennings, L.; Weatherall, M.; et al. Protocol for a Randomised, Single-Blind, Two-Arm, Parallel-Group Controlled Trial of the Efficacy of Rhinothermy Delivered by Nasal High Flow Therapy in the Treatment of the Common Cold. BMJ Open 2019, 9, e028098. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Micheli, L.; Cinci, L.; Maresca, M.; Vergelli, C.; Pacini, A.; Quinn, M.T.; Giovannoni, M.P.; Ghelardini, C. Effects of the Neutrophil Elastase Inhibitor EL-17 in Rat Adjuvant-Induced Arthritis. Rheumatology 2016, 55, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Snekhalatha, U.; Anburajan, M.; Venkatraman, B.; Menaka, M. Evaluation of Complete Freund’s Adjuvant-Induced Arthritis in a Wistar Rat Model. Z. Für Rheumatol. 2013, 72, 375–382. [Google Scholar] [CrossRef]
- Zhou, P.-H.; Liu, S.-Q.; Peng, H. The Effect of Hyaluronic Acid on IL-1β-Induced Chondrocyte Apoptosis in a Rat Model of Osteoarthritis. J. Orthop. Res. 2008, 26, 1643–1648. [Google Scholar] [CrossRef]
- Bugatti, S.; Manzo, A.; Montecucco, C.; Caporali, R. The Clinical Value of Autoantibodies in Rheumatoid Arthritis. Front. Med. 2018, 5, 339. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the Role of Cytokines in the Pathogenesis of Rheumatoid Arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Gao, Y.; Qi, D.; Zhao, L.; Zhao, L.; Liu, C.; Tao, T.; Zhou, C.; Sun, X.; et al. NR1D1 Modulates Synovial Inflammation and Bone Destruction in Rheumatoid Arthritis. Cell Death Dis. 2020, 11, 129. [Google Scholar] [CrossRef]
- Kumar, P.; Banik, S. Pharmacotherapy Options in Rheumatoid Arthritis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2013, 6, 35–43. [Google Scholar] [CrossRef]
- Feldmann, M.; Maini, R.N. Perspectives From Masters in Rheumatology and Autoimmunity: Can We Get Closer to a Cure for Rheumatoid Arthritis? Arthritis Rheumatol. 2015, 67, 2283–2291. [Google Scholar] [CrossRef]
- Combe, B.; Landewe, R.; Daien, C.I.; Hua, C.; Aletaha, D.; Álvaro-Gracia, J.M.; Bakkers, M.; Brodin, N.; Burmester, G.R.; Codreanu, C.; et al. 2016 Update of the EULAR Recommendations for the Management of Early Arthritis. Ann. Rheum. Dis. 2017, 76, 948–959. [Google Scholar] [CrossRef]
- Siebert, S.; Tsoukas, A.; Robertson, J.; McInnes, I. Cytokines as Therapeutic Targets in Rheumatoid Arthritis and Other Inflammatory Diseases. Pharmacol. Rev. 2015, 67, 280–309. [Google Scholar] [CrossRef] [PubMed]
- Ferraccioli, G.; Bracci-Laudiero, L.; Alivernini, S.; Gremese, E.; Tolusso, B.; De Benedetti, F. Interleukin-1β and Interleukin-6 in Arthritis Animal Models: Roles in the Early Phase of Transition from Acute to Chronic Inflammation and Relevance for Human Rheumatoid Arthritis. Mol. Med. 2010, 16, 552–557. [Google Scholar] [CrossRef]
- Johnson, C.I.; Argyle, D.J.; Clements, D.N. In Vitro Models for the Study of Osteoarthritis. Vet. J. 2016, 209, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, B.; Matta, C.; Fellows, C.R.; Boocock, D.J.; Smith, J.R.; Liddell, S.; Lafeber, F.; van Spil, W.E.; Mobasheri, A. Alterations in the Chondrocyte Surfaceome in Response to Pro-Inflammatory Cytokines. BMC Mol. Cell Biol. 2020, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative Stress and Inflammation in Osteoarthritis Pathogenesis: Role of Polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Martínez-Flores, K.; Martínez-Nava, G.A.; Clavijo-Cornejo, D.; Fernández-Torres, J.; Sánchez-Sánchez, R. Rheumatoid Arthritis and Oxidative Stress. Cell. Mol. Biol. 2022, 68, 174–184. [Google Scholar] [CrossRef]
