Potential Therapeutic Functions of PU-91 and Quercetin in Personalized Cybrids Derived from Patients with Age-Related Macular Degeneration, Keratoconus, and Glaucoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Methods of Cybrids Creation and Culture Condition
2.3. Cellular Metabolism Assay (MTT Assay)
2.4. Intracellular Level of Reactive Oxygen Species (ROS Assay)
2.5. RNA Isolation Process and cDNA Amplification
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analyses
3. Results
3.1. Effect of PU-91 (P), Quercetin (Q), or in Combination on Cellular Metabolism and Mitochondrial Biogenesis in Cybrids Derived from Patients with Age Macular Degeneration (AMD), Keratoconus (KC), and Glaucoma (Glc)
3.2. Effect of PU-91 (P), Quercetin (Q), or in Combination on Reactive Oxygen Species (ROS) and Redox-Sensitive Transcription Factor (NRF1, SOD2) Expression in Cybrids Derived from Patients with AMD, KC, and Glc
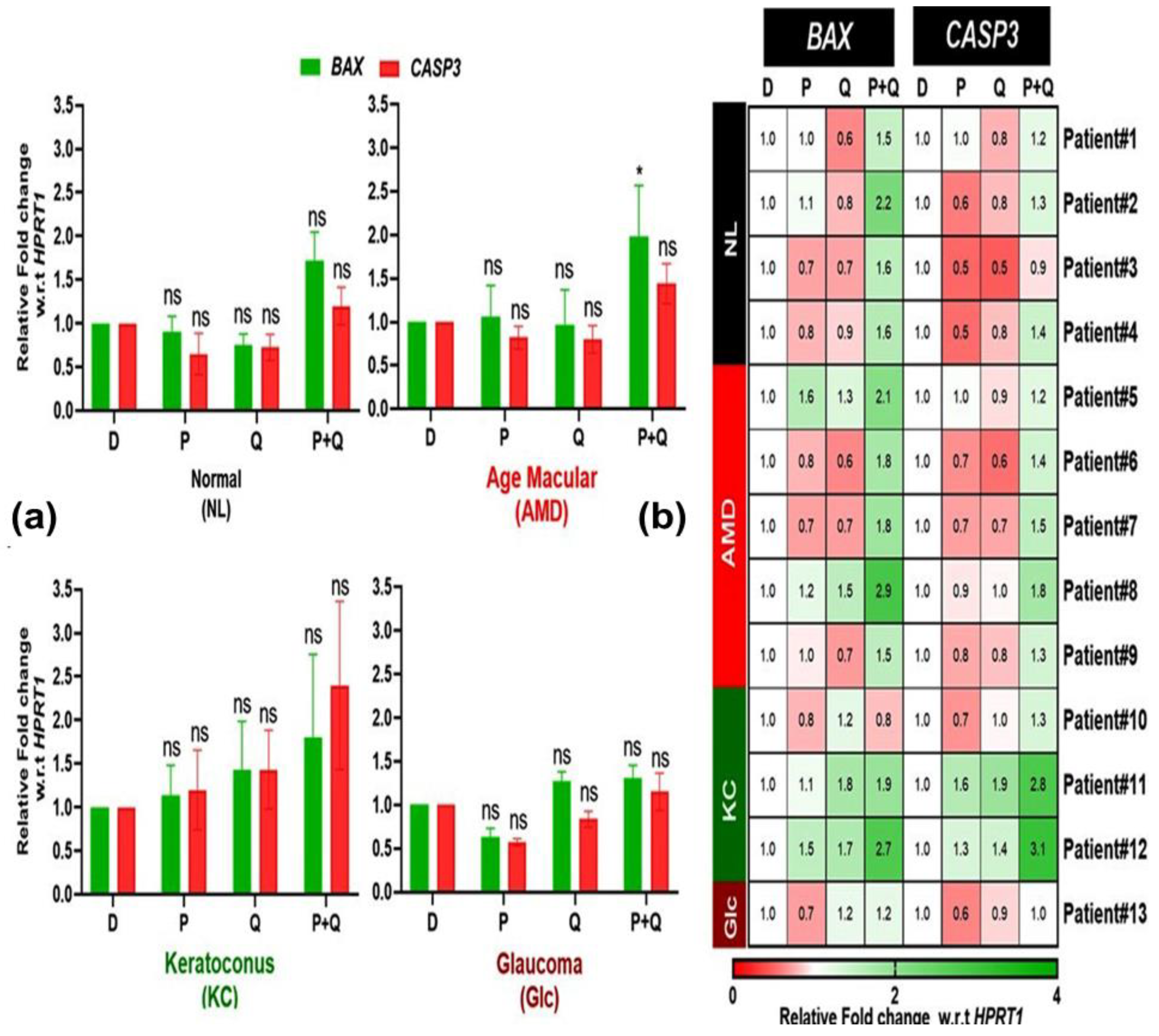
3.3. Effect of PU-91 (P), QUERCETIN (Q), or in Combination on the Expression of Apoptotic Genes in the Cybrids Derived from Patients with AMD, KC, and Glc
3.4. Effect of PU-91 (P), Quercetin (Q), or in Combination on the Expression of the Inflammatory Gene in the Cybrid Derived from Patients with AMD, KC, and Glc
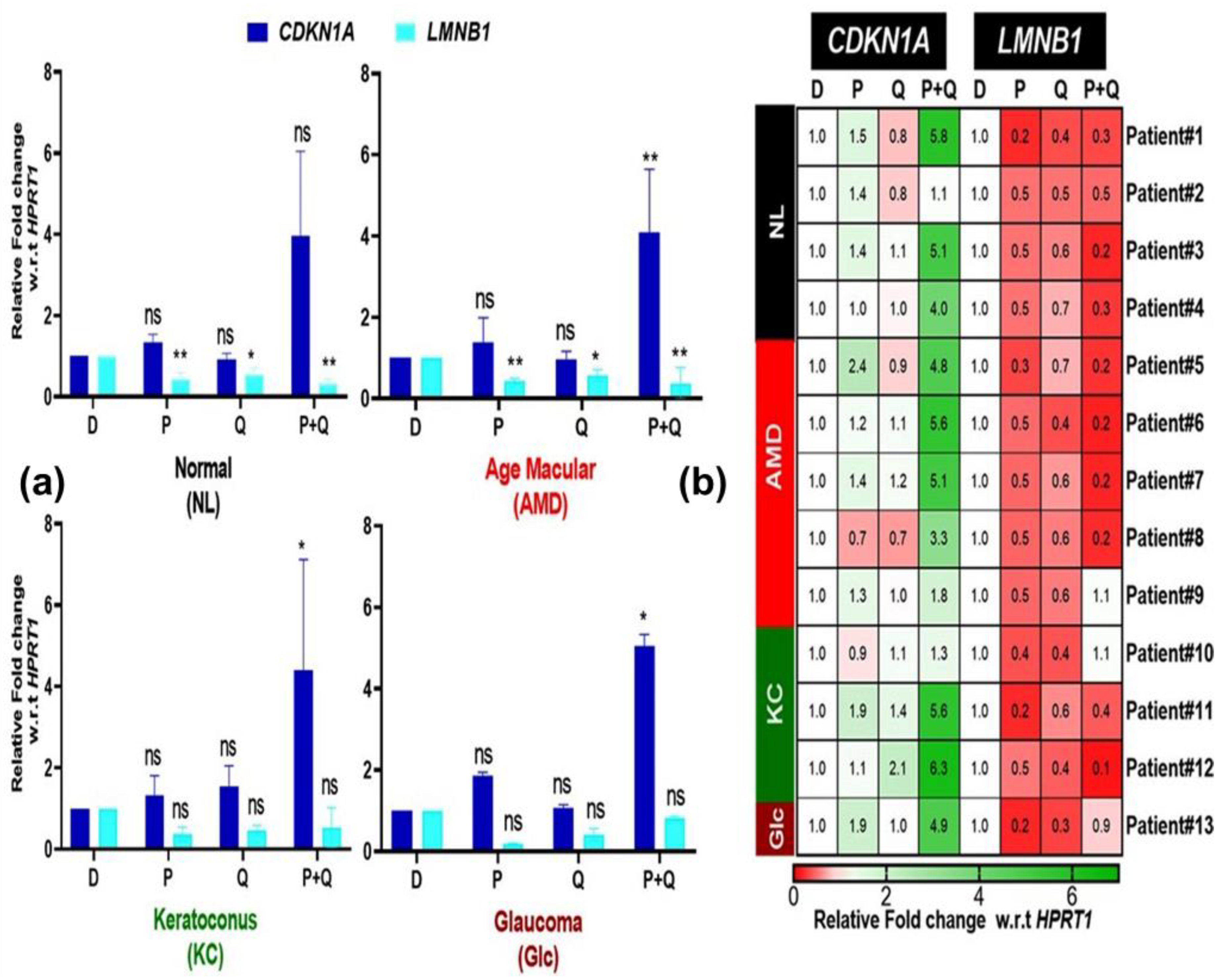
3.5. Effect of PU-91 (P), Quercetin (Q), or in Combination on the Expression of Genes Associated with Senescence in the Cybrids Derived from Patients with AMD, KC, and Glc
