Maternal Vitamin D and Inulin Supplementation in Oxidized Oil Diet Improves Growth Performance and Hepatic Innate Immunity in Offspring Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design, Diets, and Management
2.2. Preparation of Oxidized Soybean Oil
2.3. Sample Collection
2.4. Analysis of Antioxidant and Oxidant Index
2.5. Assessment of Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Effects of VD and Inulin Supplementation in an Oxidized Oil Diet during Gestation on Maternal Reproductive Performance
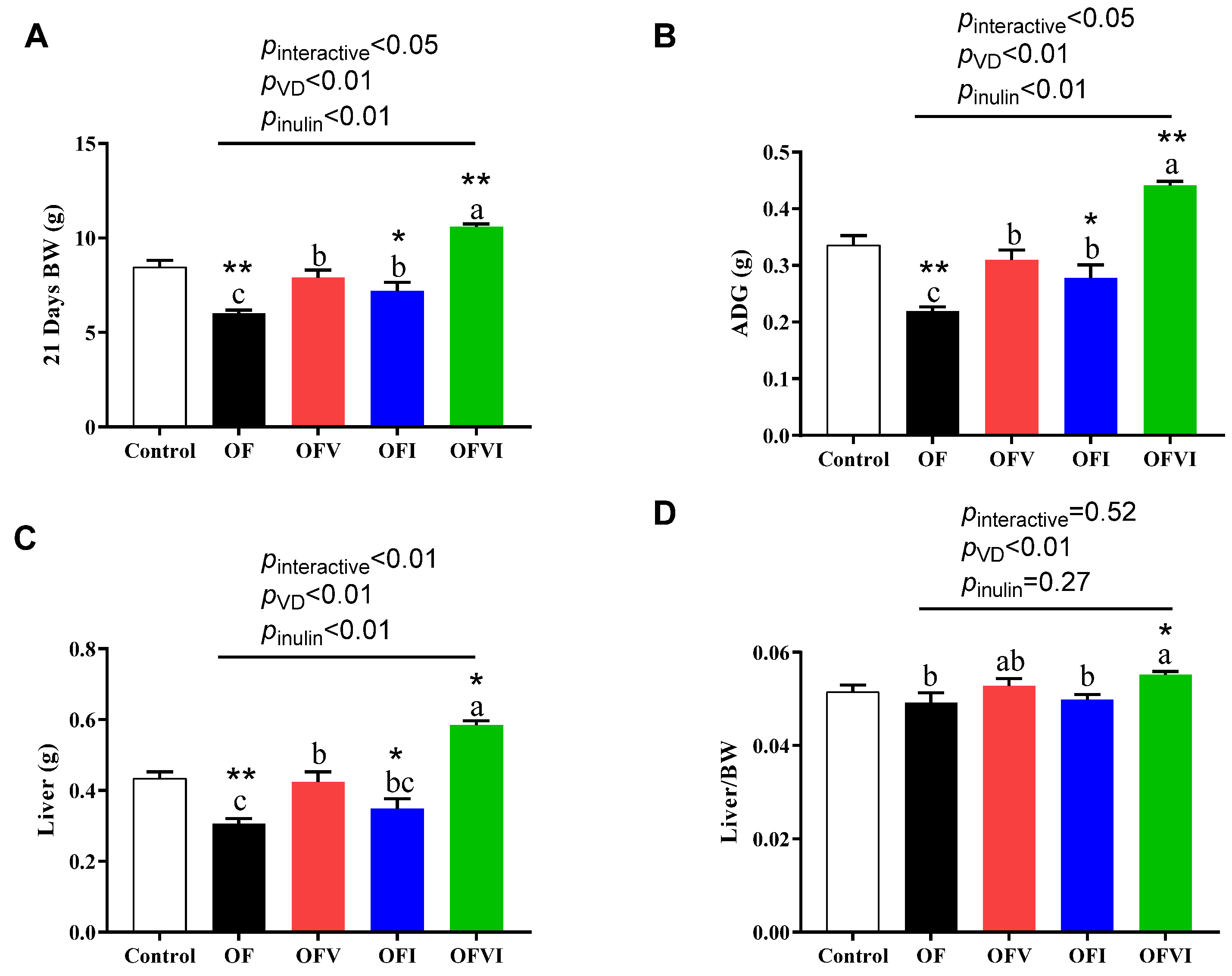
3.2. Effects of VD and Inulin Supplementation in a Maternal Oxidized Oil Diet on the Growth Performance of Offspring during the Suckling Period
3.3. Effects of VD and Inulin Supplementation in a Maternal Oxidized Oil Diet on the Oxidative/Antioxidative Condition of Offspring Mice
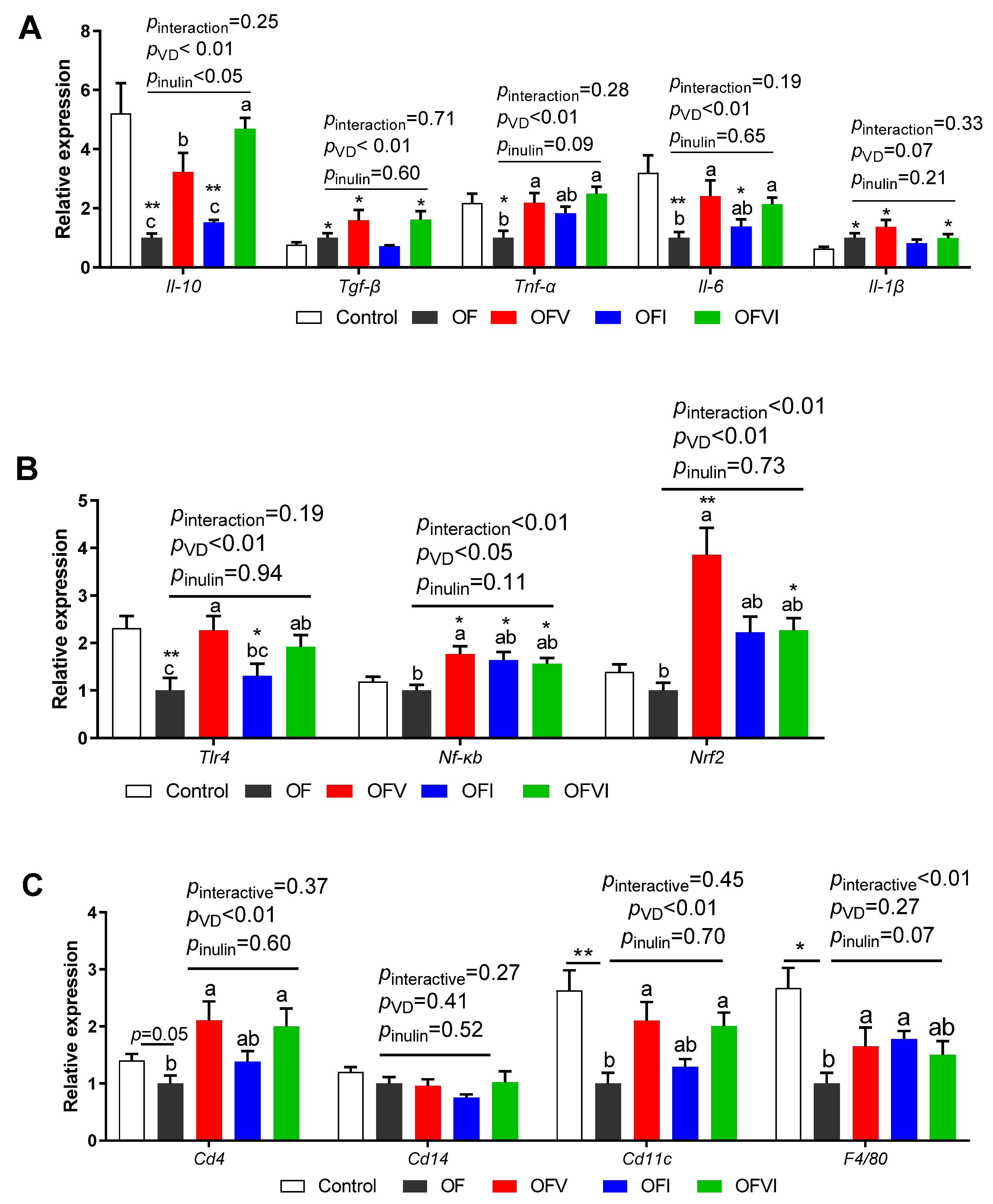
3.4. Effects of VD and Inulin Supplementation in a Maternal Oxidized Oil Diet on the Expression of Inflammatory Genes in the Liver of Offspring
3.5. Effects of VD and Inulin Supplementation in a Maternal Oxidized Oil Diet on the Expression of VD Metabolism Related Genes in Offspring Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ammouche, A.; Rouaki, F.; Bitam, A.; Bellal, M.M. Effect of ingestion of thermally oxidized sunflower oil on the fatty acid composition and antioxidant enzymes of rat liver and brain in development. Ann. Nutr. Metab. 2002, 46, 268–275. [Google Scholar] [CrossRef]
- Staprãns, I.; Rapp, J.H.; Pan, X.M.; Kim, K.Y.; Feingold, K.R. Oxidized lipids in the diet are a source of oxidized lipid in chylomicrons of human serum. Arterioscler. Thromb. A J. Vasc. Biol. 1994, 14, 1900–1905. [Google Scholar] [CrossRef] [Green Version]
- Fukase, M.; Watanabe, N.; Yamanouchi, K.; Tsutsumi, S.; Nagase, S. The Change of Oxidative Stress in Maternal Blood During Pregnancy. Reprod. Sci. 2022, 29, 2580–2585. [Google Scholar] [CrossRef]
- Watanabe, K.; Mori, T.; Iwasaki, A.; Kimura, C.; Matsushita, H.; Shinohara, K.; Wakatsuki, A. Increased oxygen free radical production during pregnancy may impair vascular reactivity in preeclamptic women. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2013, 36, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Odame Anto, E.; Owiredu, W.; Sakyi, S.A.; Turpin, C.A.; Ephraim, R.K.D.; Fondjo, L.A.; Obirikorang, C.; Adua, E.; Acheampong, E. Adverse pregnancy outcomes and imbalance in angiogenic growth mediators and oxidative stress biomarkers is associated with advanced maternal age births: A prospective cohort study in Ghana. PLoS ONE 2018, 13, e0200581. [Google Scholar] [CrossRef]
