The Cactus (Opuntia ficus-indica) Cladodes and Callus Extracts: A Study Combined with LC-MS Metabolic Profiling, In-Silico, and In-Vitro Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagents
2.2. Sample Preparation
2.3. UPLC-QTOF-MS Metabolic Profiling
2.4. In-Silico Prediction of Molecular Docking Simulation
2.4.1. Ligand and Target Protein Preparation
2.4.2. Molecular Docking
2.5. In-Vitro Anti-Inflammatory Activity
2.5.1. Cell Culture and Viability Assay
2.5.2. Nitric Oxide (NO) Assay
2.5.3. Enzyme-Linked Immunosorbent Assay
2.6. Experimental Data Analysis
3. Results and Discussion
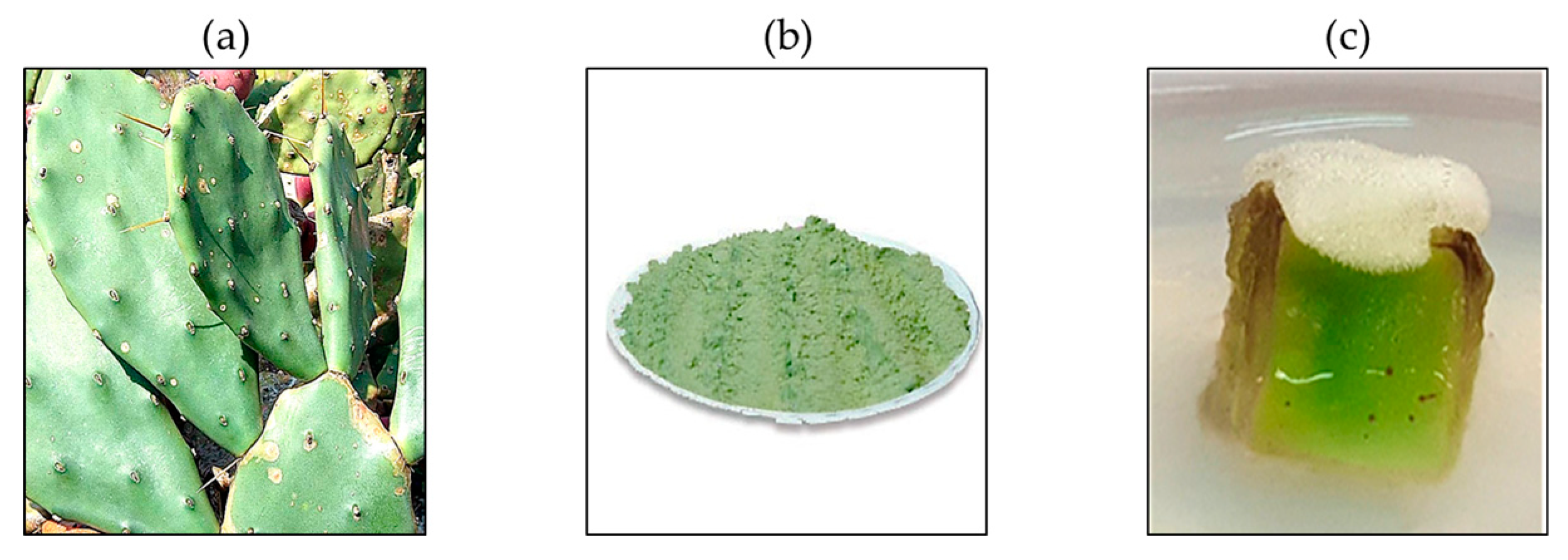
3.1. Plant Materials Used in This Study
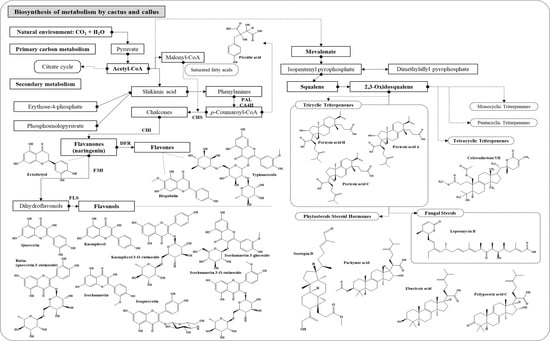
3.2. Bioactive Compounds of OFC and of Extract
3.2.1. Identification of OFC Metabolite Components
Triterpenoids
Esters and Steroids
Antifungal
Fatty Acids
Fatty Amides
3.2.2. Network and Interpretation of Phytochemicals in OFC Extract
3.2.3. Identification of OF Metabolite Components
Phenolic
Triterpenoids
Flavonols
Flavanone
Flavone
3.2.4. Network and Interpretation of Phytochemical in OF Extract
3.3. Molecular Docking Results and Interpretation
3.4. Anti-Inflammatory Activity of OFC and of Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadykalo, A.N.; López-Rodriguez, M.D.; Ainscough, J.; Droste, N.; Ryu, H.; Ávila-Flores, G.; Le Clec’h, S.; Muñozj, M.C.; Nilssone, L.; Rana, S.; et al. Disentangling ‘ecosystem services’ and ‘nature’s contributions to people’. Ecosyst. People 2019, 15, 269–287. [Google Scholar] [CrossRef]
- Richa, A.; Touil, S.; Fizir, M.; Martinez, V. Recent advances and perspectives in the treatment of hydroponic wastewater: A review. Rev. Environ. Sci. Biotechnol. 2020, 19, 945–966. [Google Scholar] [CrossRef]
- Oseni, O.M.; Pande, V.; Nailwal, T.K. A review on plant tissue culture, a technique for propagation and conservation of endangered plant species. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3778–3786. [Google Scholar] [CrossRef]
- Barker, A.V.; Bryson, G.M. Comparisons of composts with low or high nutrient status for growth of plants in containers. Commun. Soil Sci. Plant Anal. 2006, 37, 1303–1319. [Google Scholar] [CrossRef]
- Dede, O.H.; Ozdemir, S. Development of nutrient-rich growing media with hazelnut husk and municipal sewage sludge. Environ. Technol. 2018, 39, 2223–2230. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Richardson, J.S.M.; Aminudin, N.; Abd Malek, S.N. Chalepin: A compound from Ruta angustifolia L. Pers exhibits cell cycle arrest at S phase, suppresses nuclear factor-kappa B (NF-κB) pathway, signal transducer and activation of transcription 3 (STAT3) phosphorylation and extrinsic apoptotic pathway in non-small cell lung cancer carcinoma (A549). Pharmacogn. Mag. 2017, 13, S489. [Google Scholar] [CrossRef]
- Rangsinth, P.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Ung, A.T.; Chuchawankul, S. Mushroom-derived bioactive compounds potentially serve as the inhibitors of SARS-CoV-2 main protease: An in silico approach. J. Tradit. Complement. Med. 2021, 11, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Q.; Cao, G.; Zhao, H.; Chen, L.; Yang, T.; Wang, M.; Vaziri N., D.; Guo, Y.; Zhao, Y.Y. Combined melatonin and poricoic acid A inhibits renal fibrosis through modulating the interaction of Smad3 and β-catenin pathway in AKI-to-CKD continuum. Ther. Adv. Chronic Dis. 2019, 10, 2040622319869116. [Google Scholar] [CrossRef]