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tielens, A.G.; Rotte, C.; van Hellemond, J.J.; Martin, W. Mitochondria as we don’t know them. Trends Biochem. Sci. 2002, 27, 564–572. [Google Scholar] [CrossRef] [PubMed]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, T.G.; Mannella, C.A. The internal structure of mitochondria. Trends Biochem. Sci. 2000, 25, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Blum, A.; Liu, J.; Finkel, T. The role of mitochondria in aging. J. Clin. Investig. 2018, 128, 3662–3670. [Google Scholar] [CrossRef] [Green Version]
- Lane, R.K.; Hilsabeck, T.; Rea, S.L. The role of mitochondrial dysfunction in age-related diseases. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 1387–1400. [Google Scholar] [CrossRef] [Green Version]
- Castellani, R.; Hirai, K.; Aliev, G.; Drew, K.L.; Nunomura, A.; Takeda, A.; Cash, A.D.; Obrenovich, M.E.; Perry, G.; Smith, M.A. Role of mitochondrial dysfunction in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 357–360. [Google Scholar] [CrossRef]
- Kenney, M.C.; Chwa, M.; Atilano, S.R.; Falatoonzadeh, P.; Ramirez, C.; Malik, D.; Tarek, M.; Cáceres-del-Carpio, J.; Nesburn, A.B.; Boyer, D.S. Inherited mitochondrial DNA variants can affect complement, inflammation and apoptosis pathways: Insights into mitochondrial–nuclear interactions. Hum. Mol. Genet. 2014, 23, 3537–3551. [Google Scholar] [CrossRef] [Green Version]
- Martín-Jiménez, R.; Lurette, O.; Hebert-Chatelain, E. Damage in mitochondrial DNA associated with Parkinson’s Disease. DNA Cell Biol. 2020, 39, 1421–1430. [Google Scholar] [CrossRef]