- Albert, B.B.; Vickers, M.H.; Gray, C.; Reynolds, C.M.; Segovia, S.A.; Derraik, J.G.; Lewandowski, P.A.; Garg, M.L.; Cameron-Smith, D.; Hofman, P.L.; et al. Oxidized fish oil in rat pregnancy causes high newborn mortality and increases maternal insulin resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R497–R504. [Google Scholar] [CrossRef] [Green Version]
- Brandsch, C.; Eder, K. Effects of peroxidation products in thermoxidised dietary oil in female rats during rearing, pregnancy and lactation on their reproductive performance and the antioxidative status of their offspring. Br. J. Nutr. 2004, 92, 267–275. [Google Scholar] [CrossRef]
- Ndonwi, E.N.; Atogho-Tiedeu, B.; Lontchi-Yimagou, E.; Shinkafi, T.S.; Nanfa, D.; Balti, E.V.; Indusmita, R.; Mahmood, A.; Katte, J.C.; Mbanya, A.; et al. Gestational Exposure to Pesticides Induces Oxidative Stress and Lipid Peroxidation in Offspring that Persist at Adult Age in an Animal Model. Toxicol. Res. 2019, 35, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liu, Y.; Wang, H.; Gao, F.; Guan, X.; Shi, B. Maternal Exposure to Oxidized Soybean Oil Impairs Placental Development by Modulating Nutrient Transporters in a Rat Model. Mol. Nutr. Food Res. 2021, 65, e2100301. [Google Scholar] [CrossRef]
- Huang, C.F.; Lin, Y.S.; Chiang, Z.C.; Lu, S.Y.; Kuo, Y.H.; Chang, S.L.; Chao, P.M. Oxidized frying oil and its polar fraction fed to pregnant mice are teratogenic and alter mRNA expressions of vitamin A metabolism genes in the liver of dams and their fetuses. J. Nutr. Biochem. 2014, 25, 549–556. [Google Scholar] [CrossRef]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlberg, C.; Velleuer, E. Vitamin D and the risk for cancer: A molecular analysis. Biochem. Pharmacol. 2022, 196, 114735. [Google Scholar] [CrossRef]
- Bi, W.G.; Nuyt, A.M.; Weiler, H.; Leduc, L.; Santamaria, C.; Wei, S.Q. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018, 172, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fang, F.; Tang, J.; Jia, L.; Feng, Y.; Xu, P.; Faramand, A. Association between vitamin D supplementation and mortality: Systematic review and meta-analysis. BMJ (Clin. Res. Ed.) 2019, 366, l4673. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Xu, Y.; Wang, K.; Yang, Z.; Li, H.; Lei, S.; Wang, S. Maternal Vit D supplementation in AMA mice and the role of Vit D/VDR signaling in the offspring’s cognition. Am. J. Transl. Res. 2021, 13, 12650–12661. [Google Scholar]
