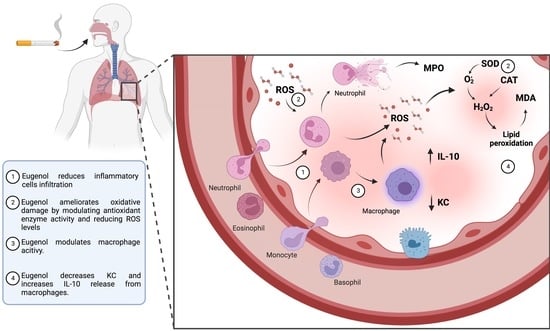
Modulation of Alveolar Macrophage Activity by Eugenol Attenuates Cigarette-Smoke-Induced Acute Lung Injury in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Experimental Design
In Vivo Cigarette Smoke Exposure and Treatment
2.4. In Vivo Experimental Protocols
2.4.1. Tissue Processing and Histological Analysis
2.4.2. Morphological Analysis
2.5. In Vitro Experimental Procedures
2.5.1. Preparation of Cigarette Smoke Extract (CSE)
2.5.2. In Vitro Exposure to Cigarette Smoke Extract (CSE) and Treatment
2.6. Analysis
2.6.1. Cytotoxicity Assay (MTT)
2.6.2. Redox Imbalance Markers
2.6.3. Inflammatory Markers
2.7. Statistical Analysis
3. Results
3.1. Morphology
3.2. Ex Vivo Inflammatory Markers
3.3. Ex Vivo Redox Markers
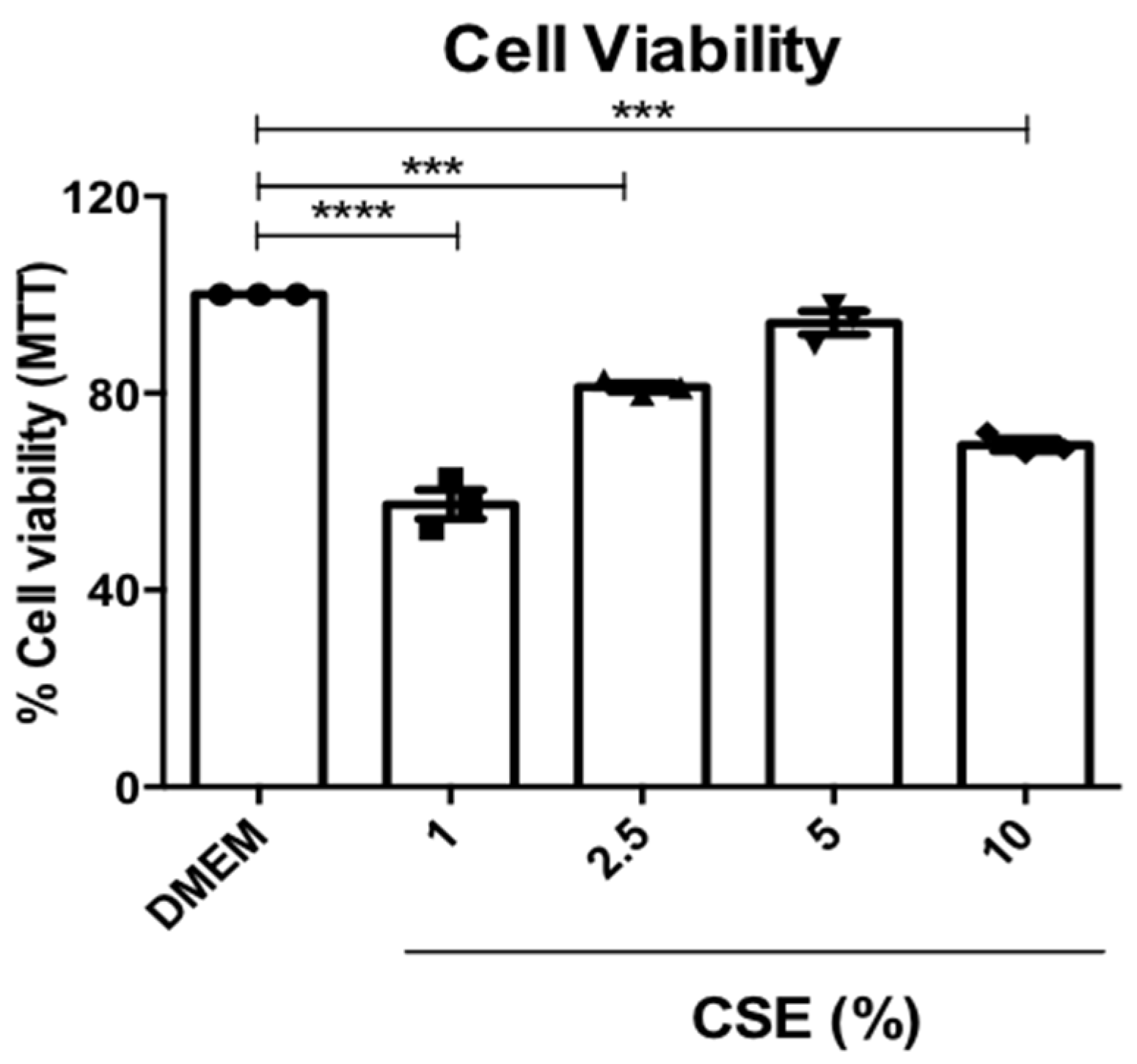
3.4. Cell Viability of RAM
3.5. In Vitro Inflammatory Markers
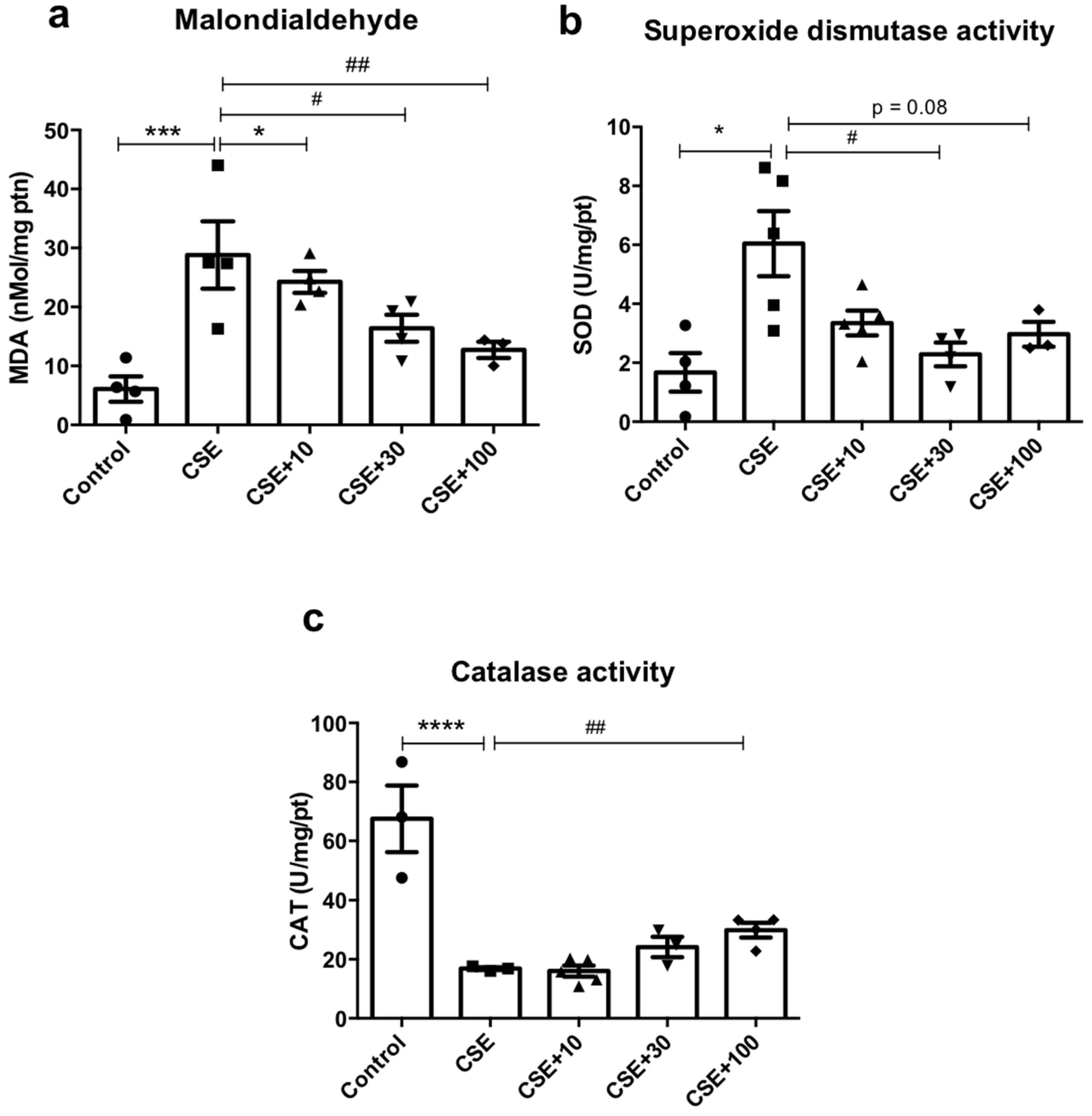
3.6. In Vitro Redox Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef]
- Rajendrasozhan, S.; Yang, S.-R.; Edirisinghe, I.; Yao, H.; Adenuga, D.; Rahman, I. Deacetylases and NF-ΚB in Redox Regulation of Cigarette Smoke-Induced Lung Inflammation: Epigenetics in Pathogenesis of COPD. Antioxid. Redox Signal. 2008, 10, 799–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuliga, M. NF-KappaB Signaling in Chronic Inflammatory Airway Disease. Biomolecules 2015, 5, 1266–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, K.; Eeden, S.F. van Lung Macrophage Phenotypes and Functional Responses: Role in the Pathogenesis of COPD. Int. J. Mol. Sci. 2018, 19, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, J.A.; Gruber, A.; Heeb, M.J.; Griffin, J.H. Protein C Pathway Impairment in Nonsymptomatic Cigarette Smokers. Blood Cells Mol. Dis. 2002, 29, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Aghapour, M.; Raee, P.; Moghaddam, S.J.; Hiemstra, P.S.; Heijink, I.H. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am. J. Respir. Cell Mol. Biol. 2018, 58, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Müller-Redetzky, H.C.; Suttorp, N.; Witzenrath, M. Dynamics of Pulmonary Endothelial Barrier Function in Acute Inflammation: Mechanisms and Therapeutic Perspectives. Cell Tissue Res. 2014, 355, 657–673. [Google Scholar] [CrossRef]