- Phull, A.-R.; Nasir, B.; ul Haq, I.; Kim, S.J. Oxidative Stress, Consequences and ROS Mediated Cellular Signaling in Rheumatoid Arthritis. Chem. Biol. Interact. 2018, 281, 121–136. [Google Scholar] [CrossRef]
- Ginter, E.; Simko, V.; Panakova, V. Antioxidants in Health and Disease. Bratisl. Med. J. 2014, 115, 603–606. [Google Scholar] [CrossRef]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of SE-Glutathione Peroxidase, Catalase, and CU/ZN-SOD for Cell Survival against Oxidative Stress. Free Radic. Biol. Med. 1994, 17, 235–248. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Labunskyy, V.M.L.; Gladyshev, V.N. Role of Reactive Oxygen Species-Mediated Signaling in Aging. Antioxid. Redox Signal. 2013, 19, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Fattori, V.; Saito, P.; Pinto, I.C.; Rodrigues, C.C.A.; Melo, C.P.B.; Bussmann, A.J.C.; Staurengo-Ferrari, L.; Bezerra, J.R.; Vignoli, J.A.; et al. The Lipoxin Receptor/FPR2 Agonist BML-111 Protects Mouse Skin Against Ultraviolet B Radiation. Molecules 2020, 25, 2953. [Google Scholar] [CrossRef] [PubMed]
- Abshirini, M.; Ilesanmi-Oyelere, B.L.; Kruger, M.C. Potential Modulatory Mechanisms of Action by Long-Chain Polyunsaturated Fatty Acids on Bone Cell and Chondrocyte Metabolism. Prog. Lipid Res. 2021, 83, 101113. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jiang, Y.; Yao, Z.; Chen, P.; Yu, B.; Wang, S. Aucubin Protects Chondrocytes Against IL-1β-Induced Apoptosis In Vitro And Inhibits Osteoarthritis In Mice Model. Drug Des. Devel. Ther. 2019, 13, 3529–3538. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, Q.; Huang, T.; Huang, C. Paeoniflorin Inhibits IL-1β-induced Expression of Inflammatory Mediators in Human Osteoarthritic Chondrocyte. Mol. Med. Rep. 2018, 17, 3306–3311. [Google Scholar] [CrossRef]
- Li, J.; Tang, R.-S.; Shi, Z.; Li, J.-Q. Nuclear Factor-ΚB in Rheumatoid Arthritis. Int. J. Rheum. Dis. 2020, 23, 1627–1635. [Google Scholar] [CrossRef]
- Gambari, L.; Cellamare, A.; Grassi, F.; Grigolo, B.; Panciera, A.; Ruffilli, A.; Faldini, C.; Desando, G. Overview of Anti-Inflammatory and Anti-Nociceptive Effects of Polyphenols to Halt Osteoarthritis: From Preclinical Studies to New Clinical Insights. Int. J. Mol. Sci. 2022, 23, 15861. [Google Scholar] [CrossRef]
- Crofford, L.J. The Role of COX-2 in Rheumatoid Arthritis Synovial Tissues. Arthritis Res. 2000, 1, S30. [Google Scholar] [CrossRef]
- Micheli, L.; Parisio, C.; Lucarini, E.; Vona, A.; Toti, A.; Pacini, A.; Mello, T.; Boccella, S.; Ricciardi, F.; Maione, S.; et al. VEGF-A/VEGFR-1 Signalling and Chemotherapy-Induced Neuropathic Pain: Therapeutic Potential of a Novel Anti-VEGFR-1 Monoclonal Antibody. J. Exp. Clin. Cancer Res. 2021, 40, 320. [Google Scholar] [CrossRef]
- Ueda, T.; Watanabe, M.; Miwa, Y.; Shibata, Y.; Kumamoto, N.; Ugawa, S. Vascular Endothelial Growth Factor-A Is Involved in Intramuscular Carrageenan-Induced Cutaneous Mechanical Hyperalgesia through the Vascular Endothelial Growth Factor-A Receptor 1 and Transient Receptor Potential Vanilloid 1 Pathways. NeuroReport 2023, 34, 238. [Google Scholar] [CrossRef]