- Riazi-Esfahani, M.; Kuppermann, B.D.; Kenney, M.C. The role of mitochondria in AMD: Current knowledge and future applications. J. Ophthalmic Vis. Res. 2017, 12, 424. [Google Scholar] [PubMed]
- Kenney, M.C.; Chwa, M.; Atilano, S.R.; Pavlis, J.M.; Falatoonzadeh, P.; Ramirez, C.; Malik, D.; Hsu, T.; Woo, G.; Soe, K. Mitochondrial DNA variants mediate energy production and expression levels for CFH, C3 and EFEMP1 genes: Implications for age-related macular degeneration. PLoS ONE 2013, 8, e54339. [Google Scholar] [CrossRef] [PubMed]
- Nashine, S.; Nesburn, A.B.; Kuppermann, B.D.; Kenney, M.C. Age-related macular degeneration (AMD) mitochondria modulate epigenetic mechanisms in retinal pigment epithelial cells. Exp. Eye Res. 2019, 189, 107701. [Google Scholar] [CrossRef] [PubMed]
- Kenney, M.C.; Hertzog, D.; Chak, G.; Atilano, S.R.; Khatibi, N.; Soe, K.; Nobe, A.; Yang, E.; Chwa, M.; Zhu, F. Mitochondrial DNA haplogroups confer differences in risk for age-related macular degeneration: A case control study. BMC Med. Genet. 2013, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atilano, S.R.; Coskun, P.; Chwa, M.; Jordan, N.; Reddy, V.; Le, K.; Wallace, D.C.; Kenney, M.C. Accumulation of mitochondrial DNA damage in keratoconus corneas. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Kalur, A.; Muste, J.; Valentim, C.C.; Iyer, A.; Singh, R.P. Novel mitochondrial therapies for the treatment of age-related macular degeneration. Ann. Eye Sci. 2021, 6, 21–27. [Google Scholar] [CrossRef]