- El-Boshy, M.; BaSalamah, M.A.; Ahmad, J.; Idris, S.; Mahbub, A.; Abdelghany, A.H.; Almaimani, R.A.; Almasmoum, H.; Ghaith, M.M.; Elzubier, M.; et al. Vitamin D protects against oxidative stress, inflammation and hepatorenal damage induced by acute paracetamol toxicity in rat. Free Radic. Biol. Med. 2019, 141, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, W.; Mao, Z.; Mou, D.; Huang, L.; Yang, M.; Ding, D.; Yan, H.; Fang, Z.; Che, L.; et al. Maternal VD(3) supplementation during gestation improves intestinal health and microbial composition of weaning piglets. Food Funct. 2022, 13, 6830–6842. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.L.; Abrams, S.A.; Osborn, D.A. Vitamin D supplementation for term breastfed infants to prevent vitamin D deficiency and improve bone health. Cochrane Database Syst. Rev. 2020, 12, CD013046. [Google Scholar] [CrossRef]
- Yepes-Nuñez, J.J.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Cuello-García, C.; Zhang, Y.; Morgano, G.P.; Agarwal, A.; Gandhi, S.; Terracciano, L.; et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy 2018, 73, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Hornsby, E.; Pfeffer, P.E.; Laranjo, N.; Cruikshank, W.; Tuzova, M.; Litonjua, A.A.; Weiss, S.T.; Carey, V.J.; O’Connor, G.; Hawrylowicz, C. Vitamin D supplementation during pregnancy: Effect on the neonatal immune system in a randomized controlled trial. J. Allergy Clin. Immunol. 2018, 141, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Huang, Y.; Wang, G.; He, Y.; Hu, L.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Li, J.; et al. Maternal Long-Term Intake of Inulin Improves Fetal Development through Gut Microbiota and Related Metabolites in a Rat Model. J. Agric. Food Chem. 2022, 70, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Bouchaud, G.; Castan, L.; Chesné, J.; Braza, F.; Aubert, P.; Neunlist, M.; Magnan, A.; Bodinier, M. Maternal exposure to GOS/inulin mixture prevents food allergies and promotes tolerance in offspring in mice. Allergy 2016, 71, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Vasconcelos, Q.; Abreu, G.; Albuquerque, A.; Vilarejo, J.; Aragão, G. Changes in nutrient absorption in children and adolescents caused by fructans, especially fructooligosaccharides and inulin. Arch. De Pediatr. Organe Off. De La Soc. Fr. De Pediatr. 2020, 27, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wang, Y.S.; Li, S.; Zhao, Y.; Deng, K.; Chao, D.D.; Jin, C.; Zhuo, Y.; Che, L.Q.; Li, J.; et al. Effects of prebiotic inulin addition to low- or high-fat diet on maternal metabolic status and neonatal traits of offspring in a pregnant sow model. J. Funct. Foods 2018, 48, 125–133. [Google Scholar] [CrossRef]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L. Oxidative stress status of highly prolific sows during gestation and lactation. Anim. Int. J. Anim. Biosci. 2011, 5, 1774–1779. [Google Scholar] [CrossRef] [Green Version]
