Hydrogen-Rich Water Ameliorates Metabolic Disorder via Modifying Gut Microbiota in Impaired Fasting Glucose Patients: A Randomized Controlled Study
Abstract
:1. Introduction
2. Methods and Materials
2.1. Screening and Grouping of Subjects
2.1.1. Subjects
2.1.2. Inclusion Criteria
2.1.3. Exclusion Criteria
- (1)
- Patients with type 1, type 2 diabetes and other special types of diabetes.
- (2)
- Patients with obvious abnormal liver and kidney function or those who have recently used drugs that affect liver and kidney function.
- (3)
- Patients during pregnancy and breastfeeding.
- (4)
- Subjects with a blood relationship with other subjects.
- (5)
- Not living in Jinzhou within 3 years before the start of the study.
- (6)
- Patients who have used antioxidants such as vitamin E, vitamin C, folate tablets, etc., within 3 months prior to the start of the study.
- (7)
- Patients who used antibiotics within 1 month of the start of the study.
- (8)
- Patients in an acute stress state caused by other diseases (such as fractures, elevated blood sugar caused by trauma, etc.).
- (9)
- Patients with a history of alcoholism, cancer, HIV infection, stroke, myocardial infarction and other serious chronic disease.
2.2. Study Design
2.2.1. Basic Index Collection
2.2.2. Glucose Metabolism-Related Index Detection
2.2.3. Lipid Metabolism-Related Index Detection
2.2.4. Inflammatory Factor Detection
2.2.5. Metabolism-Related Index Detection
2.2.6. Intestinal Flora Detection
2.3. Statistical Analysis
3. Results
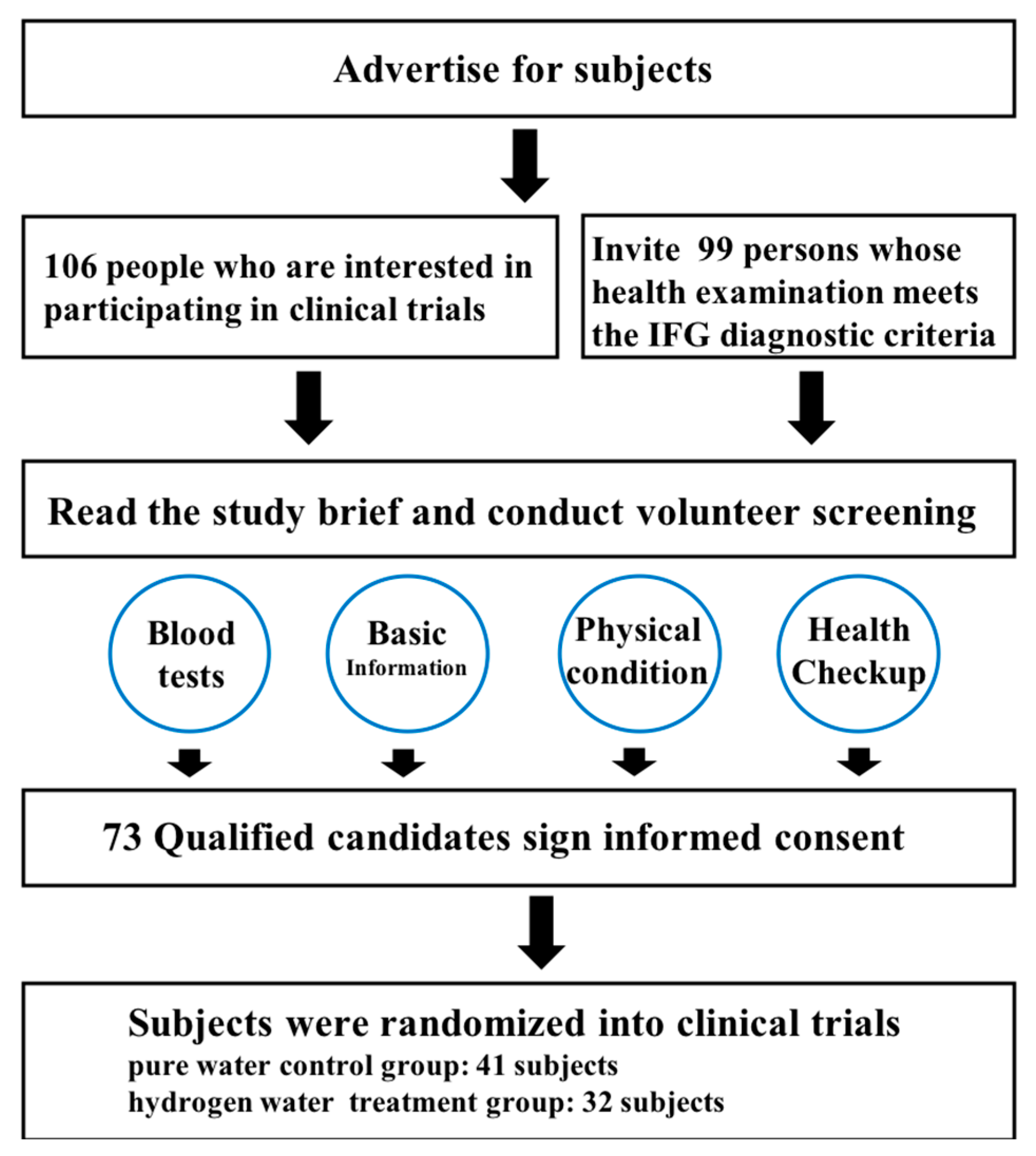
3.1. Screening of Subjects and Results of Questionnaire Survey
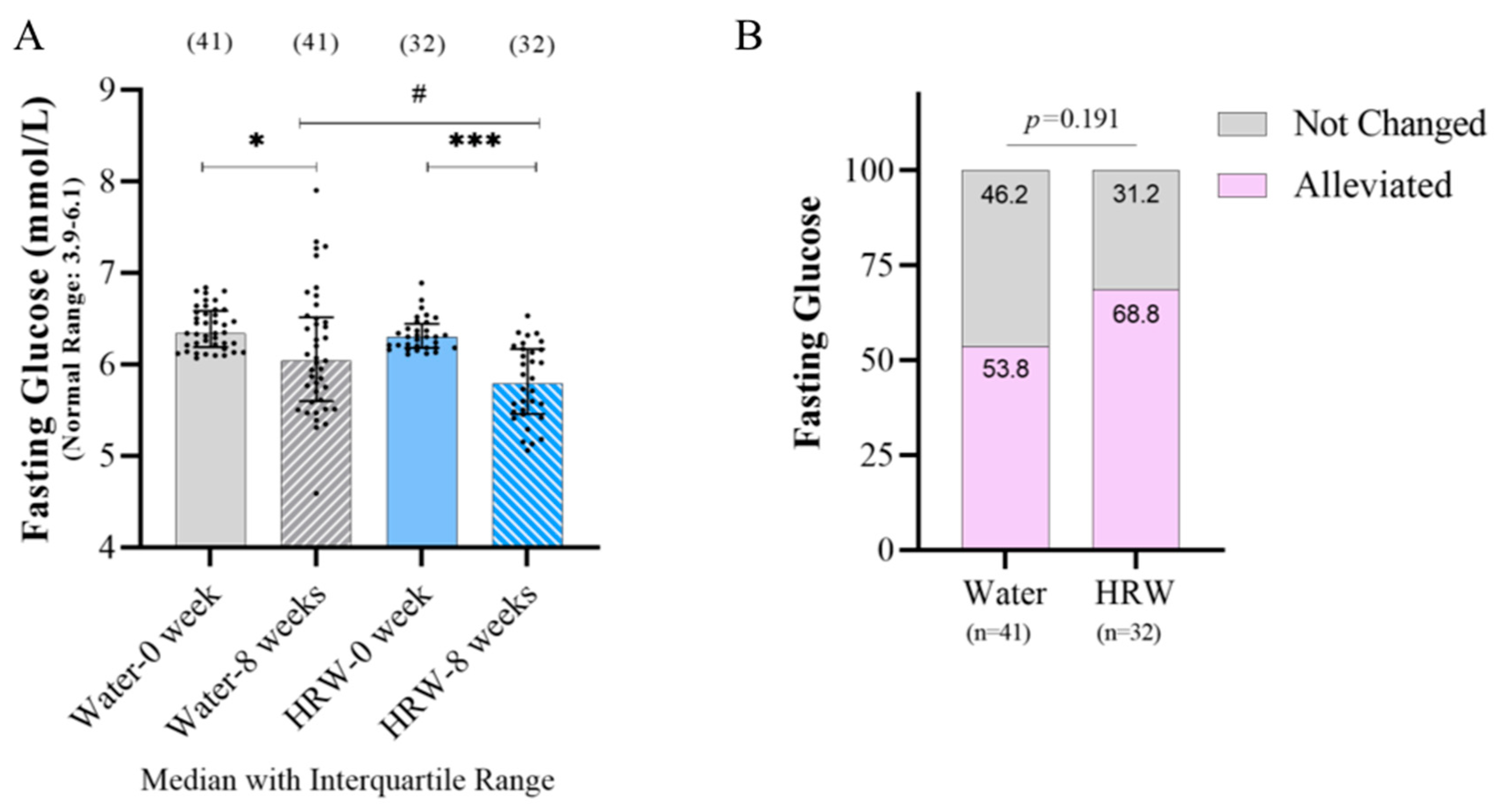
3.2. Effects of HRW on Glucose Metabolism-Related Indicators
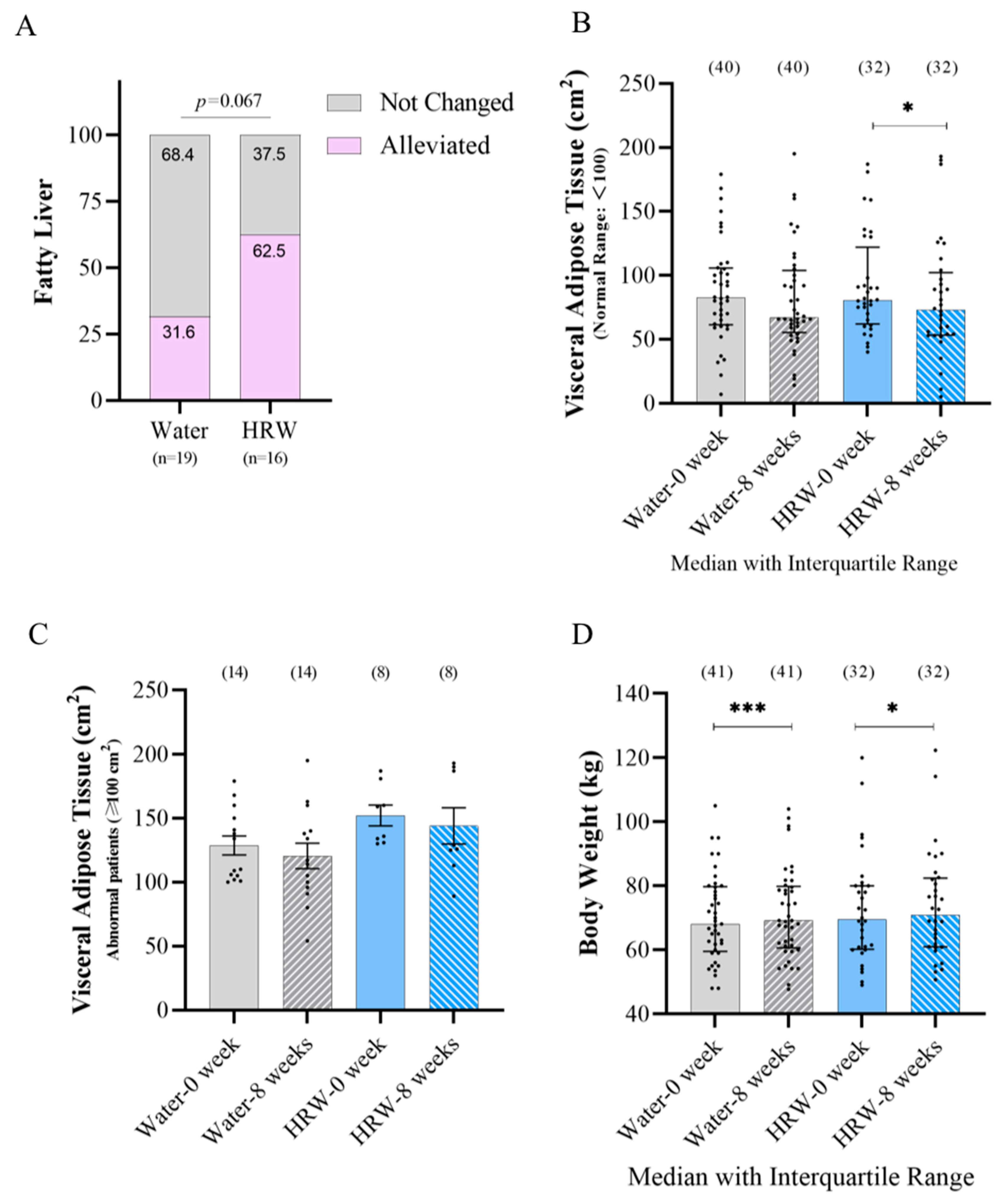
3.3. Effects of HRW on Lipid Metabolism-Related Indicators
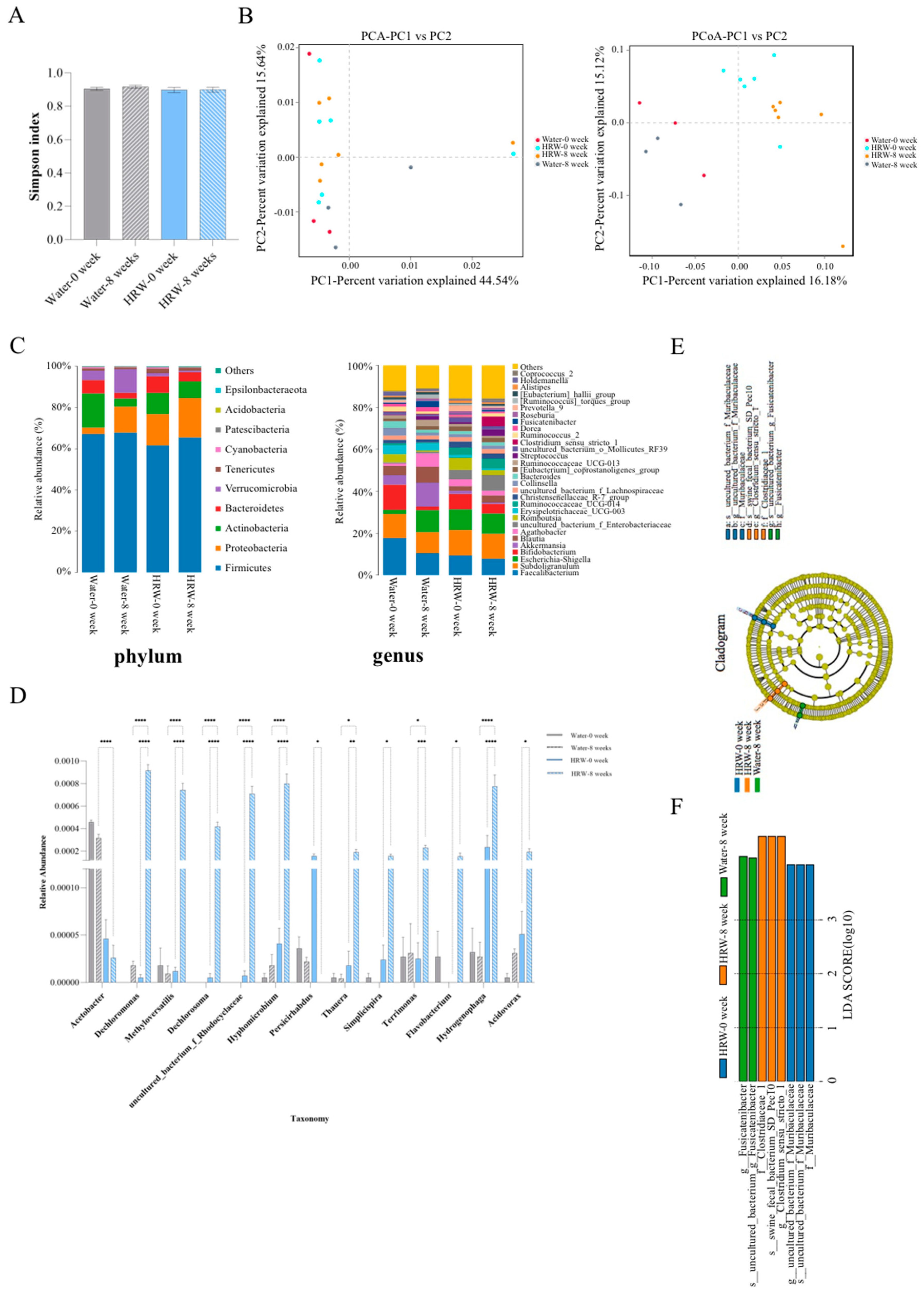
