Seed Priming Based on Iodine and Selenium Influences the Nutraceutical Compounds in Tomato (Solanum lycopersicum L.) Crop
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crop Establishment
2.1.1. Preparation of KIO3 and Na2SeO3 Treatments
2.1.2. Sowing and Planting
2.1.3. Sampling
2.2. Non-Enzymatic Compounds
2.2.1. Vitamin C
2.2.2. Total Phenols
2.2.3. Total Flavonoids
2.2.4. Chlorophyll
2.2.5. Lycopene and β-carotene
2.3. Enzymatic Activity
2.3.1. Extraction
2.3.2. Reduced Glutathione (GSH)
2.3.3. Glutathione Peroxidase (GPX) (QE 1.11.1.9)
2.3.4. Phenylalanine Ammonium Lyase (PAL) (QE 4.3.1.5)
2.3.5. Catalase (CAT) (QE 1.11.1.6)
2.3.6. Ascorbate Peroxidase (APX) (EC 1.11.1.11)
2.4. Antioxidant Capacity
2.4.1. Antioxidant Capacity of Hydrophilic and Lipophilic Compounds by ABTS
2.4.2. Antioxidant Capacity of Hydrophilic Compounds by DPPH
2.4.3. Statistical Analyses
3. Results
3.1. Non-Enzymatic Compounds in Tomato Fruits by KIO3 and Na2SeO3 Interactions
3.2. Non-Enzymatic Compounds in Tomato Fruits by KIO3 and Na2SeO3 Factors
3.3. Non-Enzymatic Compounds in Tomato Leaves by KIO3 and Na2SeO3 Interactions
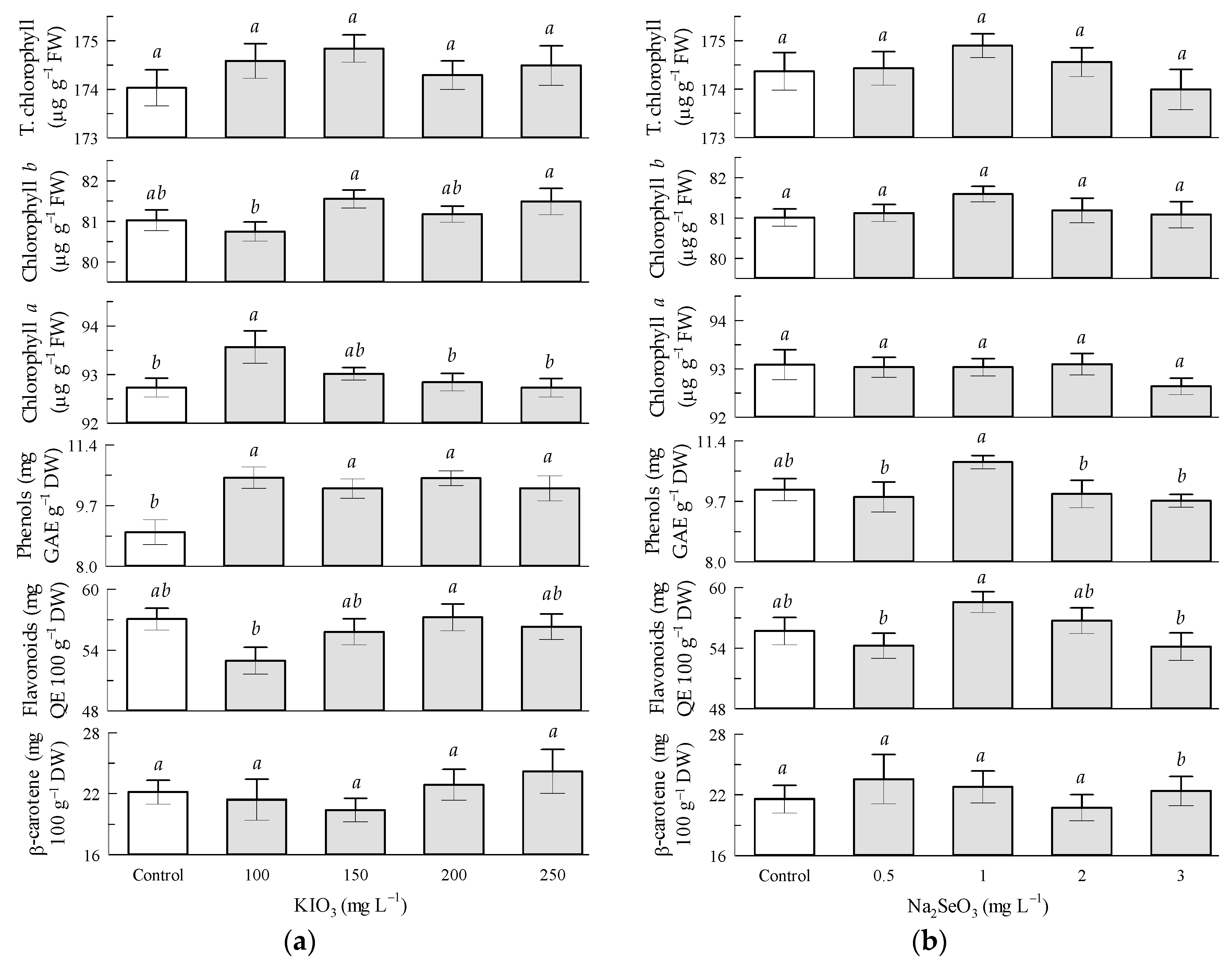
3.4. Non-Enzymatic Compounds in Tomato Leaves by KIO3 and Na2SeO3 Factors
3.5. Enzymatic Activity in Tomato Fruits by KIO3 and Na2SeO3 Interactions
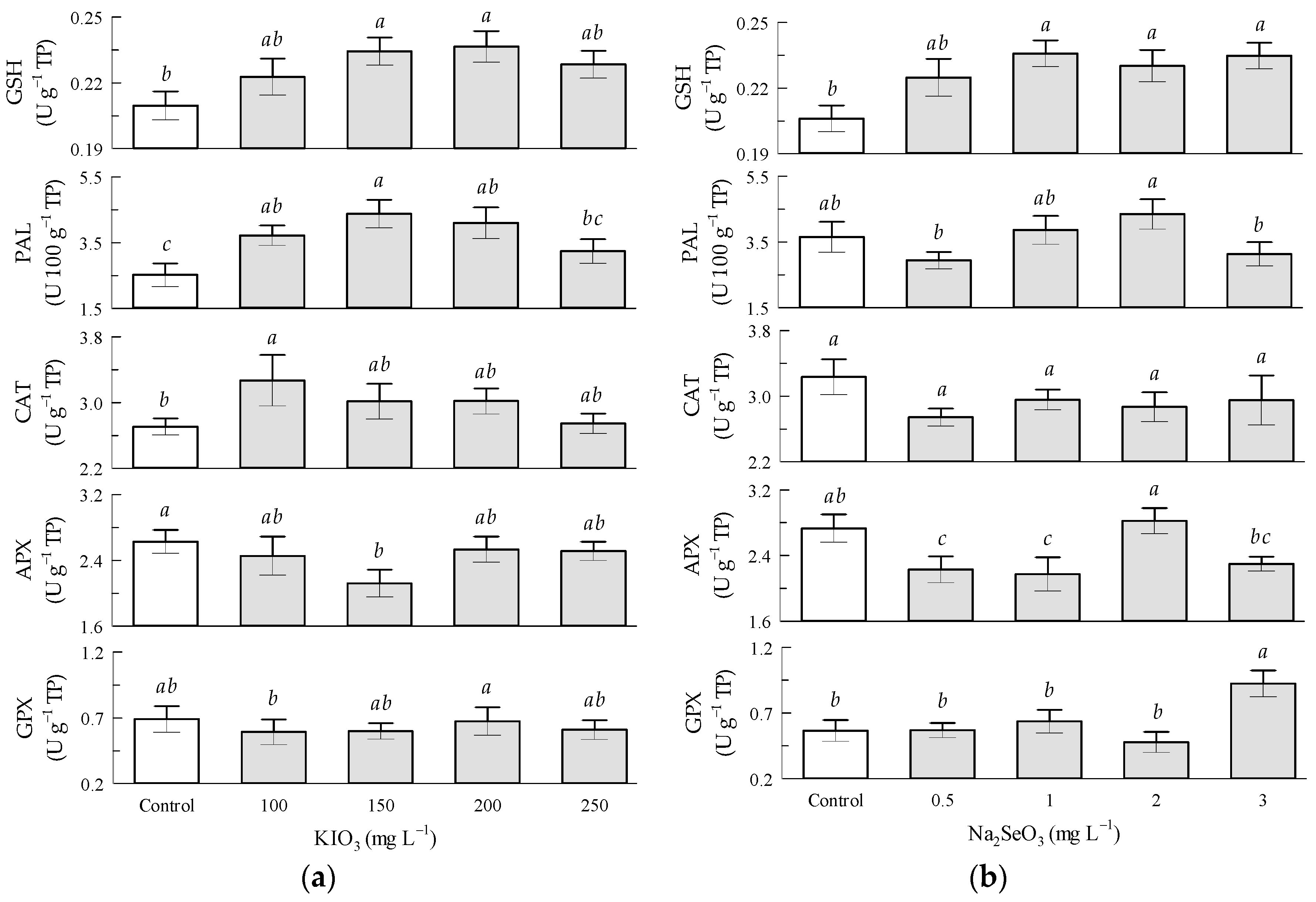
3.6. Enzymatic Activity in Tomato Fruits by KIO3 and Na2SeO3 Factors
3.7. Enzymatic Activity in Tomato Leaves by KIO3 and Na2SeO3 Interactions
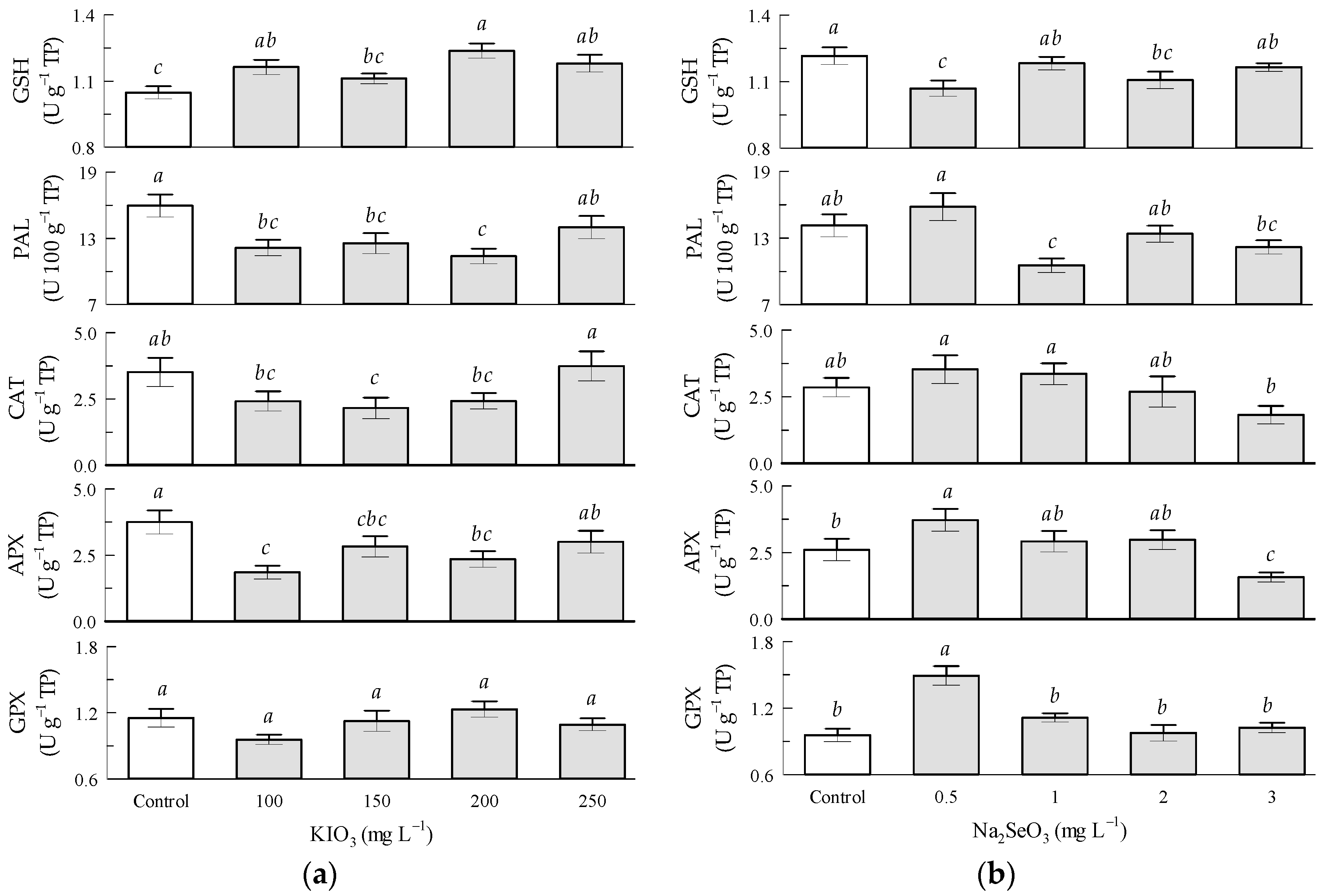