- Chatzakis, C.; Sotiriadis, A.; Tsakmaki, E.; Papagianni, M.; Paltoglou, G.; Dinas, K.; Mastorakos, G. The Effect of Dietary Supplements on Oxidative Stress in Pregnant Women with Gestational Diabetes Mellitus: A Network Meta-Analysis. Nutrients 2021, 13, 2284. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, S.W. Impacts of weaning weights and mycotoxin challenges on jejunal mucosa-associated microbiota, intestinal and systemic health, and growth performance of nursery pigs. J. Anim. Sci. Biotechnol. 2022, 13, 43. [Google Scholar] [CrossRef]
- Dawodu, A.; Tsang, R.C. Maternal vitamin D status: Effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv. Nutr. 2012, 3, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Mannion, C.A.; Gray-Donald, K.; Koski, K.G. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ Can. Med. Assoc. J. J. De L’association Med. Can. 2006, 174, 1273–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Li, Y.; Li, Y.; Zhang, J.; Zhou, C.; Wu, C.; Zhu, Q.; Shen, T. Maternal vitamin D supplementation inhibits bisphenol A-induced proliferation of Th17 cells in adult offspring. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 144, 111604. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, C.; Nass, N.; Eder, K. A thermally oxidized dietary oil does not lower the activities of lipogenic enzymes in mammary glands of lactating rats but reduces the milk triglyceride concentration. J. Nutr. 2004, 134, 631–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Guo, Z.; Gao, Y.; Wang, C.; Wang, H.; Yao, X.; Shi, B. Maternal oxidized soybean oil exposure in rats during lactation damages offspring kidneys via Nrf2/HO-1 and NF-κB signaling pathway. J. Sci. Food Agric. 2022, 102, 3119–3129. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, S.; Zhang, X.; Liu, X.; Wu, Z.; Qi, Y.; Guan, W.; Ren, M.; Zhang, S. Maternal Nutrition During Late Gestation and Lactation: Association With Immunity and the Inflammatory Response in the Offspring. Front. Immunol. 2021, 12, 758525. [Google Scholar] [CrossRef]
- Huang, L.T. Maternal and Early-Life Nutrition and Health. Int. J. Environ. Res. Public Health 2020, 17, 7982. [Google Scholar] [CrossRef]
- Beetch, M.; Harandi-Zadeh, S.; Shen, K.; Lubecka, K.; Kitts, D.D.; O’Hagan, H.M.; Stefanska, B. Dietary antioxidants remodel DNA methylation patterns in chronic disease. Br. J. Pharmacol. 2020, 177, 1382–1408. [Google Scholar] [CrossRef] [Green Version]
- De Brandt, J.; Derave, W.; Vandenabeele, F.; Pomiès, P.; Blancquaert, L.; Keytsman, C.; Barusso-Grüninger, M.S.; de Lima, F.F.; Hayot, M.; Spruit, M.A.; et al. Efficacy of 12 weeks oral beta-alanine supplementation in patients with chronic obstructive pulmonary disease: A double-blind, randomized, placebo-controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 2361–2372. [Google Scholar] [CrossRef]