- Tam, A.; Wadsworth, S.; Dorscheid, D.; Man, S.F.P.; Sin, D.D. The Airway Epithelium: More than Just a Structural Barrier. Adv. Respir. Dis. 2011, 5, 255–273. [Google Scholar] [CrossRef]
- Laskin, D.L.; Malaviya, R.; Laskin, J.D. Role of Macrophages in Acute Lung Injury and Chronic Fibrosis Induced by Pulmonary Toxicants. Toxicol. Sci. 2019, 168, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.J.; Wynn, T.A. Protective and Pathogenic Functions of Macrophage Subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [Green Version]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-Inflammatory Potential and Antioxidant Profile of Eugenol. Oxidative Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef] [PubMed]
- Retamales, I.; Elliott, W.M.; Meshi, B.; Coxson, H.O.; Pare, P.D.; Sciurba, F.C.; Rogers, R.M.; Hayashi, S.; Hogg, J.C. Amplification of Inflammation in Emphysema and Its Association with Latent Adenoviral Infection. Am. J. Respir. Crit. Care Med. 2001, 164, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.P.D.; Gomes, A.S.; Lima, F.J.B.; Brito, T.S.; Soares, P.M.G.; Pinho, J.P.M.; Silva, C.S.; Santos, A.A.; Souza, M.H.L.P.; Magalhães, P.J.C. Inhaled 1,8-Cineole Reduces Inflammatory Parameters in Airways of Ovalbumin-Challenged Guinea Pigs. Basic Clin. Pharmacol. Toxicol. 2011, 108, 34–39. [Google Scholar] [CrossRef]
- Magalhães, C.B.; Riva, D.R.; DePaula, L.J.; Brando-Lima, A.; Koatz, V.L.G.; Leal-Cardoso, J.H.; Zin, W.A.; Faffe, D.S. In Vivo Anti-Inflammatory Action of Eugenol on Lipopolysaccharide-Induced Lung Injury. J. Appl. Physiol. 2010, 108, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Kirkham, P.A.; Barnes, P.J. Oxidative Stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde Determination as Index of Lipid Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [PubMed]
- Chainy, G.B.; Manna, S.K.; Chaturvedi, M.M.; Aggarwal, B.B. Anethole Blocks Both Early and Late Cellular Responses Transduced by Tumor Necrosis Factor: Effect on NF-ΚB, AP-1, JNK, MAPKK and Apoptosis. Oncogene 2000, 19, 2943–2950. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I. Oxidative Stress and Redox Regulation of Lung Inflammation in COPD. Eur. Respir. J. 2006, 28, 219–242. [Google Scholar] [CrossRef]