- Khac Hung, N.; Quang, D.N.; Quang, L.D.; Minh, T.T.; Dung, T.N.; Duong, P.Q.; Tung, N.H.; Hoang, V.D. New cycloartane coronalyl acetate and other terpenoids with anti-inflammatory activity from the leaves of Vietnamese Gardenia philastrei. Nat. Prod. Res. 2022, 1–5. [Google Scholar] [CrossRef]
- Rios, J.L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef]
- Yu, M.; Xu, X.; Jiang, N.; Wei, W.; Li, F.; He, L.; Luo, X. Dehydropachymic acid decreases bafilomycin A1 induced β-Amyloid accumulation in PC12 cells. J. Ethnopharmacol. 2017, 198, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Huang, S.S.; Lee, C.Y.; Hou, W.C.; Wang, S.Y.; Sung, P.J.; Kuo, Y.H. Hepatoprotective effects of eburicoic acid and dehydroeburicoic acid from Antrodia camphorata in a mouse model of acute hepatic injury. Food Chem. 2013, 141, 3020–3027. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Huang, S.S.; Lin, T.H.; Lee, M.M.; Kuo, C.C.; Sung, P.J.; Kuo, Y.H. Analgesic and anti-inflammatory bioactivities of eburicoic acid and dehydroeburicoic acid isolated from Antrodia camphorata on the inflammatory mediator expression in mice. J. Agric. Food Chem. 2013, 61, 5064–5071. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Wei, B.; Cheng, S.; Ji, S. Membrane lipid metabolism in relation to core browning during ambient storage of ‘Nanguo’pears. Postharvest Biol. Technol. 2020, 169, 111288. [Google Scholar] [CrossRef]
- Kong, X.M.; Ge, W.Y.; Wei, B.D.; Zhou, Q.; Zhou, X.; Zhao, Y.B.; Ji, S.J. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol. Technol. 2020, 170, 111315. [Google Scholar] [CrossRef]
- Tsukahara, T.; Haniu, H.; Uemura, T.; Matsuda, Y. Porcine liver decomposition product-derived lysophospholipids promote microglial activation in vitro. Sci. Rep. 2020, 10, 3748. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer. 2020, 122, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shu, J.; Xue, H.; Zhang, W.; Zhang, Y.; Liu, Y.; Fang, L.; Wang, Y.; Wang, H. The gut microbiota in Camellia Weevils are influenced by plant secondary metabolites and contribute to saponin degradation. Msystems 2020, 5, e00692-19. [Google Scholar] [CrossRef]
- Zeiss, D.R.; Piater, L.A.; Dubery, I.A. Hydroxycinnamate amides: Intriguing conjugates of plant protective metabolites. Trends Plant Sci. 2021, 26, 184–195. [Google Scholar] [CrossRef]
- Mullins, A.J.; Murray, J.A.; Bull, M.J.; Jenner, M.; Jones, C.; Webster, G.; Green, A.E.; Neill, D.R.; Connor, T.R.; Parkhill, J.; et al. Genome mining identifies cepacin as a plant-protective metabolite of the biopesticidal bacterium Burkholderia ambifaria. Nat. Microbiol. 2019, 4, 996–1005. [Google Scholar] [CrossRef]
- Rogowska, A.; Szakiel, A. The role of sterols in plant response to abiotic stress. Phytochem. Rev. 2020, 19, 1525–1538. [Google Scholar] [CrossRef]