- Yilmaz, M.I.; Romano, M.; Basarali, M.K.; Elzagallaai, A.; Karaman, M.; Demir, Z.; Demir, M.F.; Akcay, F.; Seyrek, M.; Haksever, N.; et al. The Effect of Corrected Inflammation, Oxidative Stress and Endothelial Dysfunction on Fmd Levels in Patients with Selected Chronic Diseases: A Quasi-Experimental Study. Sci. Rep. 2020, 10, 9018. [Google Scholar] [CrossRef]
- Sahni, N.; Gupta, K.L.; Rana, S.V.; Prasad, R.; Bhalla, A.K. Intake of antioxidants and their status in chronic kidney disease patients. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2012, 22, 389–399. [Google Scholar] [CrossRef]
- Miralles-Pérez, B.; Nogués, M.R.; Sánchez-Martos, V.; Fortuño-Mar, À.; Ramos-Romero, S.; Torres, J.L.; Ponomarenko, J.; Amézqueta, S.; Zhang, X.; Romeu, M. Influence of Dietary Inulin on Fecal Microbiota, Cardiometabolic Risk Factors, Eicosanoids, and Oxidative Stress in Rats Fed a High-Fat Diet. Foods 2022, 11, 4072. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G.; Muralidhara. Oral supplements of inulin during gestation offsets rotenone-induced oxidative impairments and neurotoxicity in maternal and prenatal rat brain. Biomed. Pharm. 2018, 104, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Micinski, D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 2013, 437, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.; Huang, Y.; Wang, C. 1,25(OH)2D3 Inhibited Ferroptosis in Zebrafish Liver Cells (ZFL) by Regulating Keap1-Nrf2-GPx4 and NF-κB-hepcidin Axis. Int. J. Mol. Sci. 2021, 22, 1334. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.R.; Quimque, M.T.J.; Macaranas, I.T.; Khan, A.; Pastrana, A.M.; Villaflores, O.B.; Arturo, H.C.P.; Pilapil Iv, D.Y.H.; Tan, S.M.M.; Wei, D.Q.; et al. Attenuation of Lipopolysaccharide-Induced Inflammatory Responses through Inhibition of the NF-κB Pathway and the Increased NRF2 Level by a Flavonol-Enriched n-Butanol Fraction from Uvaria alba. ACS Omega 2023, 8, 5377–5392. [Google Scholar] [CrossRef]
- Yi, J.; Zhu, J.; Wu, J.; Thompson, C.B.; Jiang, X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 31189–31197. [Google Scholar] [CrossRef]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxidative Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Chen, Q.M. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic. Biol. Med. 2022, 179, 133–143. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, H.; Jiang, X.; Guan, X.; Gao, F.; Shi, B. Maternal Oxidized Soybean Oil Administration in Rats during Pregnancy and Lactation Alters the Intestinal DNA Methylation in Offspring. J. Agric. Food Chem. 2022, 70, 6224–6238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xu, M.J.; Gao, B. Hepatocytes: A key cell type for innate immunity. Cell. Mol. Immunol. 