3.8. Enzymatic Activity in Tomato Leaves by KIO3 and Na2SeO3 Factors
3.9. Antioxidant Capacity in Tomato Fruits and Leaves by KIO3 and Na2SeO3 Interactions
3.10. Antioxidant Capacity in Tomato Fruits and Leaves by KIO3 and Na2SeO3 Factors
4. Discussion
4.1. Non-Enzymatic Compounds
4.2. Enzymatic Activity
4.3. Antioxidant Capacity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, P. Seed priming: New vistas and contemporary perspectives. In Advances in Seed Priming; Springer: Singapore, 2018; pp. 3–22. [Google Scholar] [CrossRef]
- Kim, H.Y.; Hwang, I.G.; Kim, T.M.; Woo, K.S.; Park, D.S.; Kim, J.H.; Kim, D.J.; Lee, J.; Lee, Y.R.; Jeong, H.S. Chemical and functional components in different parts of rough rice (Oryza sativa L.) before and after germination. Food Chem. 2012, 134, 288–293. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Germination. In Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; pp. 133–181. [Google Scholar] [CrossRef]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Bradford, K.J.; Steiner, J.J.; Trawatha, S.E. Seed priming influence on germination and emergence of pepper seed lots. Crop Sci. 1990, 30, 718–721. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annual Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [Green Version]
- Di Girolamo, G.; Barbanti, L. Treatment conditions and biochemical processes influencing seed priming effectiveness. Ital. J. Agron. 2012, 7, e25. [Google Scholar] [CrossRef] [Green Version]