- Yu, B.; Patterson, N.; Zaharia, L.I. Saponin biosynthesis in pulses. Plants 2022, 11, 3505. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent advances in biotransformation of saponins. Molecules 2019, 24, 2365. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Seki, H.; Fujisawa, Y.; Shimoda, Y.; Hiraga, S.; Nomura, Y.; Saito, K.; Ishimoto, M.; Muranaka, T. A cellulose synthase-derived enzyme catalyses 3-O-glucuronosylation in saponin biosynthesis. Nat. Commun. 2020, 11, 5664. [Google Scholar] [CrossRef]
- Tatli Cankaya, I.; Somuncuoglu, E.I. Potential and prophylactic use of plants containing saponin-type compounds as antibiofilm agents against respiratory tract infections. Evid. Based Complement. Altern. Med. 2021, 2021, 6814215. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kaur, R.; Kumar, S.; Saini, R.K.; Sharma, S.; Pawde, S.V.; Kumar, V. Saponins: A concise review on food related aspects, applications and health implications. Food. Chem. Adv. 2023, 2, 100191. [Google Scholar] [CrossRef]
- Jiang, X.; Cao, Y.; von Gersdorff Jørgensen, L.; Strobel, B.W.; Hansen, H.C.B.; Cedergreen, N. Where does the toxicity come from in saponin extract? Chemosphere 2018, 204, 243–250. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, H.; Mu, Y.; Sun, M.; Liu, P. Pharmacological effects of Astragaloside IV: A literature review. J. Tradit. Chin. Med. 2013, 33, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Socaciu, C.; Pintea, A.; Buzoianu, A.D.; Sanders, M.G.; Gruppen, H.; Vincken, J.P. UHPLC/PDA–ESI/MS analysis of the main berry and leaf flavonol glycosides from different Carpathian Hippophaë rhamnoides L. varieties. Phytochem. Anal. 2013, 24, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.P.; Shi, Y.W.; Tang, M.; Zhang, X.C.; Gu, Y.; Liang, X.M.; Wang, Z.W.; Ding, F. Isoquercetin ameliorates cerebral impairment in focal ischemia through anti-oxidative, anti-inflammatory, and anti-apoptotic effects in primary culture of rat hippocampal neurons and hippocampal CA1 region of rats. Mol. Neurobiol. 2017, 54, 2126–2142. [Google Scholar] [CrossRef]
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Maleki Dizaj, S. Molecular mechanisms of anticancer effect of rutin. Phytother. Res. 2021, 35, 2500–2513. [Google Scholar] [CrossRef]
- Ishola, I.O.; Osele, M.O.; Chijioke, M.C.; Adeyemi, O.O. Isorhamnetin enhanced cortico-hippocampal learning and memory capability in mice with scopolamine-induced amnesia: Role of antioxidant defense, cholinergic and BDNF signaling. Brain Res. 2019, 1712, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Islam, M.S.; Rahman, M.K.; Uddin, M.N.; Akanda, M.R. The pharmacological and biological roles of eriodictyol. Arch. Pharm. Res. 2020, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Huang, Z.X.; Chen, G.Q.; Sheng, F.; Zheng, Y.S. Typhaneoside prevents acute myeloid leukemia (AML) through suppressing proliferation and inducing ferroptosis associated with autophagy. Biochem. Biophys. Res. Commun. 2019, 516, 1265–1271. [Google Scholar] [CrossRef]