2016, 13, 301–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liang, X.; Bao, X.; Xiao, W.; Chen, G. Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, C.; Wang, X.; Kim, K.; Bartolome, A.; Dongiovanni, P.; Yates, K.P.; Valenti, L.; Carrer, M.; Sadowski, T.; et al. Hepatocyte TLR4 triggers inter-hepatocyte Jagged1/Notch signaling to determine NASH-induced fibrosis. Sci. Transl. Med. 2021, 13, eabe1692. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Tsung, A.; Zheng, N.; Jeyabalan, G.; Izuishi, K.; Klune, J.R.; Geller, D.A.; Lotze, M.T.; Lu, L.; Billiar, T.R. Increasing numbers of hepatic dendritic cells promote HMGB1-mediated ischemia-reperfusion injury. J. Leukoc. Biol. 2007, 81, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.H.; Sanchez-Fueyo, A.; Samuel, D. From immunosuppression to tolerance. J. Hepatol. 2015, 62, S170–S185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Cha, H.J.; Lee, H.; Kim, G.Y.; Choi, Y.H. The regulation of the TLR4/NF-kappaB and Nrf2/HO-1 signaling pathways is involved in the inhibition of lipopolysaccharide-induced inflammation and oxidative reactions by morroniside in RAW 264.7 macrophages. Arch. Biochem. Biophys. 2021, 706, 108926. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef]
- Le Rouzic, V.; Corona, J.; Zhou, H. Postnatal development of hepatic innate immune response. Inflammation 2011, 34, 576–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallwirth, U.; Pomberger, G.; Zaknun, D.; Szepfalusi, Z.; Horcher, E.; Pollak, A.; Roth, E.; Spittler, A. Monocyte phagocytosis as a reliable parameter for predicting early-onset sepsis in very low birthweight infants. Early Hum. Dev. 2002, 67, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Förster-Waldl, E.; Sadeghi, K.; Tamandl, D.; Gerhold, B.; Hallwirth, U.; Rohrmeister, K.; Hayde, M.; Prusa, A.R.; Herkner, K.; Boltz-Nitulescu, G.; et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr. Res. 2005, 58, 121–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasoe, J.; Uchida, Y.; Kawamoto, H.; Miyauchi, T.; Watanabe, T.; Saga, K.; Tanaka, K.; Ueda, S.; Terajima, H.; Taura, K.; et al. Propionic Acid, Induced in Gut by an Inulin Diet, Suppresses Inflammation and Ameliorates Liver Ischemia and Reperfusion Injury in Mice. Front. Immunol. 2022, 13, 862503. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Song, J.; Chen, L.; Du, M.; Mao, X. Yogurt Enriched with Inulin Ameliorated Reproductive Functions and Regulated Gut Microbiota in Dehydroepiandrosterone-Induced Polycystic Ovary Syndrome Mice. Nutrients 2022, 14, 279. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef]