- Chen, L.; Lv, F.; Pei, L. Annexin 1: A Glucocorticoid-Inducible Protein That Modulates Inflammatory Pain. Eur. J. Pain 2014, 18, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Colucci, M.; Stefanucci, A.; Mollica, A.; Aloisi, A.M.; Maione, F.; Pieretti, S. New Insights on Formyl Peptide Receptor Type 2 Involvement in Nociceptive Processes in the Spinal Cord. Life 2022, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Toyoshima, S.; Miki, Y.; Taketomi, Y.; Ito, M.; Lee, H.; Saito, S.; Murakami, M.; Okayama, Y. Activation of Inflammation and Resolution Pathways of Lipid Mediators in Synovial Fluid from Patients with Severe Rheumatoid Arthritis Compared with Severe Osteoarthritis. Asia Pac. Allergy 2020, 10, e21. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Yankner, B.A. The Aging Stress Response: Molecular Cell. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.E.; Bruckner, P.; Pujol, J.-P.L. The Role of Reactive Oxygen Species in Homeostasis and Degradation of Cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef]
- Ni, S.; Li, C.; Xu, N.; Liu, X.; Wang, W.; Chen, W.; Wang, Y.; van Wijnen, A.J. Follistatin-like Protein 1 Induction of Matrix Metalloproteinase 1, 3 and 13 Gene Expression in Rheumatoid Arthritis Synoviocytes Requires MAPK, JAK/STAT3 and NF-ΚB Pathways. J. Cell. Physiol. 2019, 234, 454–463. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Xu, X.-X.; Xu, T. Ginsenoside Ro Suppresses Interleukin-1β-Induced Apoptosis and Inflammation in Rat Chondrocytes by Inhibiting NF-ΚB. Chin. J. Nat. Med. 2015, 13, 283–289. [Google Scholar] [CrossRef]
- Yoshida, Y.; Ogata, A.; Kang, S.; Ebina, K.; Shi, K.; Nojima, S.; Kimura, T.; Ito, D.; Morimoto, K.; Nishide, M.; et al. Semaphorin 4D Contributes to Rheumatoid Arthritis by Inducing Inflammatory Cytokine Production: Pathogenic and Therapeutic Implications. Arthritis Rheumatol. 2015, 67, 1481–1490. [Google Scholar] [CrossRef]
- FitzGerald, O.; Soden, M.; Yanni, G.; Robinson, R.; Bresnihan, B. Morphometric Analysis of Blood Vessels in Synovial Membranes Obtained from Clinically Affected and Unaffected Knee Joints of Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 1991, 50, 792–796. [Google Scholar] [CrossRef]
- Paleolog, E.M. The Vasculature in Rheumatoid Arthritis: Cause or Consequence? Int. J. Exp. Pathol. 2009, 90, 249–261. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.-J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Miner. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Afuwape, A.O.; Kiriakidis, S.; Paleolog, E.M. The Role of the Angiogenic Molecule VEGF in the Pathogenesis of Rheumatoid Arthritis. Histol. Histopathol. 2002, 17, 961–972. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.J.; Liu, S.-C.; Su, C.-M.; Wang, Y.-H.; Tsai, C.-H.; Tang, C.-H. Implications of Angiogenesis Involvement in Arthritis. Int. J. Mol. Sci. 2018, 19, 2012. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Tenci, B.; Micheli, L.; Vona, A.; Corti, F.; Zanardelli, M.; Lapucci, A.; Clemente, A.M.; Failli, P.; Ghelardini, C. Adipose-Derived Stem Cells Decrease Pain in a Rat Model of Oxaliplatin-Induced Neuropathy: Role of VEGF-A Modulation. Neuropharmacology 2018, 131, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Bani, D.; Bencini, A.; Brandi, M.L.; Calosi, L.; Cantore, M.; Carossino, A.M.; Ghelardini, C.; Valtancoli, B.; Failli, P. Therapeutic Effects of the Superoxide Dismutase Mimetic Compound MnIIMe2DO2A on Experimental Articular Pain in Rats. Mediators Inflamm. 