- Manonmani, V.; Begum, M.A.J.; Jayanthi, M. Halo priming of seeds. Res. J. Seed Sci. 2014, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sedghi, M.; Nemati, A.; Esmaielpour, B. Effect of seed priming on germination and seedling growth of two medicinal plants under salinity. Emir. J. Food Agric. 2010, 17, 130. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Pant, B.; Mondal, S.; Bose, B. Hydro and halo priming: Influenced germination responses in wheat Var-HUW-468 under heavy metal stress. Acta Physiol. Plant. 2016, 38, 217. [Google Scholar] [CrossRef]
- Anaytullah, S.; Bose, B. Impact of seed hardening treatment with nitrate salts on nitrogen and anti-oxidant defense metabolisms in Triticum aestivum L. under different sowing conditions. Int. J. Plant Res. 2012, 25, 292–299. [Google Scholar]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Shi, G.; Zhang, X. Selenium delays tomato fruit ripening by inhibiting ethylene biosynthesis and enhancing the antioxidant defense system. Food Chem. 2017, 219, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Breusegem, F.V. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Aceves, C.; Anguiano, B.; Delgado, G. Is iodine a gatekeeper of the integrity of the mammary gland? J. Mammary Gland Biol. 2005, 10, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, selenoproteins, and immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, selenoproteins and viral infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture (USDA). Index of Official Visual Aids. 2017. Available online: https://www.ams.usda.gov/sites/default/files/media/Official%20Inventory%20of%20FV%20Inspection%20Aids.pdf (accessed on 19 September 2022).
