Phytochemical and Bioactivity Studies on Hedera helix L. (Ivy) Flower Pollen and Ivy Bee Pollen
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Preparation of Standard Solutions
2.4. Preparation of Sample Test Solutions
2.5. Isolation and Structure Elucidation of the Marker Compounds from the Bee Pollen Sample
2.6. MS/MS Analyses

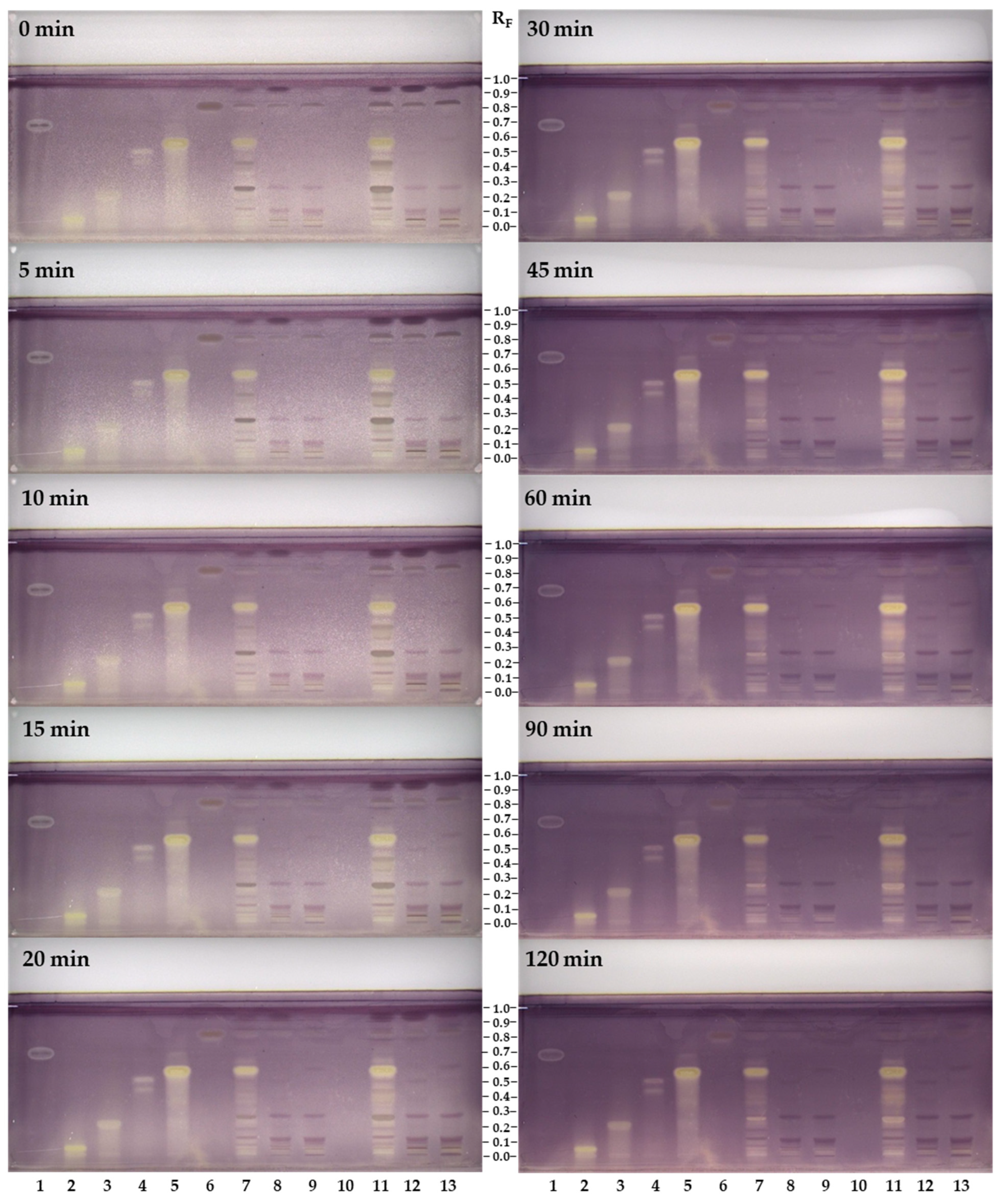
2.7. HPTLC Analyses
2.8. HPLC Analyses
2.9. In Vitro Bioactivity Analyses
2.9.1. DPPH• Assay
2.9.2. FRAP Assay
2.9.3. CUPRAC Assay
2.9.4. ABTS Assay
2.9.5. Xanthine Oxidase Inhibitory Activity
2.9.6. Superoxide Radical Scavenging (SOD) Activity
2.10. HPTLC-Effect-Directed Analyses (EDA)
2.10.1. HPTLC-DPPH•
2.10.2. HPTLC-Xanthine Oxidase Inhibitory Activity
2.11. Statistical Analyses
3. Results and Discussion
3.1. Isolation and Structural Elucidation of Compounds Isolated from BP-SI
3.2. HPTLC Chemical Profiling
3.3. HPLC Analyses
3.3.1. HPLC Method Validation
Specificity
Linearity of the Calibration Curve, Limit of Detection (LOD), and Limit of Quantification (LOQ)
Precision
Accuracy
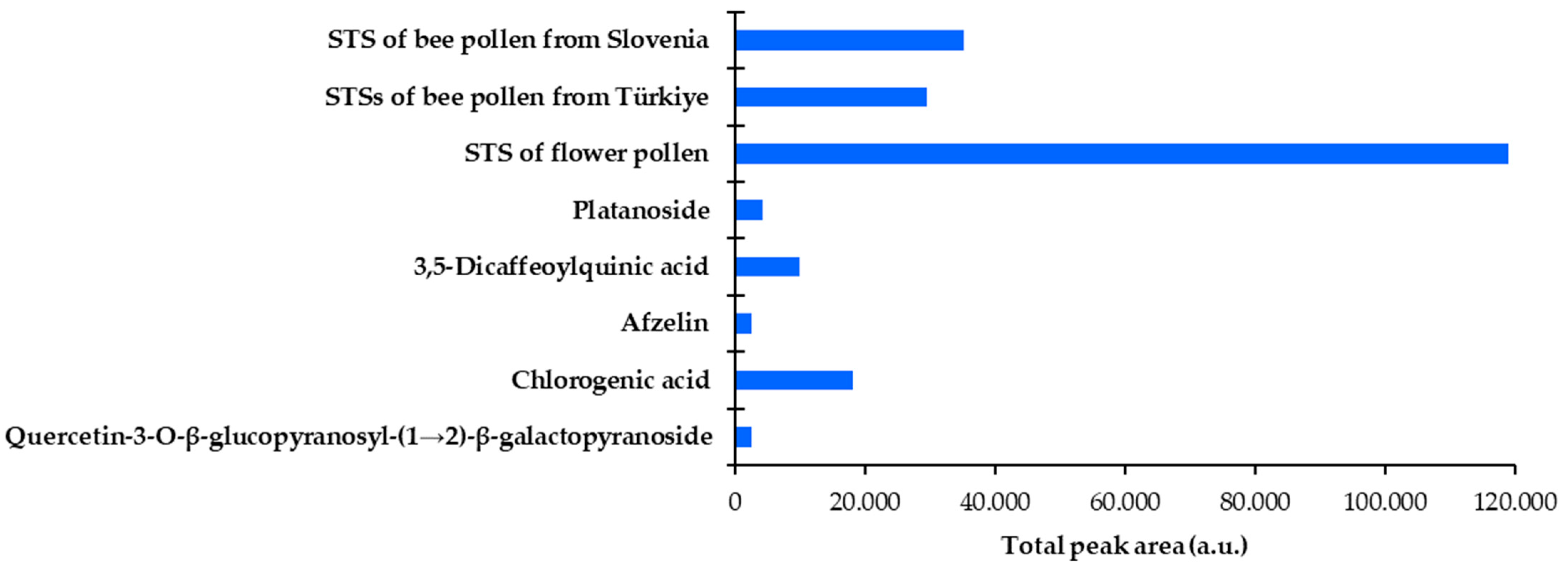
3.3.2. Quantitative Analyses
3.4. In Vitro Bioactivity Analyses
3.4.1. Antioxidant Activity Determined by DPPH, FRAP, ABTS, and CUPRAC Assays
3.4.2. Xanthine Oxidase Inhibitory Activity and Superoxide Radicals Scavenging Activity
3.5. HPTLC-Effect-Directed Analyses
3.5.1. HPTLC-DPPH Analyses of Antioxidant Activity
3.5.2. HPTLC-XO Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bezruk, I.; Marksa, M.; Georgiyants, V.; Ivanauskas, L.; Raudone, L. Phytogeographical Profiling of Ivy Leaf (Hedera Helix L.). Ind. Crops Prod. 2020, 154, 112713. [Google Scholar] [CrossRef]
- Sierocinski, E.; Holzinger, F.; Chenot, J.-F. Ivy Leaf (Hedera Helix) for Acute Upper Respiratory Tract Infections: An Updated Systematic Review. Eur. J. Clin. Pharmacol. 2021, 77, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Parvu, M.; Vlase, L.; Parvu, A.E.; Rosca-Casian, O.; Gheldiu, A.-M.; Parvu, O. Phenolic Compounds and Antifungal Activity of Hedera Helix L. (Ivy) Flowers and Fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 53–58. [Google Scholar] [CrossRef]