3.4. Effects of HRW on the Gut Microbiota
3.5. Correlation Analysis between Gut Microbiota and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinese Diabetes Socitey. Guideline for the prevention and treatment of 2 diabetes mellitus in China (2020 edition). Chin. J. Diabetes Mellit. 2021, 13, 315–409. [Google Scholar]
- Zhang, J.J.; Liu, C.; Zhou, L.; Qu, K.C.; Wang, R.T.; Tai, M.H.; Wei, J.C.; Qi, L.L.; Wu, F.; Wang, Z.X. A review of hydrogen as a new medical therapy. Hepatogastroenterology 2012, 59, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Bao, C.; He, Y.; Tian, X.; Yang, Y.; Zhang, T.; Xu, K.F. Hydrogen gas (XEN) inhalation ameliorates airway inflammation in asthma and COPD patients. QJM Int. J. Med. 2020, 113, 870–875. [Google Scholar] [CrossRef]
- Ceriello, A.; Testa, R. Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. S2), S232–S236. [Google Scholar] [CrossRef] [Green Version]
- Tamasawa, A.; Mochizuki, K.; Hariya, N.; Saito, M.; Ishida, H.; Doguchi, S.; Yanagiya, S.; Osonoi, T. Hydrogen gas production is associated with reduced interleukin-1β mRNA in peripheral blood after a single dose of acarbose in Japanese type 2 diabetic patients. Eur. J. Pharmacol. 2015, 762, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Nosaka, N.; Yumoto, T.; Knaup, E.; Naito, H.; Nishiyama, C.; Yamakawa, Y.; Tsukahara, K.; Terado, M.; Sato, K.; et al. The clinical application of hydrogen as a medical treatment. Acta Med. Okayama 2016, 70, 331–337. [Google Scholar]
- Corkey, B.E. Banting lecture 2011: Hyperinsulinemia: Cause or consequence? Diabetes 2012, 61, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity 2011, 19, 1396–1403. [Google Scholar] [CrossRef]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Li, M.; Sang, H.; Zhang, L.; Li, X.; Yao, S.; Yu, Y.; Zong, C.; Xue, Y.; Qin, S. Hydrogen-rich