- Yang, X.; He, F.; Zhang, Y.; Xue, J.; Li, K.; Zhang, X.; Zhu, L.; Wang, Z.; Wang, H.; Yang, S. Inulin Ameliorates Alcoholic Liver Disease via Suppressing LPS-TLR4-Mψ Axis and Modulating Gut Microbiota in Mice. Alcohol. Clin. Exp. Res. 2019, 43, 411–424. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Rugyendo, A.; Morrison, D.J.; Preston, T.; Tedford, C.; Bell, J.D.; Thomas, L.; Akbar, A.N.; Riddell, N.E.; et al. The effects of dietary supplementation with inulin and inulin-propionate ester on hepatic steatosis in adults with non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 372–376. [Google Scholar] [CrossRef] [Green Version]
- Farhangi, M.A.; Javid, A.Z.; Dehghan, P. The effect of enriched chicory inulin on liver enzymes, calcium homeostasis and hematological parameters in patients with type 2 diabetes mellitus: A randomized placebo-controlled trial. Prim. Care Diabetes 2016, 10, 265–271. [Google Scholar] [CrossRef]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2020, 22, 362. [Google Scholar] [CrossRef]
- Dong, B.; Zhou, Y.; Wang, W.; Scott, J.; Kim, K.; Sun, Z.; Guo, Q.; Lu, Y.; Gonzales, N.M.; Wu, H.; et al. Vitamin D Receptor Activation in Liver Macrophages Ameliorates Hepatic Inflammation, Steatosis, and Insulin Resistance in Mice. Hepatology 2020, 71, 1559–1574. [Google Scholar] [CrossRef] [PubMed]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef] [PubMed]
- Fathi, N.; Ahmadian, E.; Shahi, S.; Roshangar, L.; Khan, H.; Kouhsoltani, M.; Maleki Dizaj, S.; Sharifi, S. Role of vitamin D and vitamin D receptor (VDR) in oral cancer. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 391–401. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, G.; Zhang, Q.; Fang, Z.; Che, L.; Lin, Y.; Xu, S.; Zhuo, Y.; Hua, L.; Jiang, X.; Li, J.; et al. Maternal Vitamin D and Inulin Supplementation in Oxidized Oil Diet Improves Growth Performance and Hepatic Innate Immunity in Offspring Mice. Antioxidants 2023, 12, 1355. https://doi.org/10.3390/antiox12071355
Xie G, Zhang Q, Fang Z, Che L, Lin Y, Xu S, Zhuo Y, Hua L, Jiang X, Li J, et al. Maternal Vitamin D and Inulin Supplementation in Oxidized Oil Diet Improves Growth Performance and Hepatic Innate Immunity in Offspring Mice. Antioxidants. 2023; 12(7):1355. https://doi.org/10.3390/antiox12071355
Chicago/Turabian StyleXie, Guangrong, Qipeng Zhang, Zhengfeng Fang, Lianqiang Che, Yan Lin, Shengyu Xu, Yong Zhuo, Lun Hua, Xuemei Jiang, Jian Li, and et al. 2023. "Maternal Vitamin D and Inulin Supplementation in Oxidized Oil Diet Improves Growth Performance and Hepatic Innate Immunity in Offspring Mice" Antioxidants 12, no. 7: 1355. https://doi.org/10.3390/antiox12071355