- Pop, C.E.; Pârvu, M.; Leti, A.; Arsene, Ț.I.A.; Vlase, L. Investigation of Antioxidant and Antimicrobial Potential of Some Extracts from Hedera Helix L. Farmacia 2017, 65, 624–629. [Google Scholar]
- Temİzer, İ.K. Pollen Morphology of Hedera Helix L. Mellifera 2019, 19, 1–6. [Google Scholar]
- Villanueva, M.T.O.; Marquina, A.D.; Serrano, R.B.; Abellán, G.B. The Importance of Bee-Collected Pollen in the Diet: A Study of Its Composition. Int. J. Food Sci. Nutr. 2002, 53, 217–224. [Google Scholar] [CrossRef]
- El Ghouizi, A.; Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Menyiy, N.; Hano, C.; Lyoussi, B. Bee Pollen as Functional Food: Insights into Its Composition and Therapeutic Properties. Antioxidants 2023, 12, 557. [Google Scholar] [CrossRef]
- Molan, P.C. The Limitations of the Methods of Identifying the Floral Source of Honeys. Bee World 1998, 79, 59–68. [Google Scholar] [CrossRef]
- Bensa, M.; Glavnik, V.; Vovk, I. Leaves of Invasive Plants—Japanese, Bohemian and Giant Knotweed—The Promising New Source of Flavan-3-ols and Proanthocyanidins. Plants 2020, 9, 118. [Google Scholar] [CrossRef]
- Bensa, M.; Glavnik, V.; Vovk, I. Flavan-3-ols and Proanthocyanidins in Japanese, Bohemian and Giant Knotweed. Plants 2021, 10, 402. [Google Scholar] [CrossRef]
- Zekič, J.; Vovk, I.; Glavnik, V. Extraction and Analyses of Flavonoids and Phenolic Acids from Canadian Goldenrod and Giant Goldenrod. Forests 2020, 12, 40. [Google Scholar] [CrossRef]
- Baglyas, M.; Ott, P.G.; Garádi, Z.; Glavnik, V.; Béni, S.; Vovk, I.; Móricz, Á.M. High-Performance Thin-Layer Chromatography—Antibacterial Assay First Reveals Bioactive Clerodane Diterpenes in Giant Goldenrod (Solidago Gigantea Ait.). J. Chromatogr. A 2022, 1677, 463308. [Google Scholar] [CrossRef] [PubMed]