- Padayatty, S.; Daruwala, R.; Wang, Y.; Eck, P.; Song, J.; Koh, W.; Levine, M. Vitamin C: From molecular mechanisms to optimum intake. In Handbook of Antioxidants; Elsevier: Amsterdam, The Netherlands, 2001; pp. 117–146. [Google Scholar]
- Yu, Z.; Dahlgren, R.A. Evaluation of methods for measuring polyphenols in conifer foliage. J. Chem. Ecol. 2000, 26, 2119–2140. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Munira, S.; Hossain, M.M.; Zakaria, M.; Ahmed, J.U.; Islam, M.M. Evaluation of potato varieties against salinity stress in Bangladesh. Int. J. Plant Soil Sci. 2015, 6, 73–81. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J. Food Sci. Tech. 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. In Methods in Enzymology, Oxygen Radicals in Biological Systems; Academic Press: New York, NY, USA, 1984; pp. 114–120. [Google Scholar] [CrossRef]
- Sykłowska-Baranek, K.; Pietrosiuk, A.; Naliwajski, M.R.; Kawiak, A.; Jeziorek, M.; Wyderska, S.; Łojkowska, E.; Chinou, I. Effect of l-phenylalanine on PAL activity and production of naphthoquinone pigments in suspension cultures of Arnebia euchroma (Royle) Johnst. Vitr. Cell. Dev.-Plant 2012, 48, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Medrano Macías, J.; López Caltzontzit, M.G.; Rivas Martínez, E.N.; Narváez Ortiz, W.A.; Benavides Mendoza, A.; Martínez Lagunes, P. Enhancement to salt stress tolerance in strawberry plants by iodine products application. Agronomy 2021, 11, 602. [Google Scholar] [CrossRef]
- Revelou, P.-K.; Xagoraris, M.; Kokotou, M.G.; Constantinou-Kokotou, V. Cruciferous vegetables as functional foods: Effects of selenium biofortification. Int. J. Veg. Sci. 2022, 28, 191–210. [Google Scholar] [CrossRef]
- Kathpalia, R.; Bhatla, S.C. Plant mineral nutrition. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2018; pp. 37–81. [Google Scholar] [CrossRef]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta BBA Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Narvaéz-Ortiz, W.A. Selenium and nano-selenium as a new frontier of plant biostimulant. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Sustainable Plant Nutrition in a Changing World; Springer: Cham, Switzerland, 2022; pp. 41–54. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Singh, K.; Iqbal, N.; Nisha, N.; Rani, A.; Kumar, M. Iodine: An emerging biostimulant of growth and stress responses in plants. Plant Soil 2023, 486, 119–133. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singh, D.; Pandey, H.; Jatav, R.B.; Singh, V.; Pandey, D. An overview of roles of enzymatic and nonenzymatic antioxidants in plant. In Antioxidant Defense in Plants; Springer: Singapore, 2022; pp. 1–13. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Khaliq, A.; Ali, S.; Khan, I. Physiological, biochemical, and molecular aspects of seed priming. In Priming and Pretreatment of Seeds and Seedlings; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Millar, A.H.; Mittova, V.; Kiddle, G.; Heazlewood, J.L.; Bartoli, C.G.; Theodoulou, F.L.; Foyer, C.H. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol. 2003, 133, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaucin-Delgado, J.M.; Hernández-Montiel, L.G.; Sánchez-Chavez, E.; Ortega-Ortiz, H.; Fortis-Hernández, M.; Reyes-Pérez, J.J.; Preciado-Rangel, P. Agronomic biofortification with selenium improves the yield and nutraceutical quality in tomato under soilless conditions. Not. Bot. Horti Agrobot. 2020, 48, 1221–1232. [Google Scholar] [CrossRef]
- Cunha, M.L.O.; Oliveira, L.C.A.; de Silva, V.M.; Montanha, G.S.; dos Reis, A.R. Selenium increases photosynthetic capacity, daidzein biosynthesis, nodulation and yield of peanuts plants (Arachis hypogaea L.). Plant Physiol. Biochem. 2022, 190, 231–239. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Hussain, T.; Hussain, S.A.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Seed priming with the selenium nanoparticles maintains the redox status in the water stressed tomato plants by modulating the antioxidant defense enzymes. Plants 2023, 12, 1556. [Google Scholar] [CrossRef]