- Nashine, S.; Subramaniam, S.R.; Chwa, M.; Nesburn, A.; Kuppermann, B.D.; Federoff, H.; Kenney, M.C. PU-91 drug rescues human age-related macular degeneration RPE cells; implications for AMD therapeutics. Aging (Albany NY) 2019, 11, 6691. [Google Scholar] [CrossRef]
- Bao, A.; Nashine, S.; Atilano, S.; Chwa, M.; Federoff, H.; Kenney, M.C. Differential responses of AMD mitochondrial DNA haplogroups to PU-91, a mitochondria-targeting drug. Mitochondrion 2021, 60, 189–200. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Kristo, A.S.; Klimis-Zacas, D.; Sikalidis, A.K. Protective role of dietary berries in cancer. Antioxidants 2016, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.V.; Jazwinski, S.M. Nuclear gene expression changes due to mitochondrial dysfunction in ARPE-19 cells: Implications for age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1765–1773. [Google Scholar] [CrossRef]
- Udar, N.; Atilano, S.R.; Memarzadeh, M.; Boyer, D.S.; Chwa, M.; Lu, S.; Maguen, B.; Langberg, J.; Coskun, P.; Wallace, D.C.; et al. Mitochondrial DNA haplogroups associated with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2966–2974. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Hertel, M.; Braun, S.; Durka, S.; Alzheimer, C.; Werner, S. Upregulation and activation of the Nrf-1 transcription factor in the lesioned hippocampus. Eur. J. Neurosci. 2002, 15, 1707–1711. [Google Scholar] [CrossRef] [PubMed]