water decreases serum LDL-cholesterol levels and improves HDL function in patients with potential metabolic syndrome. J. Lipid Res. 2013, 54, 1884–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahng, J.; Jung, I.S.; Choi, E.J.; Conklin, J.L.; Park, H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol. Motil. 2012, 24, 185–190.e192. [Google Scholar] [CrossRef]
- Higashimura, Y.; Baba, Y.; Inoue, R.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Hirai, Y.; Ushiroda, C.; Tanaka, Y.; Naito, Y. Effects of molecular hydrogen-dissolved alkaline electrolyzed water on intestinal environment in mice. Med. Gas Res. 2018, 8, 6–11. [Google Scholar]
- Seo, T.; Kurokawa, R.; Sato, B. A convenient method for determining the concentration of hydrogen in water: Use of methylene blue with colloidal platinum. Med. Gas Res. 2012, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Chilicka, K.; Rogowska, A.M.; Szygula, R. Effects of topical hydrogen purification on skin parameters and acne vulgaris in adult women. Healthcare 2021, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bai, Q.; Peng, B.; Wu, X.; Cao, Y.; Sun, X.; Hong, M.; Na, R.; Liu, B.; Li, Q.; Li, Z.; et al. Metabolomic study for essential hypertension patients based on dried blood spot mass spectrometry approach. IUBMB Life 2018, 70, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Qin, P.F.; Yin, J.C.; Shi, Y.; Xuan, Y.; Chen, Z.Q.; Zhou, X.; Yu, H.; Peng, D.H.; Wang, B. Clinical manifestations of polycystic ovary syndrome and associations with the vaginal microbiome: A cross-sectional based exploratory study. Front. Endocrinol. 2021, 12, 662725. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Singh, R.B.; Fatima, G.; Kartikey, K.; Sharma, J.P.; Ostojic, S.M.; Gvozdjakova, A.; Kura, B.; Noda, M.; Mojto, V.; et al. The effects of 24-week, high-concentration hydrogen-rich water on body composition, blood lipid profiles and inflammation biomarkers in men and women with metabolic syndrome: A randomized controlled trial. Diabetes Metab. Syndr. Obes. 2020, 13, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Liu, H.F.; Xiao, M.F.; Liu, B.; Liao, J.; Zhang, J.N. Analysis of prevalence of thyroid cancer in patients with type 2 diabetes mellitus and the relevant factors. Chin. J. Gen. Surg. 2019, 28, 558–564. [Google Scholar]
- Enhörning, S.; Brunkwall, L.; Tasevska, I.; Ericson, U.; Tholin, J.P.; Persson, M.; Lemetais, G.; Vanhaecke, T.; Dolci, A.; Perrier, E.T.; et al. Water supplementation reduces copeptin and plasma glucose in adults with high copeptin: The H2O metabolism pilot study. J. Clin. Endocrinol. Metab. 2019, 104, 1917–1925. [Google Scholar] [CrossRef] [Green Version]
- Muscogiuri, G.; Barrea, L.; Annunziata, G.; Vecchiarini, M.; Orio, F.; Somma, C.D.; Colao, A.; Savastano, S. Water intake keeps type 2 diabetes away? Focus on copeptin. Endocrine 2018, 62, 292–298. [Google Scholar] [CrossRef]
- Sedaghat, G.; Montazerifar, F.; Keykhaie, M.A.; Karajibani, M.; Shourestani, S.; Dashipour, A. Effect of pre-meal water intake on the serum levels of copeptin, glycemic control, lipid profile and anthropometric indices in patients with type 2 diabetes mellitus: A randomized, controlled trial. J. Diabetes Metab. Disord. 2021, 20, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Trivic, T.; Drid, P.; Ostojic, S.M. Molecular hydrogen affects body composition, metabolic profiles, and mitochondrial function in middle-aged overweight women. Ir. J. Med. Sci. 2018, 187, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhu, F.; Chen, L.; Chen, K. Proteomics, metabolomics and metagenomics for type 2 diabetes and its complications. Life Sci. 2018, 212, 194–202. [Google Scholar] [CrossRef]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.I.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [Green Version]