- Yang, L.; Yu, Z.; Qu, H.; Li, M. Comparative effects of hispidulin, genistein, and icariin with estrogen on bone tissue in ovariectomized rats. Cell Biochem. Biophys. 2014, 70, 485–490. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic acid pathway in biosynthesis of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; Volume 1, pp. 1–15. [Google Scholar] [CrossRef]
- Wohl, J.; Petersen, M. Phenolic metabolism in the hornwort Anthoceros agrestis: 4-coumarate CoA ligase and 4-hydroxybenzoate CoA ligase. Plant Cell Rep. 2020, 39, 1129–1141. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Komla, M.G.; Adam Mariod, A. Nopal cactus (Opuntia ficus-indica (L.) Mill) as a source of bioactive compounds. In Wild Fruits: Composition, Nutritional Value and Products; Springer: Berlin/Heidelberg, Germany,, 2019; pp. 333–358. [Google Scholar] [CrossRef]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural features of small molecule antioxidants and strategic modifications to improve potential bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Extractable and macromolecular antioxidants of Opuntia ficus-indica cladodes: Phytochemical profiling, antioxidant and antibacterial activities. S. Afr. J. Bot. 2019, 125, 402–410. [Google Scholar] [CrossRef]
- Smith, R.D.; Engdahl, A.L.; Dunbar, J.B., Jr.; Carlson, H.A. Biophysical limits of protein–ligand binding. J. Chem. Inf. Model. 2012, 52, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Kairys, V.; Baranauskiene, L.; Kazlauskiene, M.; Matulis, D.; Kazlauskas, E. Binding affinity in drug design: Experimental and computational techniques. Expert Opin, Drug Discov. 2019, 14, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Preskorn, S.H. Drug-Drug Interactions (DDIs) in Psychiatric Practice, Part 7: Relative receptor binding affinity as a way of understanding the differential pharmacology of currently available antipsychotics. J. Psychiatr. Pract. 2019, 25, 461–465. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.H.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, D.-G.; Yang, H.-S.; Bae, U.-J.; Park, E.; Choi, A.-J.; Choe, J.-S. The Cactus (Opuntia ficus-indica) Cladodes and Callus Extracts: A Study Combined with LC-MS Metabolic Profiling, In-Silico, and In-Vitro Analyses. Antioxidants 2023, 12, 1329. https://doi.org/10.3390/antiox12071329
Nam D-G, Yang H-S, Bae U-J, Park E, Choi A-J, Choe J-S. The Cactus (Opuntia ficus-indica) Cladodes and Callus Extracts: A Study Combined with LC-MS Metabolic Profiling, In-Silico, and In-Vitro Analyses. Antioxidants. 2023; 12(7):1329. https://doi.org/10.3390/antiox12071329
Chicago/Turabian StyleNam, Dong-Geon, Hee-Sun Yang, Ui-Jin Bae, Eunmi Park, Ae-Jin Choi, and Jeong-Sook Choe. 2023. "The Cactus (Opuntia ficus-indica) Cladodes and Callus Extracts: A Study Combined with LC-MS Metabolic Profiling, In-Silico, and In-Vitro Analyses" Antioxidants 12, no. 7: 1329. https://doi.org/10.3390/antiox12071329
APA StyleNam, D. -G., Yang, H. -S., Bae, U. -J., Park, E., Choi, A. -J., & Choe, J. -S. (2023). The Cactus (Opuntia ficus-indica) Cladodes and Callus Extracts: A Study Combined with LC-MS Metabolic Profiling, In-Silico, and In-Vitro Analyses. Antioxidants, 12(7), 1329. https://doi.org/10.3390/antiox12071329