- Li, W.; Tsubouchi, R.; Qiao, S.; Haneda, M.; Murakami, K.; Yoshino, M. Inhibitory Action of Eugenol Compounds on the Production of Nitric Oxide in RAW264.7 Macrophages. Biomed. Res. 2006, 27, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Kennedy-Feitosa, E.; Cattani-Cavalieri, I.; Barroso, M.V.; Romana-Souza, B.; Brito-Gitirana, L.; Valenca, S.S. Eucalyptol Promotes Lung Repair in Mice Following Cigarette Smoke-Induced Emphysema. Phytomedicine 2019, 55, 70–79. [Google Scholar] [CrossRef]
- de Moura, R.S.; Ferreira, T.S.; Lopes, A.A.; Pires, K.M.P.; Nesi, R.T.; Resende, A.C.; Souza, P.J.C.; da Silva, A.J.R.; Borges, R.M.; Porto, L.C.; et al. Effects of Euterpe Oleracea Mart. (AÇAÍ) Extract in Acute Lung Inflammation Induced by Cigarette Smoke in the Mouse. Phytomedicine 2012, 19, 262–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tornling, G.; Eklund, A.; Engstrom-Laurent, A.; Hallgren, R.; Unge, G.; Westman, B. Hyaluronic Acid in Bronchoalveolar Lavage in Rats Exposed to Quartz. Occup. Environ. Med. 1987, 44, 443–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, S.; Zou, Y.; Wang, B.; Li, Y.; Zhu, J.; Luo, Y.; Li, J. Ginsenoside Rg1 Improves Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting Inflammatory Responses and Modulating Infiltration of M2 Macrophages. Int. Immunopharmacol. 2015, 28, 429–434. [Google Scholar] [CrossRef]
- Sakae, R.S.; Leme, A.S.; Dolhnikoff, M.; Pereira, P.M.; do Patrocinio, M.; Warth, T.N.; Zin, W.A.; Saldiva, P.H.; Martins, M.A. Neonatal Capsaicin Treatment Decreases Airway and Pulmonary Tissue Responsiveness to Methacholine. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1994, 266, L23–L29. [Google Scholar] [CrossRef]
- Kode, A.; Yang, S.-R.; Rahman, I. Differential Effects of Cigarette Smoke on Oxidative Stress and Proinflammatory Cytokine Release in Primary Human Airway Epithelial Cells and in a Variety of Transformed Alveolar Epithelial Cells. Respir. Res. 2006, 7, 132. [Google Scholar] [CrossRef] [Green Version]
- Bannister, J.V.; Bannister, W.H.; Rotilio, G. Aspects of the Structure, Function, and Applications of Superoxide Dismutas. Crit. Rev. Biochem. 1987, 22, 111–180. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Sim Choi, H.; Woo Kim, J.; Cha, Y.; Kim, C. A Quantitative Nitroblue Tetrazolium Assay for Determining Intracellular Superoxide Anion Production in Phagocytic Cells. J. Immunoass. Immunochem. 2006, 27, 31–44. [Google Scholar] [CrossRef]