- Pathak, P.; Xie, C.; Nichols, R.G.; Ferrell, J.M.; Boehme, S.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J.; Chiang, J.Y.L. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 2018, 68, 1574–1588. [Google Scholar] [CrossRef] [Green Version]
- Dou, Y.; Ma, C.; Wang, K.; Liu, S.; Sun, J.; Tan, W.; Neckenig, M.; Wang, Q.; Dong, Z.; Gao, W.; et al. Dysbiotic tumor microbiota associates with head and neck squamous cell carcinoma outcomes. Oral Oncol. 2022, 124, 105657. [Google Scholar] [CrossRef]
- Nistal, E.; Sáenz de Miera, L.E.; Ballesteros Pomar, M.; Sánchez-Campos, S.; García-Mediavilla, M.V.; Álvarez-Cuenllas, B.; Linares, P.; Olcoz, J.L.; Arias-Loste, M.T.; García-Lobo, J.M.; et al. An altered fecal microbiota profile in patients with non-alcoholic fatty liver disease (NAFLD) associated with obesity. Rev. Esp. Enferm. Dig. 2019, 111, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Porras, D.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Juarez-Fernández, M.; Cuevas, M.J.; Mauriz, J.L.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis. Model. Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oba, P.M.; Holscher, H.D.; Mathai, R.A.; Kim, J.; Swanson, K.S. Diet Influences the oral microbiota of infants during the first six months of life. Nutrients 2020, 12, 3400. [Google Scholar] [CrossRef]
- Inanova, A.Y.; Shirokov, I.V.; Toshchanko, S.V.; Kozlova, A.D.; Obolenskaya, O.N.; Mariasina, S.S.; Ivlev, V.A.; Gartseev, I.B.; Medvedev, O.S. Effects of coenzyme Q10 on the biomarkers (hydrogen, methane, SCFA and TMA) and compositon of the gut microbiome in rats. Pharmaceuticals 2023, 16, 686. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A novel option in human disease treatment. Oxid. Med. Cell. Longev. 2020, 2020, 8384742. [Google Scholar] [CrossRef] [PubMed]