- Inarejos-Garcia, A.M.; Heil, J.; Martorell, P.; Álvarez, B.; Llopis, S.; Helbig, I.; Liu, J.; Quebbeman, B.; Nemeth, T.; Holmgren, D.; et al. Effect-Directed, Chemical and Taxonomic Profiling of Peppermint Proprietary Varieties and Corresponding Leaf Extracts. Antioxidants 2023, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Vovk, I.; Glavnik, V.; Jug, U.; Corradini, D. HPTLC, HPTLC-MS/MS and HPTLC-DPPH Methods for Analyses of Flavonoids and Their Antioxidant Activity in Cyclanthera Pedata Leaves, Fruits and Dietary Supplement. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 290–301. [Google Scholar] [CrossRef]
- Jug, U.; Glavnik, V.; Kranjc, E.; Vovk, I. High-Performance Thin-Layer Chromatography and High-Performance Thin-Layer Chromatography–Mass Spectrometry Methods for the Analysis of Phenolic Acids. J. Planar Chromatogr. Mod. TLC 2018, 31, 13–22. [Google Scholar] [CrossRef]
- Jug, U.; Glavnik, V.; Kranjc, E.; Vovk, I. HPTLC–Densitometric and HPTLC–MS Methods for Analysis of Flavonoids. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 329–341. [Google Scholar] [CrossRef]
- Guzelmeric, E.; Özdemir, D.; Sen, N.B.; Celik, C.; Yesilada, E. Quantitative Determination of Phenolic Compounds in Propolis Samples from the Black Sea Region (Türkiye) Based on HPTLC Images Using Partial Least Squares and Genetic Inverse Least Squares Methods. J. Pharm. Biomed. Anal. 2023, 229, 115338. [Google Scholar] [CrossRef]
- Mosić, M.; Trifković, J.; Vovk, I.; Gašić, U.; Tešić, Ž.; Šikoparija, B.; Milojković-Opsenica, D. Phenolic Composition Influences the Health-Promoting Potential of Bee-Pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef]
- Morlock, G.E.; Heil, J.; Inarejos-Garcia, A.M.; Maeder, J. Effect-Directed Profiling of Powdered Tea Extracts for Catechins, Theaflavins, Flavonols and Caffeine. Antioxidants 2021, 10, 117. [Google Scholar] [CrossRef]
- Vovk, I.; Glavnik, V. Applications of Thin-Layer Chromatography to the Quality Control of Dietary Supplements. In Instrumental Thin-Layer Chromatography; Poole, C.F., Ed.; Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 615–663. ISBN 978-0-323-99970-0. [Google Scholar]