2013, 2013, e905360. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Micheli, L.; Cinci, L.; Bilia, A.R.; Ghelardini, C.; Di Cesare Mannelli, L. Pain Relieving and Protective Effects of Astragalus Hydroalcoholic Extract in Rat Arthritis Models. J. Pharm. Pharmacol. 2017, 69, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Wang, F.; He, X.; Zhou, K.; Wu, X.; Wu, X. Protective Effect of Corynoline on the CFA Induced Rheumatoid Arthritis via Attenuation of Oxidative and Inflammatory Mediators. Mol. Cell. Biochem. 2021, 476, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Koop, S.M.W.; ten Klooster, P.M.; Vonkeman, H.E.; Steunebrink, L.M.M.; van de Laar, M.A.F.J. Neuropathic-like Pain Features and Cross-Sectional Associations in Rheumatoid Arthritis. Arthritis Res. Ther. 2015, 17, 237. [Google Scholar] [CrossRef]
- DeLeo, J.A.; Yezierski, R.P. The Role of Neuroinflammation and Neuroimmune Activation in Persistent Pain. PAIN 2001, 90, 1–6. [Google Scholar] [CrossRef]
- Guo, W.; Imai, S.; Zou, S.; Yang, J.; Watanabe, M.; Wang, J.; Dubner, R.; Wei, F.; Ren, K. Altered Glial Glutamate Transporter Expression in Descending Circuitry and the Emergence of Pain Chronicity. Mol. Pain 2019, 15, 1744806918825044. [Google Scholar] [CrossRef]
- Hidayati, H.B.; Machfoed, M.H.; Subadi, I.; Khaerunnisa, S. Widjiati Increase in the Glutamate Transporter 1 and Time Withdrawal Latency Following Wet Cupping Therapy in Chronic Constriction Injury in Rats. Anaesth. Pain Intensive Care 2021, 25, 50–56. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, V.; Toti, A.; Lucarini, E.; Parisio, C.; Micheli, L.; Ciampi, C.; Margiotta, F.; Crocetti, L.; Vergelli, C.; Giovannoni, M.P.; et al. Protective and Pain-Killer Effects of AMC3, a Novel N-Formyl Peptide Receptors (FPRs) Modulator, in Experimental Models of Rheumatoid Arthritis. Antioxidants 2023, 12, 1207. https://doi.org/10.3390/antiox12061207
Ferrara V, Toti A, Lucarini E, Parisio C, Micheli L, Ciampi C, Margiotta F, Crocetti L, Vergelli C, Giovannoni MP, et al. Protective and Pain-Killer Effects of AMC3, a Novel N-Formyl Peptide Receptors (FPRs) Modulator, in Experimental Models of Rheumatoid Arthritis. Antioxidants. 2023; 12(6):1207. https://doi.org/10.3390/antiox12061207
Chicago/Turabian StyleFerrara, Valentina, Alessandra Toti, Elena Lucarini, Carmen Parisio, Laura Micheli, Clara Ciampi, Francesco Margiotta, Letizia Crocetti, Claudia Vergelli, Maria Paola Giovannoni, and et al. 2023. "Protective and Pain-Killer Effects of AMC3, a Novel N-Formyl Peptide Receptors (FPRs) Modulator, in Experimental Models of Rheumatoid Arthritis" Antioxidants 12, no. 6: 1207. https://doi.org/10.3390/antiox12061207
APA StyleFerrara, V., Toti, A., Lucarini, E., Parisio, C., Micheli, L., Ciampi, C., Margiotta, F., Crocetti, L., Vergelli, C., Giovannoni, M. P., Di Cesare Mannelli, L., & Ghelardini, C. (2023). Protective and Pain-Killer Effects of AMC3, a Novel N-Formyl Peptide Receptors (FPRs) Modulator, in Experimental Models of Rheumatoid Arthritis. Antioxidants, 12(6), 1207. https://doi.org/10.3390/antiox12061207