| Hydrogen-Rich Water Group | Placebo Water Group | Values Presented | |||
|---|---|---|---|---|---|
| (n = 32) | (n = 41) | ||||
| 0 Week | 8 Weeks | 0 Week | 8 Weeks | ||
| Weight (kg) | 69.50 ± 19.78 | 70.85 ± 21.53 * | 68.10 ± 20.30 | 69.20 ± 19.30 *** | Median ± IR |
| Body mass index (kg/m2) | 25.26 ± 4.29 | 25.09 ± 4.15 | 24.66 ± 3.41 | 24.63 ± 3.36 | Mean ± SD |
| Abdominal girth (cm) | 85.37 ± 12.59 | 84.47 ± 12.65 | 83.90 ± 10.94 | 84.22 ± 11.32 | Mean ± SD |
| Visceral Adipose Tissue (cm2) | 80.50 ± 60.00 | 73.00 ± 49.00 * | 82.50 ± 44.50 | 66.00 ± 49.00 | Median ± IR |
| Fasting glucose (mmol/L) | 6.30 ± 0.26 | 5.79 ± 0.71 *** # | 6.34 ± 0.40 | 6.04 ± 0.92 * | Median ± IR |
| Creatinine (μmol/L) | 67.96 ± 18.19 | 69.51 ± 18.47 | 69.22 ± 10.64 | 69.49 ± 11.91 | Mean ± SD |
| Uric acid (μmol/L) | 354.43 ± 134.35 | 356.06 ± 117.36 | 381.02 ± 126.73 | 355.96 ± 109.44 ** | Mean ± SD |
| Total protein (g/L) | 73.13 ± 4.43 | 73.12 ± 3.95 | 71.77 ± 4.19 | 72.42 ± 3.73 | Mean ± SD |
| Globulin (g/L) | 27.54 ± 3.66 | 27.04 ± 3.26 | 26.20 ± 3.11 | 26.18 ± 3.29 | Mean ± SD |
| Albumin (g/L) | 45.59 ± 2.36 | 46.07 ± 2.34 | 45.56 ± 2.52 | 46.23 ± 2.08 | Mean ± SD |
| AST (IU/L) | 20.50 ± 7.75 | 19.00 ± 7.25 | 20.00 ± 8.50 | 21.00 ± 6.50 | Median ± IR |
| ALT (IU/L) | 24.50 ± 17.50 | 20.00 ± 19.75 | 25.00 ± 19.50 | 23.00 ± 20.00 | Median ± IR |
| Total bilirubin (μmol/L) | 11.20 ± 7.75 | 11.60 ± 8.40 | 11.10 ± 8.15 | 11.70 ± 6.20 | Median ± IR |
| Direct bilirubin (μmol/L) | 2.55 ± 1.28 | 2.75 ± 1.20 | 2.40 ± 1.40 | 2.60 ± 1.55 | Median ± IR |
| TC (mmol/L) | 5.26 ± 0.69 | 5.11 ± 0.73 | 5.14 ± 0.96 | 5.22 ± 0.96 | Mean ± SD |