- Böhmdorfer, S. Effect-Directed Detection: Chemical and Enzyme-Based Assays. In Instrumental Thin-Layer Chromatography; Poole, C.F., Ed.; Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 297–324. ISBN 978-0-323-99970-0. [Google Scholar]
- Móricz, Á.M.; Ott, P.G. Effects-Directed Detection: Cell-Based Assays. In Instrumental Thin-Layer Chromatography; Poole, C.F., Ed.; Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 259–296. ISBN 978-0-323-99970-0. [Google Scholar]
- Rahaman, M.S.; Siraj, M.A.; Islam, M.A.; Shanto, P.C.; Islam, O.; Islam, M.A.; Simal-Gandara, J. Crosstalk between Xanthine Oxidase (XO) Inhibiting and Cancer Chemotherapeutic Properties of Comestible Flavonoids—A Comprehensive Update. J. Nutr. Biochem. 2022, 110, 109147. [Google Scholar] [CrossRef]
- Fagugli, R.M.; Gentile, G.; Ferrara, G.; Brugnano, R. Acute Renal and Hepatic Failure Associated with Allopurinol Treatment. Clin. Nephrol. 2008, 70, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Guzelmeric, E.; Ristivojević, P.; Trifković, J.; Dastan, T.; Yilmaz, O.; Cengiz, O.; Yesilada, E. Authentication of Turkish Propolis through HPTLC Fingerprints Combined with Multivariate Analysis and Palynological Data and Their Comparative Antioxidant Activity. LWT 2018, 87, 23–32. [Google Scholar] [CrossRef]
- Barth, O.M. Pollen Analysis of Brazilian Propolis. Grana 1998, 37, 97–101. [Google Scholar] [CrossRef]
- Guzelmeric, E.; Ugurlu, P.; Celik, C.; Sen, N.B.; Helvacıoglu, S.; Charehsaz, M.; Erdogan, M.; Ockun, M.A.; Kırmızıbekmez, H.; Aydın, A.; et al. Myrtus Communis L. (Myrtle) Plant Parts: Comparative Assessment of Their Chemical Compositions and Antioxidant, Anticancer, and Antimutagenic Activities. South African J. Bot. 2022, 150, 711–720. [Google Scholar] [CrossRef]
- Reich, E.; Schibli, A. High-Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants; Thieme: New York, NY, USA, 2007. [Google Scholar]
- International Conference on Harmonisation ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2(R1). 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 1 January 2021).