- Suzuki, K.; Ota, H.; Sasagawa, S.; Sakatani, T.; Fujikura, T. Assay Method for Myeloperoxidase in Human Polymorphonuclear Leukocytes. Anal. Biochem. 1983, 132, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-Y.; Hung, S.-L.; Pai, S.-F.; Lee, Y.-H.; Yang, S.-F. Eugenol Suppressed the Expression of Lipopolysaccharide-Induced Proinflammatory Mediators in Human Macrophages. J. Endod. 2007, 33, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.C. Asthma and Cigarette Smoking. Eur. Respir. J. 2004, 24, 822–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsico, A.; Milanese, M.; Baraldo, S.; Casoni, G.L.; Papi, A.; Riccio, A.M.; Cerveri, I.; Saetta, M.; Brusasco, V. Small Airway Morphology and Lung Function in the Transition from Normality to Chronic Airway Obstruction. J. Appl. Physiol. 2003, 95, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, K.M.; Teixeira, T.O.; Lima, A.T.C.; Costa, R.S.; Carneiro, T.C.B.; Silva, D.F.; Barreto, M.L.; Pontes-de-Carvalho, L.C.; Alcantatara, N.M.N.; Figueiredo, C.A. Antiasthmatic Effect of Eugenol (4-Allyl-2- Methoxyophenol) Mediated by Both Bronchodilator and Immunomodulatory Properties. J. Pharm. Pharmacol. 2014, 2, 38–49. [Google Scholar]
- Johnson, E.R.; Matthay, M.A. Acute Lung Injury: Epidemiology, Pathogenesis, and Treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef]
- Heijink, I.H.; Brandenburg, S.M.; Postma, D.S.; van Oosterhout, A.J.M. Cigarette Smoke Impairs Airway Epithelial Barrier Function and Cell-Cell Contact Recovery. Eur. Respir. J. 2012, 39, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Cundall, M.; Sun, Y.; Miranda, C.; Trudeau, J.B.; Barnes, S.; Wenzel, S.E. Neutrophil-Derived Matrix Metalloproteinase-9 Is Increased in Severe Asthma and Poorly Inhibited by Glucocorticoids. J. Allergy Clin. Immunol. 2003, 112, 1064–1071. [Google Scholar] [CrossRef]
- Butler, A.; Walton, G.M.; Sapey, E. Neutrophilic Inflammation in the Pathogenesis of Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 392–404. [Google Scholar] [CrossRef]
- Foronjy, R.; D’Armiento, J. The Effect of Cigarette Smoke–Derived Oxidants on the Inflammatory Response of the Lung. Clin. Appl. Immunol. Rev. 2006, 6, 53–72. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Liu, Y.; Lu, Y.; Ma, C. Anti-Inflammatory Effects of Eugenol on Lipopolysaccharide-Induced Inflammatory Reaction in Acute Lung Injury via Regulating Inflammation and Redox Status. Int. Immunopharmacol. 2015, 26, 265–271. [Google Scholar] [CrossRef]