| HDL (mmol/L) | 1.25 ± 0.30 | 1.24 ± 0.32 | 1.24 ± 0.25 | 1.22 ± 0.27 | Mean ± SD |
| LDL (mmol/L) | 3.13 ± 0.63 | 3.13 ± 0.61 | 3.04 ± 0.73 | 3.11 ± 0.74 | Mean ± SD |
| Triglycerides (mmol/L) | 1.30 ± 1.05 | 1.14 ± 1.00 | 1.19 ± 0.80 | 1.42 ± 1.05 | Median ± IR |
| GHb (%) | 5.41 ± 0.56 | 5.32 ± 0.53 | 5.51 ± 0.51 | 5.36 ± 0.49 * | Mean ± SD |
| Insulin (U/mL) | 12.57 ± 9.85 | 10.48 ± 8.25 | 10.45 ± 7.91 | 10.39 ± 9.55 | Median ± IR |
| ALP (IU/L) | 63.91 ± 16.07 | 65.56 ± 17.26 | 71.78 ± 17.09 | 73.59 ± 18.04 | Mean ± SD |
| γ-GT (IU/L) | 29.00 ± 21.00 | 26.00 ± 27.50 | 27.00 ± 28.00 | 25.00 ± 25.00 * | Median ± IR |
| LDH (U/L) | 158.00 ± 46.75 | 144.00 ± 36.25 *** | 161.00 ± 38.50 | 152.00 ± 30.50 * | Median ± IR |
| Creatine kinase (U/L) | 82.00 ± 55.75 | 86.00 ± 52.00 | 99.00 ± 49.50 | 91.00 ± 56.00 | Median ± IR |
| ApoA1 (mg/dL) | 113.10 ± 26.95 | 117.90 ± 24.33 * | 117.20 ± 25.00 | 115.60 ± 26.30 * | Median ± IR |
| ApoB (mg/dL) | 96.33 ± 19.86 | 98.13 ± 19.14 | 94.51 ± 23.60 | 99.07 ± 23.53 * | Mean ± SD |
| Lipoprotein a (mg/L) | 137.80 ± 270.85 | 137.95 ± 230.95 | 90.30 ± 205.35 | 138.60 ± 221.75 | Median ± IR |
| RBC (109/L) | 4.63 ± 0.52 | 4.70 ± 0.62 | 4.69 ± 0.44 | 4.68 ± 0.47 | Mean ± SD |
| Hb (g/L) | 138.03 ± 14.91 | 141.81 ± 17.25 | 139.24 ± 16.72 | 140.10 ± 17.07 | Mean ± SD |
| WBC (109/L) | 5.40 ± 1.55 | 5.15 ± 1.55 | 5.70 ± 1.70 | 5.30 ± 1.45 | Median ± IR |
| Platelet (109/L) | 243.06 ± 53.51 | 232.38 ± 50.87 * | 227.71 ± 43.20 | 226.29 ± 45.56 | Mean ± SD |
| IL-1β (pg/mL) | 26.38 ± 12.68 | 20.71 ± 6.24 *** | 22.53 ± 12.34 | 18.86 ± 10.71 *** | Median ± IR |
| IL-2 (pg/mL) | 31.97 ± 11.33 | 37.70 ± 13.59 *** | 30.53 ± 6.98 | 38.37 ± 10.78 *** | Median ± IR |
| IL-6 (pg/mL) | 171.76 ± 34.17 | 151.43 ± 27.06 *** | 176.28 ± 25.30 | 150.07 ± 25.68 *** | Mean ± SD |