- Tugba Degirmencioglu, H.; Guzelmeric, E.; Yuksel, P.I.; Kırmızıbekmez, H.; Deniz, I.; Yesilada, E. A New Type of Anatolian Propolis: Evaluation of Its Chemical Composition, Activity Profile and Botanical Origin. Chem. Biodivers. 2019, 16, e1900492. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Gülü, K.; Demirata, B.; Apak, R. A Novel Antioxidant Assay of Ferric Reducing Capacity Measurement Using Ferrozine as the Colour Forming Complexation Reagent. Anal. Methods 2010, 2, 1770–1778. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of Antioxidant Capacity Assays and the CUPRAC (Cupric Ion Reducing Antioxidant Capacity) Assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Ockun, M.A.; Baranauskaite, J.; Uner, B.; Kan, Y.; Kırmızıbekmez, H. Preparation, Characterization and Evaluation of Liposomal-Freeze Dried Anthocyanin-Enriched Vaccinium Arctostaphylos L. Fruit Extract Incorporated into Fast Dissolving Oral Films. J. Drug Deliv. Sci. Technol. 2022, 72, 103428. [Google Scholar] [CrossRef]
- Azmi, S.M.N.; Jamal, P.; Amid, A. Xanthine Oxidase Inhibitory Activity from Potential Malaysian Medicinal Plant as Remedies for Gout. Int. Food Res. J. 2012, 19, 159–165. [Google Scholar]