- Sabatino, L.; La Bella, S.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C.; Consentino, B.B.; Rouphael, Y. Selenium biofortification and grafting modulate plant performance and functional features of cherry tomato grown in a soilless system. Sci. Hortic. 2021, 285, 110095. [Google Scholar] [CrossRef]
- Smoleń, S.; Wierzbińska, J.; Sady, W.; Kołton, A.; Wiszniewska, A.; Liszka-Skoczylas, M. Iodine biofortification with additional application of salicylic acid affects yield and selected parameters of chemical composition of tomato fruits (Solanum lycopersicum L.). Sci. Hortic. 2015, 188, 89–96. [Google Scholar] [CrossRef]
- Jerše, A.; Kacjan-Maršić, N.; Šircelj, H.; Germ, M.; Kroflič, A.; Stibilj, V. Seed soaking in I and Se solutions increases concentrations of both elements and changes morphological and some physiological parameters of pea sprouts. Plant Physiol. Biochem. 2017, 118, 285–294. [Google Scholar] [CrossRef]
- Smoleń, S.; Czernicka, M.; Kowalska, I.; Kȩska, K.; Halka, M.; Grzebelus, D.; Grzanka, M.; Skoczylas, Ł.; Pitala, J.; Koronowicz, A.; et al. New aspects of uptake and metabolism of non-organic and organic iodine compounds—The role of vanadium and plant-derived thyroid hormone analogs in lettuce. Front. Plant Sci. 2021, 12, 653168. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Vicas, S.I.; Cavalu, S.; Laslo, V.; Tocai, M.; Costea, T.O.; Moldovan, L. Growth, photosynthetic pigments, phenolic, glucosinolates content and antioxidant capacity of broccoli sprouts in response to nanoselenium particles supply. Not. Bot. Horti Agrobot. 2019, 47, 821–828. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.-J.; Kang, H.-M.; Lee, Y.-T. Influence of selenium biofortification on the bioactive compounds and antioxidant activity of wheat microgreen extract. Food Chem. 2020, 309, 125763. [Google Scholar] [CrossRef] [PubMed]
- Shohag, M.J.I.; Wei, Y.; Yang, X. Changes of folate and other potential health-promoting phytochemicals in legume seeds as affected by germination. J. Agric. Food Chem. 2012, 60, 9137–9143. [Google Scholar] [CrossRef]
- Deng, B.; Tian, S.; Li, S.; Guo, M.; Liu, H.; Li, Y.; Wang, Q.; Zhao, X. A simple, rapid and efficient method for essential element supplementation based on seed germination. Food Chem. 2020, 325, 126827. [Google Scholar] [CrossRef]
- Radawiec, A.; Szulc, W.; Rutkowska, B. Agrotechnical biofortification as a method to increase selenium content in spring wheat. Agronomy 2021, 11, 541. [Google Scholar] [CrossRef]
- Blasco, B.; Ríos, J.J.; Leyva, R.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Ruiz, J.M.; Romero, L. Does iodine biofortification affect oxidative metabolism in lettuce plants? Biol. Trace Elem. Res. 2011, 142, 831–842. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Cao, F.; Wang, N.; Zhang, M.; Dai, H.; Dawood, M.; Zhang, G.; Wu, F. Comparative study of alleviating effects of GSH, Se and Zn under combined contamination of cadmium and chromium in rice (Oryza sativa). BioMetals 2013, 26, 297–308. [Google Scholar] [CrossRef]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H. Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Nawaz, F.; Zulfiqar, B.; Ahmad, K.S.; Majeed, S.; Shehzad, M.A.; Javeed, H.M.R.; Tahir, M.N.; Ahsan, M. Pretreatment with selenium and zinc modulates physiological indices and antioxidant machinery to improve drought tolerance in maize (Zea mays L.). S. Afr. J. Bot. 2021, 138, 209–216. [Google Scholar] [CrossRef]