| GSH-Px (U/mL) | 61.44 ± 25.05 ### | 66.00 ± 26.95 * ### | 48.18 ± 19.17 | 54.89 ± 17.03 *** | Median ± IR |
| TNF-α (pg/mL) | 212.05 ± 46.81 | 161.67 ± 37.64 *** | 208.95 ± 36.10 | 162.34 ± 34.72 *** | Mean ± SD |
| TAC (U/mL) | 7.17 ± 3.23 # | 8.02 ± 2.49 *** | 5.70 ± 2.29 | 7.93 ± 3.49 *** | Median ± IR |
| MDA (nmol/mL) | 9.00 ± 4.00 | 6.68 ± 3.34 *** | 10.70 ± 4.96 | 6.64 ± 2.53 *** | Median ± IR |
| LPO (nmol/L) | 2.61 ± 1.15 | 1.62 ± 1.30 *** | 2.25 ± 0.98 | 1.94 ± 1.11 * | Median ± IR |
| C-RP (mg/dL) | 0.27 ± 0.20 | 0.25 ± 0.15 | 0.25 ± 0.16 | 0.24 ± 0.15 | Mean ± SD |
| Adiponectin (μg/mL) | 7.64 ± 3.06 | 8.36 ± 2.05 * | 6.96 ± 1.94 | 9.11 ± 1.99 *** | Mean ± SD |
| VLDL (ug/dL) | 0.44 ± 0.10 | 0.52 ± 0.12 * | 0.45 ± 0.12 | 0.51 ± 0.12 * | Mean ± SD |
| EC-SOD (U/mL) | 19.78 ± 3.11 | 19.40 ± 4.13 | 19.86 ± 3.30 | 17.75 ± 2.70 ** | Mean ± SD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, B.; Shi, L.; Du, D.; Li, H.; Yi, N.; Xi, Y.; Cui, J.; Li, P.; Kang, H.; Noda, M.; et al. Hydrogen-Rich Water Ameliorates Metabolic Disorder via Modifying Gut Microbiota in Impaired Fasting Glucose Patients: A Randomized Controlled Study. Antioxidants 2023, 12, 1245. https://doi.org/10.3390/antiox12061245
Liang B, Shi L, Du D, Li H, Yi N, Xi Y, Cui J, Li P, Kang H, Noda M, et al. Hydrogen-Rich Water Ameliorates Metabolic Disorder via Modifying Gut Microbiota in Impaired Fasting Glucose Patients: A Randomized Controlled Study. Antioxidants. 2023; 12(6):1245. https://doi.org/10.3390/antiox12061245
Chicago/Turabian StyleLiang, Bing, Le Shi, Dongyue Du, Hua Li, Ning Yi, Yue Xi, Jianjiao Cui, Ping Li, Hongbin Kang, Mami Noda, and et al. 2023. "Hydrogen-Rich Water Ameliorates Metabolic Disorder via Modifying Gut Microbiota in Impaired Fasting Glucose Patients: A Randomized Controlled Study" Antioxidants 12, no. 6: 1245. https://doi.org/10.3390/antiox12061245