- Lugg, S.T.; Scott, A.; Parekh, D.; Naidu, B.; Thickett, D.R. Cigarette Smoke Exposure and Alveolar Macrophages: Mechanisms for Lung Disease. Thorax 2022, 77, 94–101. [Google Scholar] [CrossRef]
- Kirkham, P. Oxidative Stress and Macrophage Function: A Failure to Resolve the Inflammatory Response. Biochem. Soc. Trans. 2007, 35, 284–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively Activated Macrophages; a Double-Edged Sword in Allergic Asthma. J. Transl. Med. 2020, 18, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, R.L.; Borges, T.J.; Zanin, R.F.; Bonorino, C. IL-10 Is Required for Polarization of Macrophages to M2-like Phenotype by Mycobacterial DnaK (Heat Shock Protein 70). Cytokine 2016, 85, 123–129. [Google Scholar] [CrossRef]
- Yao, T.-C.; Chang, S.-W.; Hua, M.-C.; Liao, S.-L.; Tsai, M.-H.; Lai, S.-H.; Tseng, Y.-L.; Yeh, K.-W.; Tsai, H.-J.; Huang, J.-L. Tobacco Smoke Exposure and Multiplexed Immunoglobulin E Sensitization in Children: A Population-Based Study. Allergy 2016, 71, 90–98. [Google Scholar] [CrossRef]
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Env. Res. Public Health 2018, 15, 1033. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, E.; Patel, A.; Ball, D.; Klapwijk, J.; Millar, V.; Kumar, A.; Martin, A.; Mahendran, R.; Dailey, L.A.; Forbes, B.; et al. Morphometric Characterization of Rat and Human Alveolar Macrophage Cell Models and Their Response to Amiodarone Using High Content Image Analysis. Pharm. Res. 2017, 34, 2466–2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasper, A.E.; McIver, W.J.; Sapey, E.; Walton, G.M. Understanding the Role of Neutrophils in Chronic Inflammatory Airway Disease. F1000Research 2019, 8, 557. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, A.G.d.M.; de Bruin, P.F.C. O Papel Do Estresse Oxidativo Na DPOC: Conceitos Atuais e Perspectivas. J. Bras. Pneumol. 2009, 35, 1227–1237. [Google Scholar] [CrossRef]