- Justilien, V.; Pang, J.J.; Renganathan, K.; Zhan, X.; Crabb, J.W.; Kim, S.R.; Sparrow, J.R.; Hauswirth, W.W.; Lewin, A.S. SOD2 knockdown mouse model of early AMD. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4407–4420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nashine, S.; Cohen, P.; Chwa, M.; Lu, S.; Nesburn, A.B.; Kuppermann, B.D.; Kenney, M.C. Humanin G (HNG) protects age-related macular degeneration (AMD) transmitochondrial ARPE-19 cybrids from mitochondrial and cellular damage. Cell Death Dis. 2017, 8, e2951. [Google Scholar] [CrossRef]
- Kohli, J.; Wang, B.; Brandenburg, S.M.; Basisty, N.; Evangelou, K.; Varela-Eirin, M.; Campisi, J.; Schilling, B.; Gorgoulis, V.; Demaria, M. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat. Protoc. 2021, 16, 2471–2498. [Google Scholar] [CrossRef]
- Dib, B.; Lin, H.; Maidana, D.E.; Tian, B.; Miller, J.B.; Bouzika, P.; Miller, J.W.; Vavvas, D.G. Mitochondrial DNA has a pro-inflammatory role in AMD. Biochim. Et Biophys. Acta 2015, 1853, 2897–2906. [Google Scholar] [CrossRef] [Green Version]
- Kong, G.Y.; Van Bergen, N.J.; Trounce, I.A.; Crowston, J.G. Mitochondrial dysfunction and glaucoma. J. Glaucoma 2009, 18, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Van Bergen, N.J.; Kong, G.Y.; Chrysostomou, V.; Waugh, H.S.; O’Neill, E.C.; Crowston, J.G.; Trounce, I.A. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp. Eye Res. 2011, 93, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Saccà, S.C.; Longobardi, M.; Cartiglia, C. Mitochondrial damage in the trabecular meshwork of patients with glaucoma. Arch. Ophthalmol. 