- Gainche, M.; Ogeron, C.; Ripoche, I.; Senejoux, F.; Cholet, J.; Decombat, C.; Delort, L.; Berthon, J.; Saunier, E.; Caldefie Chezet, F.; et al. Xanthine Oxidase Inhibitors from Filipendula Ulmaria (L.) Maxim. and Their Efficient Detections by HPTLC and HPLC Analyses. Molecules 2021, 26, 1939. [Google Scholar] [CrossRef]
- Sikorska, M. Flavonoids in the Leaves of Asclepias Incarnata L. Acta Pol. Pharm. 2003, 60, 471–476. [Google Scholar] [PubMed]
- Li, S.C.; Jin, Y.J.; Xue, X.; Xu, G.H. Bioactive Chemical Constituents of Cercis Chinensis. Chem. Nat. Compd. 2022, 58, 138–140. [Google Scholar] [CrossRef]
- Kaouadji, M. Acylated and Non-Acylated Kaempferol Monoglycosides from Platanus Acerifolia Buds. Phytochemistry 1990, 29, 2295–2297. [Google Scholar] [CrossRef]
- Tian, Q.; Li, D.; Patil, B.S. Identification and Determination of Flavonoids in Buckwheat (Fagopyrum Esculentum Moench, Polygonaceae) by High-Performance Liquid Chromatography with Electrospray Ionisation Mass Spectrometry and Photodiode Array Ultraviolet Detection. Phytochem. Anal. 2002, 13, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Cuyckens, F.; Claeys, M. Mass Spectrometry in the Structural Analysis of Flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- March, R.E.; Lewars, E.G.; Stadey, C.J.; Miao, X.