- Kuyumcu, F.; Aycan, A. Evaluation of Oxidative Stress Levels and Antioxidant Enzyme Activities in Burst Fractures. Med. Sci. Monit. 2018, 24, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, K.B.F.; Costa, N.M.B.; Alfenas, R.d.C.G.; De Paula, S.O.; Minim, V.P.R.; Bressan, J. Estresse Oxidativo: Conceito, Implicações e Fatores Modulatórios. Rev. Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef] [Green Version]
- Nagababu, E.; Rifkind, J.M.; Boindala, S.; Nakka, L. Assessment of Antioxidant Activity of Eugenol In Vitro and In Vivo. Methods Mol. Biol. 2010, 610, 165–180. [Google Scholar] [PubMed] [Green Version]
- Gülçin, İ. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa-de-Oliveira, M.C.; Oliveira-Melo, P.; Gonçalves da Silva, M.H.; Santos da Silva, F.; Andrade Carvalho da Silva, F.; Silva de Araujo, B.V.; Franco de Oliveira, M.; Tadeu Correia, A.; Miyoshi Sakamoto, S.; Valença, S.S.; et al. Modulation of Alveolar Macrophage Activity by Eugenol Attenuates Cigarette-Smoke-Induced Acute Lung Injury in Mice. Antioxidants 2023, 12, 1258. https://doi.org/10.3390/antiox12061258
Barbosa-de-Oliveira MC, Oliveira-Melo P, Gonçalves da Silva MH, Santos da Silva F, Andrade Carvalho da Silva F, Silva de Araujo BV, Franco de Oliveira M, Tadeu Correia A, Miyoshi Sakamoto S, Valença SS, et al. Modulation of Alveolar Macrophage Activity by Eugenol Attenuates Cigarette-Smoke-Induced Acute Lung Injury in Mice. Antioxidants. 2023; 12(6):1258. https://doi.org/10.3390/antiox12061258
Chicago/Turabian StyleBarbosa-de-Oliveira, Maria Clara, Paolo Oliveira-Melo, Marcos Henrique Gonçalves da Silva, Flávio Santos da Silva, Felipe Andrade Carvalho da Silva, Bruno Vinicios Silva de Araujo, Moacir Franco de Oliveira, Aristides Tadeu Correia, Sidnei Miyoshi Sakamoto, Samuel Santos Valença, and et al. 2023. "Modulation of Alveolar Macrophage Activity by Eugenol Attenuates Cigarette-Smoke-Induced Acute Lung Injury in Mice" Antioxidants 12, no. 6: 1258. https://doi.org/10.3390/antiox12061258
APA StyleBarbosa-de-Oliveira, M. C., Oliveira-Melo, P., Gonçalves da Silva, M. H., Santos da Silva, F., Andrade Carvalho da Silva, F., Silva de Araujo, B. V., Franco de Oliveira, M., Tadeu Correia, A., Miyoshi Sakamoto, S., Valença, S. S., Lanzetti, M., Schmidt, M., & Kennedy-Feitosa, E. (2023). Modulation of Alveolar Macrophage Activity by Eugenol Attenuates Cigarette-Smoke-Induced Acute Lung Injury in Mice. Antioxidants, 12(6), 1258. https://doi.org/10.3390/antiox12061258