2010, 128, 724–730. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Xu, H.; Liang, F.Q.; Liang, H.; Gupta, P.; Havey, A.N.; Boulton, M.E.; Godley, B.F. Mitochondrial DNA damage and repair in RPE associated with aging and age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.D.; Chen, Z.L.; Qu, M.L.; Zhao, X.W.; Li, S.X.; Chen, P. Decreased Integrity, Content, and Increased Transcript Level of Mitochondrial DNA Are Associated with Keratoconus. PLoS ONE 2016, 11, e0165580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallabh, N.A.; Romano, V.; Willoughby, C.E. Mitochondrial dysfunction and oxidative stress in corneal disease. Mitochondrion 2017, 36, 103–113. [Google Scholar] [CrossRef]
- McKay, T.B.; Lyon, D.; Sarker-Nag, A.; Priyadarsini, S.; Asara, J.M.; Karamichos, D. Quercetin attenuates lactate production and extracellular matrix secretion in keratoconus. Sci. Rep. 2015, 5, 9003. [Google Scholar] [CrossRef] [Green Version]
- McKay, T.B.; Karamichos, D. Quercetin and the ocular surface: What we know and where we are going. Exp. Biol. Med. (Maywood) 2017, 242, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, J.; Ngema, M.; Nkomozepi, P.; Erlwanger, K. Quercetin administration post-weaning attenuates high-fructose, high-cholesterol diet-induced hepatic steatosis in growing, female, Sprague Dawley rat pups. J. Sci. Food Agric. 2019, 99, 6954–6961. [Google Scholar] [CrossRef]