- Hu, F.; Jiang, S.; Wang, Z.; Hu, K.; Xie, Y.; Zhou, L.; Zhu, J.; Xing, D.; Du, B. Seed priming with selenium: Effects on germination, seedling growth, biochemical attributes, and grain yield in rice growing under flooding conditions. Plant Direct 2022, 6, e378. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Castellanos, B.; Rivas, E.; Narvaez-Ortiz, W.; Benavides-Mendoza, A.; Medrano, J. Outcomes of foliar iodine application on growth, minerals and antioxidants in tomato plants under salt stress. Folia Hortic. 2022, 34, 27–37. [Google Scholar] [CrossRef]
- Sarrou, E.; Siomos, A.S.; Riccadona, S.; Aktsoglou, D.-C.; Tsouvaltzis, P.; Angeli, A.; Franceschi, P.; Chatzopoulou, P.; Vrhovsek, U.; Martens, S. Improvement of sea fennel (Crithmum maritimum L.) nutritional value through iodine biofortification in a hydroponic floating system. Food Chem. 2019, 296, 150–159. [Google Scholar] [CrossRef]



| Variation Factor | Concentration (mg L−1) |
|---|---|
| KIO3 | 0, 100, 150, 200, 250 |
| Na2SeO3 | 0, 0.5, 1, 2, 3 |
| Na2SeO3 | KIO3 | Vitamin C | Phenols | Flavonoids | Lycopene | β-carotene |
|---|---|---|---|---|---|---|
| (mg L−1) | (mg L−1) | (mg 100 g−1 FW) | (mg GAE g−1 DW) | (mg QE 100 g−1 DW) | (mg 100 g−1 DW) | (mg 100 g−1 DW) |
| 0 | 0 | 18.8 abcdef | 3.1 ab | 18.0 ab | 6.6 cd | 2.1 efg |
| 0 | 100 | 17.6 bcdef | 2.9 abc | 17.2 abc | 12.4 b | 3.8 abcde |
| 0 | 150 | 21.5 a | 2.7 abc | 12.6 fghi | 4.0 efg | 1.9 fgh |
| 0 | 200 | 20.5 abc | 2.6 abc | 15.8 bcdef | 4.3 efg | 1.6 fgh |
| 0 | 250 | 18.0 bcdef | 2.6 abc | 12.4 hi | 2.9 gh | 1.5 fgh |
| 0.5 | 0 | 19.2 abcdef | 2.7 abc | 13.4 defghi | 4.3 efg | 0.2 h |
| 0.5 | 100 | 16.2 f | 3.0 abc | 15.4 bcdefgh | 6.6 cd | 2.1 efg |
| 0.5 | 150 | 16.5 ef | 2.6 abc | 17.3 abc | 13.8 a | 3.9 abcd |
| 0.5 | 200 | 17.9 bcdef | 2.8 abc | 15.7 bcdefg | 4.9 ef | 0.8 gh |
| 0.5 | 250 | 19.5 abcde | 2.9 abc | 18.0 ab | 7.8 c | 1.7 fgh |
| 1 | 0 | 19.1 abcdef | 2.8 abc | 12.9 efghi | 3.8 fg | 2.0 fg |
| 1 | 100 | 20.6 ab | 2.7 abc | 15.1 bcdefgh | 6.4 cd | 3.0 cdef |
| 1 | 150 | 17.1 def | 2.1 c | 15.9 bcde | 6.6 cd | 4.2 abc |
| 1 | 200 | 19.8 abcd | 3.4 a | 16.8 bc | 11.6 b | 5.4 a |
| 1 | 250 | 19.07 abcdef | 3.0 abc | 20.4 a | 6.9 c | 4.1 abcd |
| 2 | 0 | 19.3 abcde | 2.3 bc | 17.2 abc | 3.0 gh | 2.6 cdef |
| 2 | 100 | 18.2 bcdef | 2.7 abc | 14.8 cdefghi | 3.2 gh | 2.7 cdef |
| 2 | 150 | 18 bcdef | 3.0 ab | 17.5 abc | 11.2 b | 5.2 ab |
| 2 | 200 | 16.8 def | 3.0 abc | 17.1 bc | 13.9 a | 4.9 ab |
| 2 | 250 | 19.3 abcdef | 2.9 abc | 15.8 bcdef | 6.4 cd | 3.7 be |
| 3 | 0 | 19.2 abcdef | 2.6 abc | 15.3 bcdefgh | 2.3 hi | 2.5 def |
| 3 | 100 | 19.5 abcde | 2.9 abc | 16.2 bcd | 7.5 c | 3.8 abcde |
| 3 | 150 | 17.4 cdef | 2.8 abc | 14.9 bcdefgh | 5.4 de | 3.8 abcd |
| 3 | 200 | 18.8 abcdef | 2.3 bc | 11.7 i | 1.5 i | 1.7 fgh |
| 3 | 250 | 18.9 abcdef | 2.7 abc | 12.5 ghi | 3.1 gh | 2.6 cdef |
| Na2SeO3 | KIO3 | Phenols | Flavonoids | Chlorophyll a | Chlorophyll b | Total chl. | β-carotene |
|---|---|---|---|---|---|---|---|
| (mg L−1) | (mg L−1) | (mg GAE g−1 DW) | (mg QE 100 g−1 DW) | (µg g−1 FW) | (µg g−1 FW) | (µg g−1 FW) | (mg 100 g−1 DW) |
| 0 | 0 | 9.5 cdef | 60.4 ab | 93.0 abc | 81.3 a | 174.7 ab | 0.22 a |
| 0 | 100 | 10.3 abcde | 58.2 abc | 94.4 a | 81.0 a | 175.8 a | 0.15 a |
| 0 | 150 | 10.5 abcde | 52.8 abc | 93.1 abc | 81.3 a | 174.7 ab | 0.22 a |
| 0 | 200 | 11.1 abcd | 58.1 abc | 92.8 abc | 80.8 a | 174.0 ab | 0.25 a |
| 0 | 250 | 8.5 efg | 48.8 abc | 91.9 bc | 80.3 a | 172.5 ab | 0.23 a |
| 0.5 | 0 | 6.8 g | 51.4 abc | 92.7 abc | 80.6 a | 173.6 ab | 0.23 a |
| 0.5 | 100 | 12.5 a | 48.4 bc | 94.0 ab | 81.0 a | 175.4 a | 0.24 a |
| 0.5 | 150 | 9.16 efg | 55.4 abc | 92.9 abc | 81.3 a | 174.5 ab | 0.19 a |
| 0.5 | 200 | 11.13 bcd | 58.9 abc | 92.3 abc | 81.1 a | 173.7 ab | 0.24 a |
| 0.5 | 250 | 9.4 def | 56.8 abc | 93.0 abc | 81.4 a | 174.7 ab | 0.28 a |
| 1 | 0 | 10.7 abcde | 58.1 abc | 92.9 abc | 81.4 a | 174.6 ab | 0.23 a |
| 1 | 100 | 10.1 abcde | 54.2 abc | 93.2 abc | 80.6 a | 174.1 ab | 0.24 a |
| 1 | 150 | 10.7 abcde | 59.6 abc | 92.8 abc | 81.8 a | 175.0 ab | 0.21 a |
| 1 | 200 | 10.5 abcde | 61.0 ab | 93.5 abc | 81.8 a | 175.7 a | 0.21 a |
| 1 | 250 | 11.8 abc | 59.6 abc | 92.5 abc | 82.1 a | 174.9 ab | 0.25 a |
| 2 | 0 | 7.6 fg | 58.1 abc | 93.3 abc | 81.8 a | 175.4 a | 0.19 a |
| 2 | 100 | 9.4 cdef | 56.3 abc | 93.6 abc | 80.1 a | 174.0 ab | 0.18 a |
| 2 | 150 | 11.0 abcd | 50.4 abc | 92.9 abc | 81.0 a | 174.1 ab | 0.20 a |
| 2 | 200 | 9.3 def | 57.2 abc | 92.9 abc | 81.3 a | 174.6 ab | 0.21 a |
| 2 | 250 | 12.1 ab | 61.4 a | 92.6 abc | 81.6 a | 174.5 ab | 0.27 a |
| 3 | 0 | 10.0 bcdef | 57.1 abc | 91.5 c | 79.8 a | 171.6 b | 0.24 a |
| 3 | 100 | 9.9 bcdef | 47.5 c | 92.4 abc | 80.8 a | 173.5 ab | 0.26 a |
| 3 | 150 | 9.3 def | 60.7 ab | 93.2 abc | 82.2 a | 175.7 a | 0.20 a |
| 3 | 200 | 10.2 abcde | 50.7 abc | 92.4 abc | 80.6 a | 173.3 ab | 0.23 a |
| 3 | 250 | 9.0 defg | 54.7 abc | 93.5 abc | 81.9 a | 175.6 a | 0.18 a |
| Na2SeO3 | KIO3 | GSH | GPX | PAL | CAT | APX |
|---|---|---|---|---|---|---|
| (mg L−1) | (mg L−1) | (U g−1 TP) | (U g−1 TP) | (U 100 g−1 TP) | (U g−1 TP) | (U g−1 TP) |
| 0 | 0 | 0.20 bc | 0.36 ab | 1.2 b | 2.7 ab | 2.8 ab |
| 0 | 100 | 0.22 abc | 0.59 ab | 4.1 ab | 2.9 ab | 2.3 ab |
| 0 | 150 | 0.21 abc | 0.54 ab | 4.4 ab | 4.0 ab | 2.7 ab |
| 0 | 200 | 0.20 abc | 0.77 ab | 4.7 ab | 3.1 ab | 2.9 ab |
| 0 | 250 | 0.20 abc | 0.57 ab | 3.7 ab | 3.2 ab | 2.7 ab |
| 0.5 | 0 | 0.20 bc | 0.47 ab | 3.0 ab | 2.6 ab | 3.0 ab |
| 0.5 | 100 | 0.20 abc | 0.48 ab | 3.6 ab | 2.6 ab | 1.9 ab |
| 0.5 | 150 | 0.27 a | 0.71 ab | 3.8 ab | 2.9 ab | 1.6 ab |
| 0.5 | 200 | 0.25 abc | 0.72 ab | 2.3 ab | 3.0 ab | 2.2 ab |
| 0.5 | 250 | 0.21 abc | 0.46 ab | 1.7 b | 2.5 ab | 2.3 ab |
| 1 | 0 | 0.20 abc | 0.73 ab | 3.0 ab | 3.0 ab | 2.0 ab |
| 1 | 100 | 0.25 abc | 0.43 ab | 2.3 ab | 2.8 ab | 2.4 ab |
| 1 | 150 | 0.22 abc | 0.72 ab | 4.1 ab | 3.0 ab | 1.5 b |
| 1 | 200 | 0.26 a | 0.70 ab | 6.5 a | 3.4 ab | 2.4 ab |
| 1 | 250 | 0.25 abc | 0.60 ab | 3.2 ab | 2.4 ab | 2.3 ab |
| 2 | 0 | 0.21 abc | 0.68 ab | 2.4 ab | 2.7 ab | 2.8 ab |
| 2 | 100 | 0.19 c | 0.25 b | 5.0 ab | 3.3 ab | 3.4 a |
| 2 | 150 | 0.24 abc | 0.47 ab | 5.4 ab | 2.5 ab | 2.4 ab |
| 2 | 200 | 0.26 ab | 0.61 ab | 4.4 ab | 2.8 ab | 2.3 ab |
| 2 | 250 | 0.25 abc | 0.38 ab | 4.4 ab | 2.9 ab | 2.9 ab |
| 3 | 0 | 0.24 abc | 1.22 a | 2.8 ab | 2.3 b | 2.2 ab |
| 3 | 100 | 0.26 abc | 1.22 ab | 3.3 ab | 4.6 a | 1.9 ab |
| 3 | 150 | 0.23 abc | 0.57 ab | 3.9 ab | 2.5 ab | 2.2 ab |
| 3 | 200 | 0.22 abc | 0.58 ab | 2.5 ab | 2.6 ab | 2.7 ab |
| 3 | 250 | 0.23 abc | 1.04 ab | 3.0 ab | 2.5 ab | 2.2 ab |
| Na2SeO3 | KIO3 | GSH | GPX | PAL | CAT | APX |
|---|---|---|---|---|---|---|
| (mg L−1) | (mg L−1) | (U g−1 TP) | (U g−1 TP) | (U 100 g−1 TP) | (U g−1 TP) | (U g−1 TP) |
| 0 | 0 | 1.1 cdefg | 1.0 bcd | 13.9 abc | 3.5 abc | 4.6 ab |
| 0 | 100 | 1.1 bcdefg | 0.9 cd | 12.4 abc | 2.5 abc | 2.1 ab |
| 0 | 150 | 1.1 bcdefg | 1.0 cd | 11.7 abc | 1.8 bc | 2.4 ab |
| 0 | 200 | 1.4 a | 0.9 cd | 13.8 abc | 2.2 abc | 1.5 ab |
| 0 | 250 | 1.2 abcd | 0.7 d | 18.6 ab | 4.1 abc | 2.1 ab |
| 0.5 | 0 | 1.0 defg | 1.4 abc | 19.9 a | 2.1 abc | 4.2 ab |
| 0.5 | 100 | 1.2 abcd | 1.1 abcd | 17.0 abc | 2.5 abc | 2.1 ab |
| 0.5 | 150 | 1.0 defg | 1.8 a | 18.7 ab | 4.4 abc | 4.6 ab |
| 0.5 | 200 | 1.0 cdefg | 1.7 ab | 9.5 c | 2.2 abc | 3.2 ab |
| 0.5 | 250 | 0.9 g | 1.2 abcd | 13.7 abc | 6.3 a | 4.2 ab |
| 1 | 0 | 1.0 cdefg | 1.0 cd | 13.9 abc | 3.9 abc | 2.7 ab |
| 1 | 100 | 1.2 abcdef | 1.0 cd | 9.5 c | 3.5 abc | 1.2 ab |
| 1 | 150 | 1.1 abcdefg | 1.1 bcd | 8.7 c | 1.8 bc | 3.1 ab |
| 1 | 200 | 1.3 abc | 1.1 bcd | 8.7 c | 2.4 abc | 3.1 ab |