- Zoico, E.; Nori, N.; Darra, E.; Tebon, M.; Rizzatti, V.; Policastro, G.; De Caro, A.; Rossi, A.P.; Fantin, F.; Zamboni, M. Senolytic effects of quercetin in an in vitro model of pre-adipocytes and adipocytes induced senescence. Sci. Rep. 2021, 11, 23237. [Google Scholar] [CrossRef]
- Nogueira-Recalde, U.; Lorenzo-Gómez, I.; Blanco, F.J.; Loza, M.I.; Grassi, D.; Shirinsky, V.; Shirinsky, I.; Lotz, M.; Robbins, P.D.; Domínguez, E.; et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine 2019, 45, 588–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
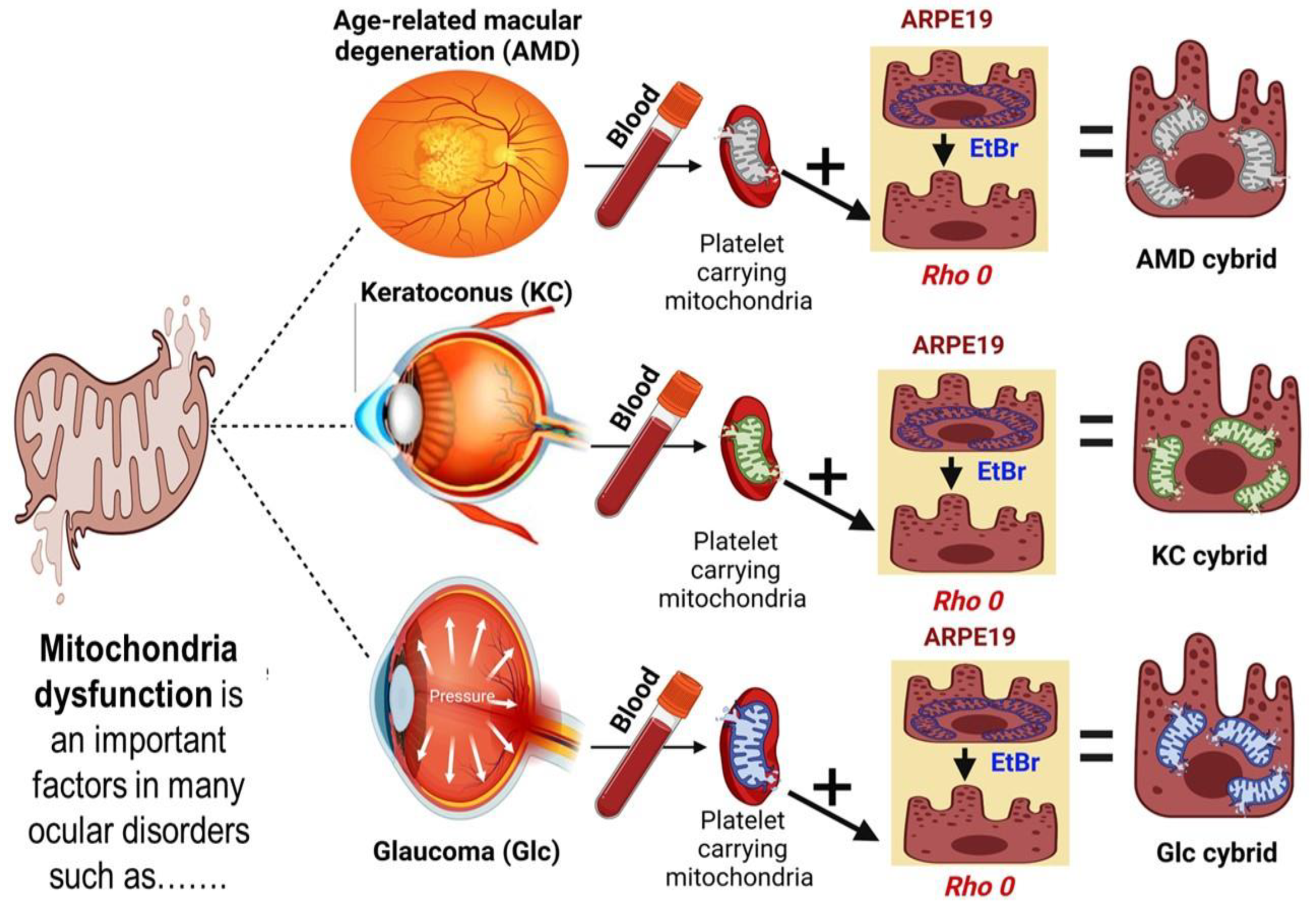

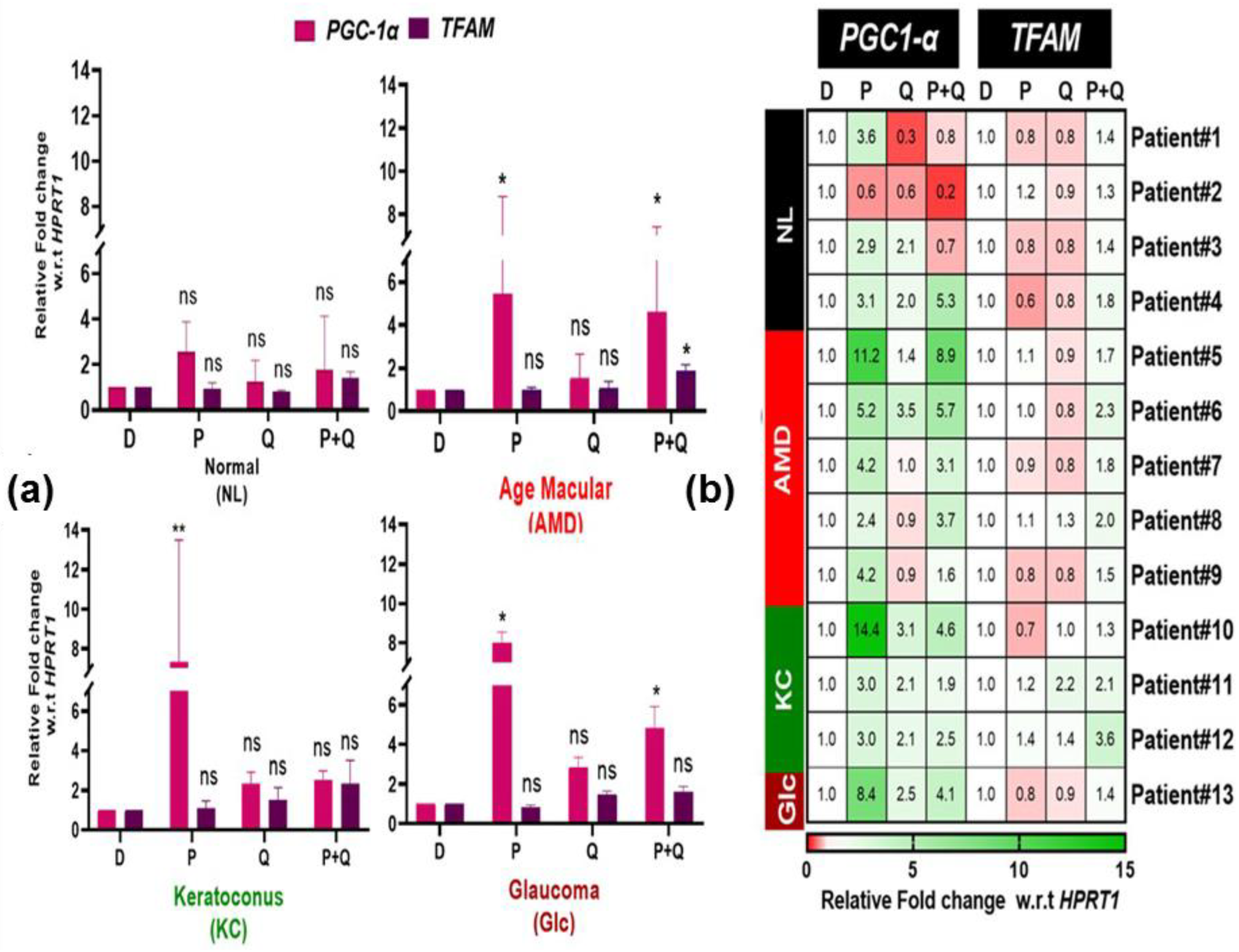
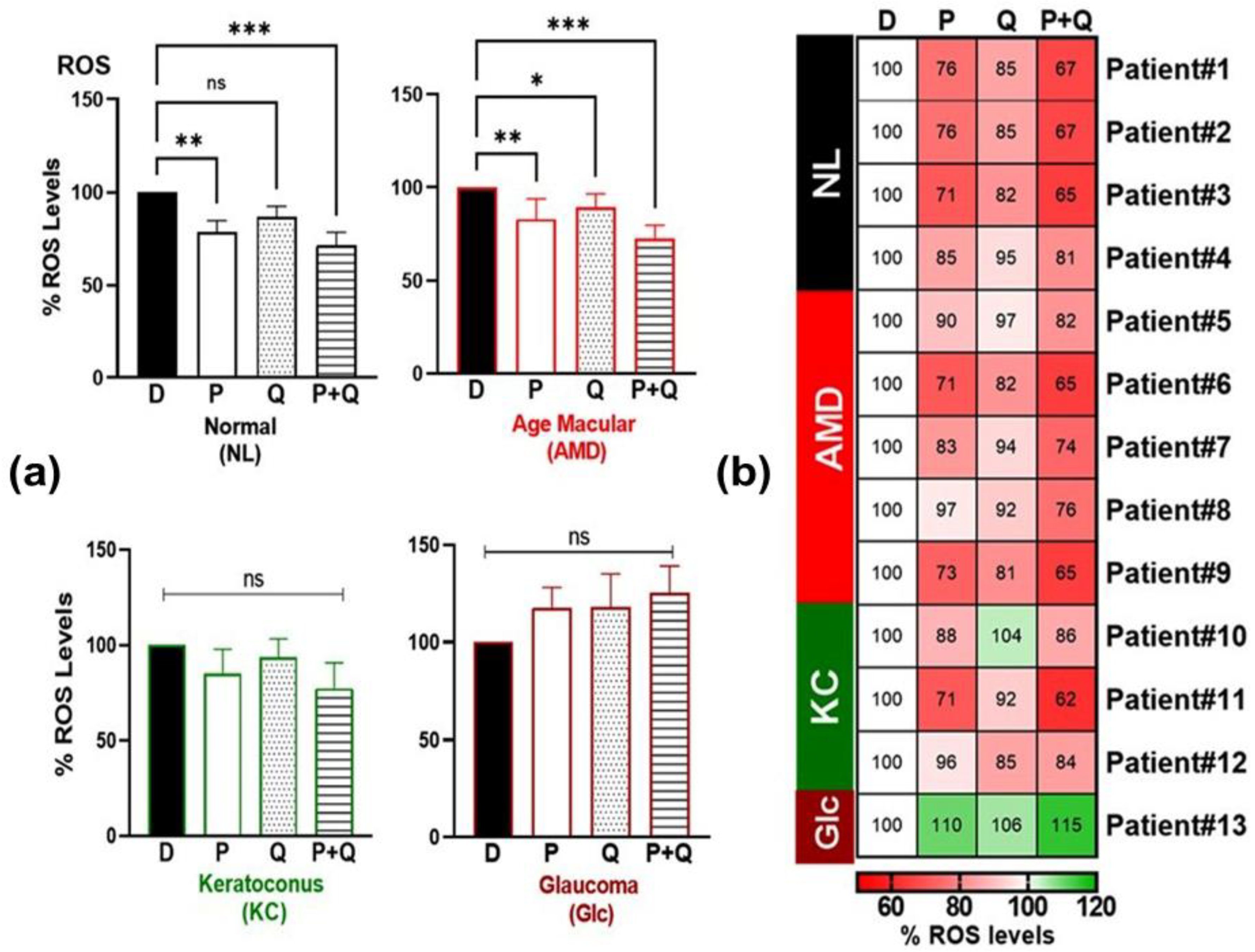
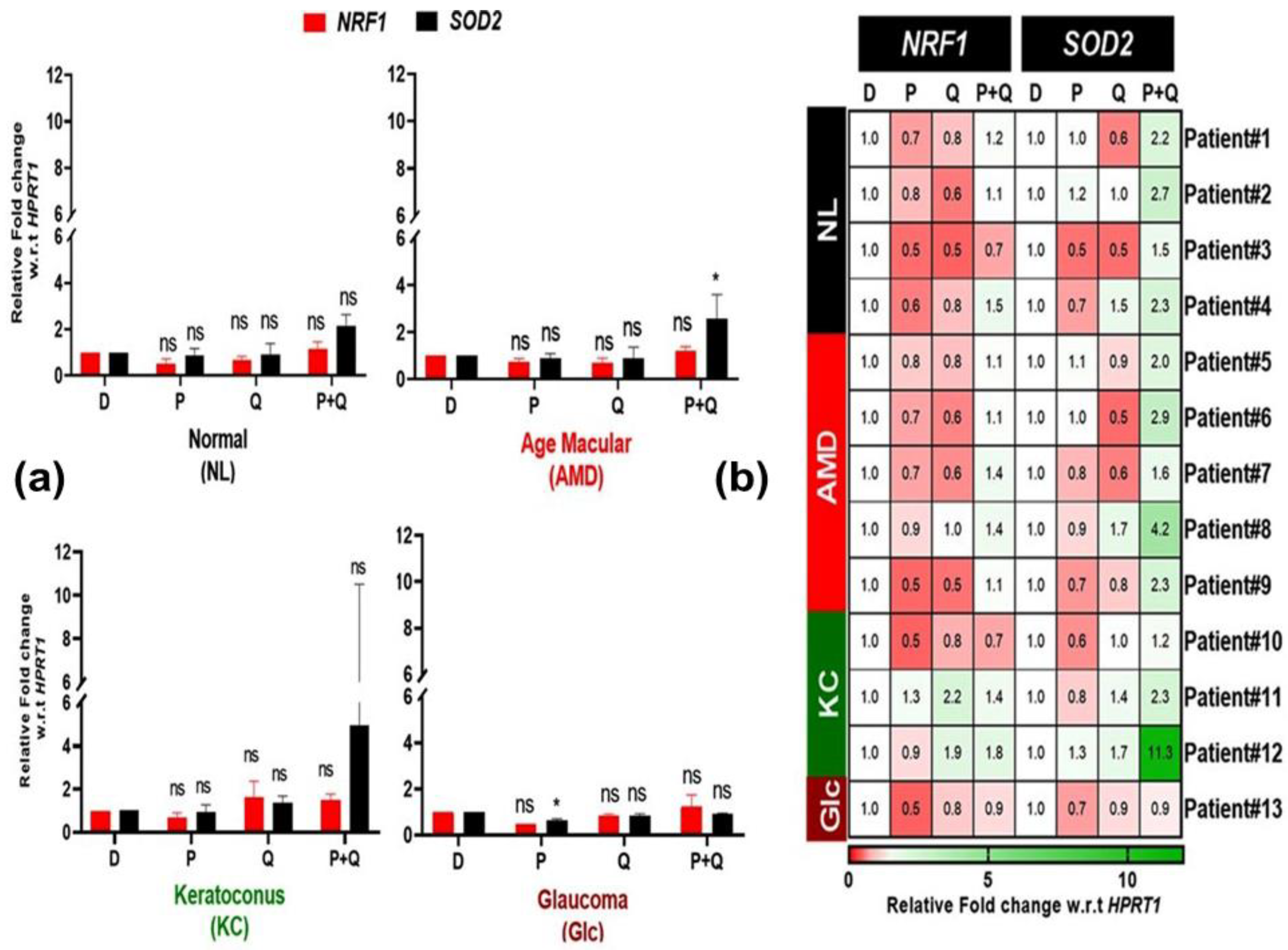


| Patient # | Cybrid | Haplogroup | Age (y) | Sex | Ethnicity | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 17.201 | H | 77 | M | White | NL |
| 2 | 21.264 | H | 89 | M | White | NL |
| 3 | 15.150 | K1a1b1a | 59 | M | White | NL |
| 4 | 19.245 | A2e | 62 | F | White | NL |
| 5 | 13.128 | H7e | 86 | M | White | Early dry AMD |
| 6 | 17.199 | H | 83 | M | White | dry AMD |
| 7 | 19.256 | H | 86 | M | White | dry AMD |
| 8 | 21.263 | H | 84 | F | White | dry AMD |
| 9 | 14.139 | H17b | 81 | F | White | wet AMD |
| 10 | 16.188 | K2a2a1 | 90 | M | White | KC |
| 11 | 18.220 | K | 78 | M | White | KC |
| 12 | 19.259 | H | 73 | M | White | KC |