| 1 | 250 | 1.1 abcdefg | 1.2 abcd | 11.6 abc | 4.9 abc | 4.2 ab |
| 2 | 0 | 0.9 fg | 1.2 abcd | 19.1 ab | 6.1 ab | 4.9 a |
| 2 | 100 | 1.0 defg | 0.6 d | 10.5 bc | 2.8 abc | 1.6 ab |
| 2 | 150 | 0.9 efg | 0.7 d | 10.9 abc | 0.9 c | 2.5 ab |
| 2 | 200 | 1.1 abcdefg | 1.3 abcd | 12.5 abc | 2.9 bc | 2.8 ab |
| 2 | 250 | 1.3 ab | 1.0 cd | 13.7 abc | 0.7 c | 2.8 ab |
| 3 | 0 | 1.1 bcdefg | 0.9 cd | 12.8 abc | 1.8 bc | 2.2 ab |
| 3 | 100 | 1.1 abcdefg | 0.9 cd | 11.1 abc | 0.6 c | 2.1 ab |
| 3 | 150 | 1.2 abcde | 0.9 cd | 12.5 abc | 1.7 c | 1.2 ab |
| 3 | 200 | 1.1 abcdefg | 1.0 cd | 12.3 abc | 2.3 abc | 0.8 b |
| 3 | 250 | 1.1 abcdefg | 1.1 abcd | 12.1 abc | 2.6 abc | 1.4 ab |
| Na2SeO3 | KIO3 | Fruits | Fruits | Fruits | Leaves | Leaves | Leaves |
|---|---|---|---|---|---|---|---|
| (mg L−1) | (mg L−1) | ABTS-H | ABTS-L | DPPH-H | ABTS-H | ABTS-L | DPPH-H |
| 0 | 0 | 28.7 a | 6.6 a | 43.7 abc | 47.8 ab | 20.1 abc | 21.5 e |
| 0 | 100 | 27.5 ab | 8.4 a | 34.1 abc | 64.7 ab | 14.6 bc | 22.3 de |
| 0 | 150 | 27.0 ab | 4.0 a | 41.0 abc | 44.2 ab | 17.7 abc | 34.4 bcde |
| 0 | 200 | 25.3 ab | 3.2 a | 42.0 abc | 61.6 ab | 15.8 abc | 26.0 cde |
| 0 | 250 | 27.1 ab | 7.1 a | 44.9 abc | 55.3 ab | 19.0 abc | 36.0 abcde |
| 0.5 | 0 | 20.3 abc | 8.8 a | 50.2 a | 47.0 ab | 13.1 bc | 28.5 cde |
| 0.5 | 100 | 19.3 abc | 8.4 a | 36.6 abc | 56.3 ab | 10.9 c | 36.1 abcde |
| 0.5 | 150 | 16.8 abc | 4.7 a | 41.3 abc | 59.4 ab | 10.3 c | 40.0 abcde |
| 0.5 | 200 | 26.4 ab | 1.4 a | 41.9 abc | 45.7 ab | 26.2 a | 28.9 cde |
| 0.5 | 250 | 27.4 ab | 3.6 a | 36.5 abc | 42.7 ab | 13.6 bc | 22.3 de |
| 1 | 0 | 17.3 abc | 1.8 a | 26.2 abc | 52.1 ab | 18.1 abc | 28.0 cde |
| 1 | 100 | 16.3 abc | 2.3 a | 35.6 abc | 44.4 ab | 12.7 bc | 27.0 cde |
| 1 | 150 | 27.8 ab | 4.3 a | 46.9 ab | 55.3 ab | 19.9 abc | 48.3 abcd |
| 1 | 200 | 18.1 abc | 4.3 a | 32.2 abc | 44.5 ab | 14.0 bc | 49.7 abc |
| 1 | 250 | 15.3 bc | 2.6 a | 32.1 abc | 46.2 ab | 14.0 bc | 36.2 abcde |
| 2 | 0 | 17.0 abc | 1.7 a | 29.4 abc | 40.4 b | 18.9 abc | 36.1 abcde |
| 2 | 100 | 17.2 abc | 4.2 a | 23.1 b | 44.8 ab | 19.7 abc | 61.5 a |
| 2 | 150 | 12.1 c | 6.6 a | 20.8 c | 44.9 ab | 22.5 ab | 29.0 cde |
| 2 | 200 | 10.3 c | 3.4 a | 28.3 abc | 50.0 ab | 18.9 abc | 61.7 a |
| 2 | 250 | 15.6 abc | 2.7 a | 26.5 abc | 50.0 ab | 16.4 abc | 52.5 abc |
| 3 | 0 | 18.2 abc | 9.7 a | 28.3 abc | 53.0 ab | 22.3 ab | 56.1 ab |
| 3 | 100 | 22.3 abc | 9.6 a | 30.7 abc | 48.0 ab | 19.3 abc | 49.1 abc |
| 3 | 150 | 11.8 c | 2.3 a | 29.4 abc | 67.0 a | 18.2 abc | 42.7 abcde |
| 3 | 200 | 14.9 bc | 5.3 a | 25.5 abc | 54.4 ab | 13.4 bc | 21.6 e |
| 3 | 250 | 23.2 abc | 2.4 a | 30.9 abc | 57.5 ab | 20.0 abc | 46.9 abcde |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía-Ramírez, F.; Benavides-Mendoza, A.; González-Morales, S.; Juárez-Maldonado, A.; Lara-Viveros, F.M.; Morales-Díaz, A.B.; Morelos-Moreno, Á. Seed Priming Based on Iodine and Selenium Influences the Nutraceutical Compounds in Tomato (Solanum lycopersicum L.) Crop. Antioxidants 2023, 12, 1265. https://doi.org/10.3390/antiox12061265
Mejía-Ramírez F, Benavides-Mendoza A, González-Morales S, Juárez-Maldonado A, Lara-Viveros FM, Morales-Díaz AB, Morelos-Moreno Á. Seed Priming Based on Iodine and Selenium Influences the Nutraceutical Compounds in Tomato (Solanum lycopersicum L.) Crop. Antioxidants. 2023; 12(6):1265. https://doi.org/10.3390/antiox12061265
Chicago/Turabian StyleMejía-Ramírez, Fernando, Adalberto Benavides-Mendoza, Susana González-Morales, Antonio Juárez-Maldonado, Francisco Marcelo Lara-Viveros, América Berenice Morales-Díaz, and Álvaro Morelos-Moreno. 2023. "Seed Priming Based on Iodine and Selenium Influences the Nutraceutical Compounds in Tomato (Solanum lycopersicum L.) Crop" Antioxidants 12, no. 6: 1265. https://doi.org/10.3390/antiox12061265