| 13 | 18.241 | H | 80 | M | White | Glc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salimiaghdam, N.; Singh, L.; Singh, M.K.; Chwa, M.; Atilano, S.; Mohtashami, Z.; Nesburn, A.; Kuppermann, B.D.; Kenney, M.C. Potential Therapeutic Functions of PU-91 and Quercetin in Personalized Cybrids Derived from Patients with Age-Related Macular Degeneration, Keratoconus, and Glaucoma. Antioxidants 2023, 12, 1326. https://doi.org/10.3390/antiox12071326
Salimiaghdam N, Singh L, Singh MK, Chwa M, Atilano S, Mohtashami Z, Nesburn A, Kuppermann BD, Kenney MC. Potential Therapeutic Functions of PU-91 and Quercetin in Personalized Cybrids Derived from Patients with Age-Related Macular Degeneration, Keratoconus, and Glaucoma. Antioxidants. 2023; 12(7):1326. https://doi.org/10.3390/antiox12071326
Chicago/Turabian StyleSalimiaghdam, Nasim, Lata Singh, Mithalesh Kumar Singh, Marilyn Chwa, Shari Atilano, Zahra Mohtashami, Anthony Nesburn, Baruch D. Kuppermann, and M. Cristina Kenney. 2023. "Potential Therapeutic Functions of PU-91 and Quercetin in Personalized Cybrids Derived from Patients with Age-Related Macular Degeneration, Keratoconus, and Glaucoma" Antioxidants 12, no. 7: 1326. https://doi.org/10.3390/antiox12071326
APA StyleSalimiaghdam, N., Singh, L., Singh, M. K., Chwa, M., Atilano, S., Mohtashami, Z., Nesburn, A., Kuppermann, B. D., & Kenney, M. C. (2023). Potential Therapeutic Functions of PU-91 and Quercetin in Personalized Cybrids Derived from Patients with Age-Related Macular Degeneration, Keratoconus, and Glaucoma. Antioxidants, 12(7), 1326. https://doi.org/10.3390/antiox12071326