-S.; Zhao, X.; Metcalfe, C.D. A Comparison of Flavonoid Glycosides by Electrospray Tandem Mass Spectrometry. Int. J. Mass Spectrom. 2006, 248, 61–85. [Google Scholar] [CrossRef]
- Li, X.; Jin, W.; Zhang, W.; Zheng, G. The Inhibitory Kinetics and Mechanism of Quercetin-3-O-Rhamnoside and Chlorogenic Acid Derived from Smilax China L. EtOAc Fraction on Xanthine Oxidase. Int. J. Biol. Macromol. 2022, 213, 447–455. [Google Scholar] [CrossRef]
- Dai, H.; Lv, S.; Fu, X.; Li, W. Identification of Scopoletin and Chlorogenic Acid as Potential Active Components in Sunflower Calathide Enzymatically Hydrolyzed Extract towards Hyperuricemia. Appl. Sci. 2021, 11, 10306. [Google Scholar] [CrossRef]
- Mehmood, A.; Li, J.; Rehman, A.U.; Kobun, R.; Llah, I.U.; Khan, I.; Althobaiti, F.; Albogami, S.; Usman, M.; Alharthi, F.; et al. Xanthine Oxidase Inhibitory Study of Eight Structurally Diverse Phenolic Compounds. Front. Nutr. 2022, 9, 966557. [Google Scholar] [CrossRef]
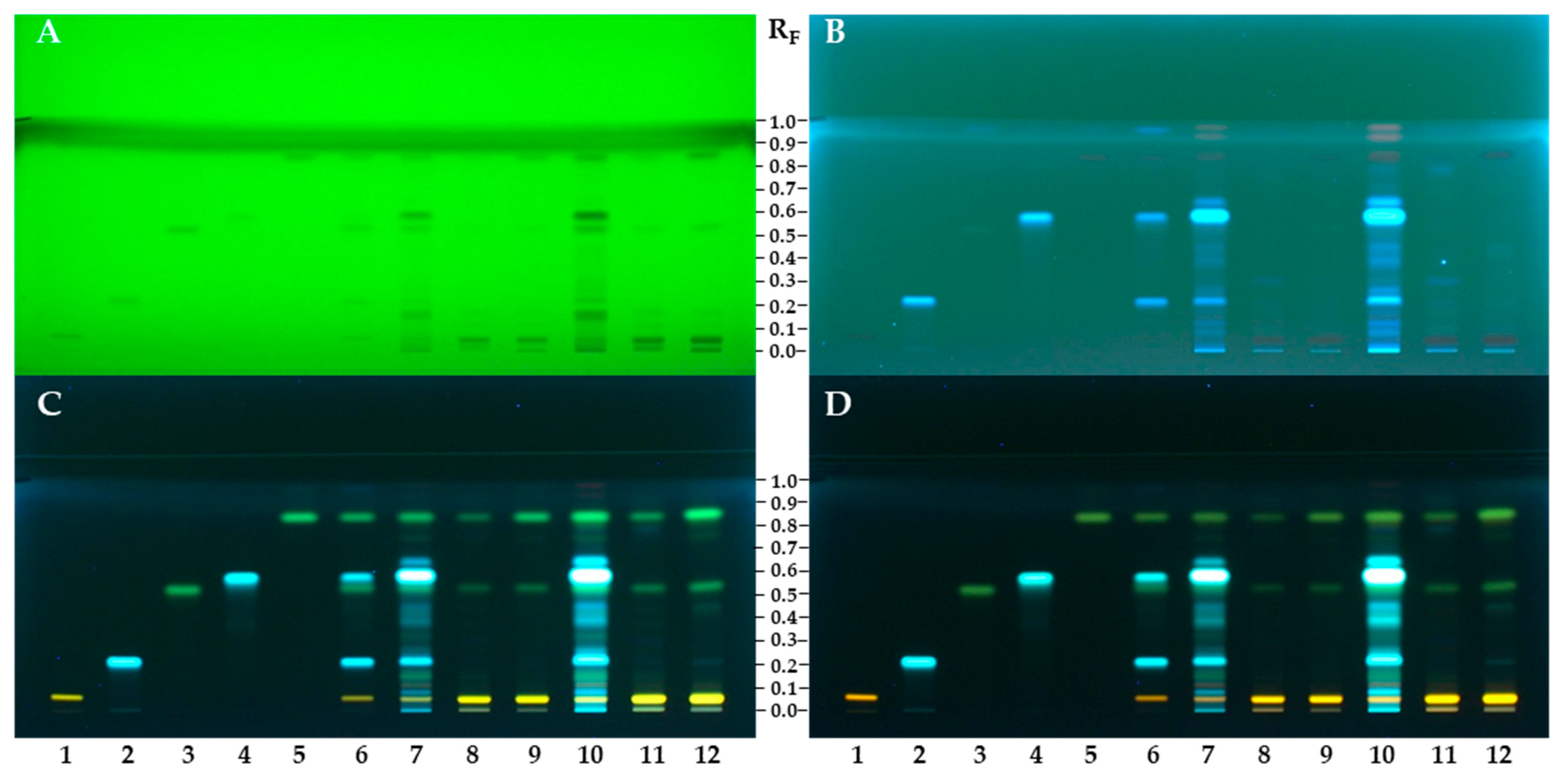
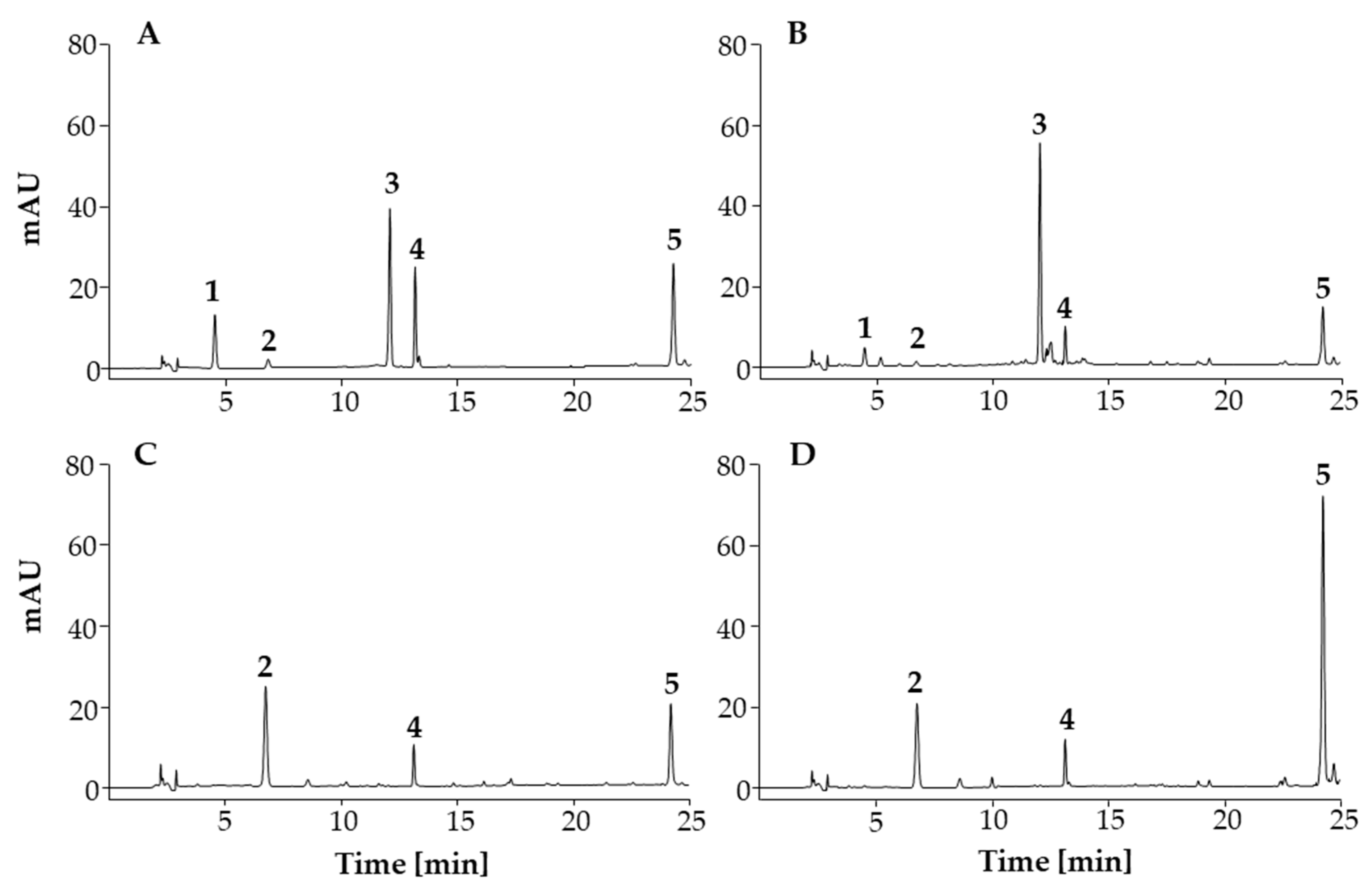

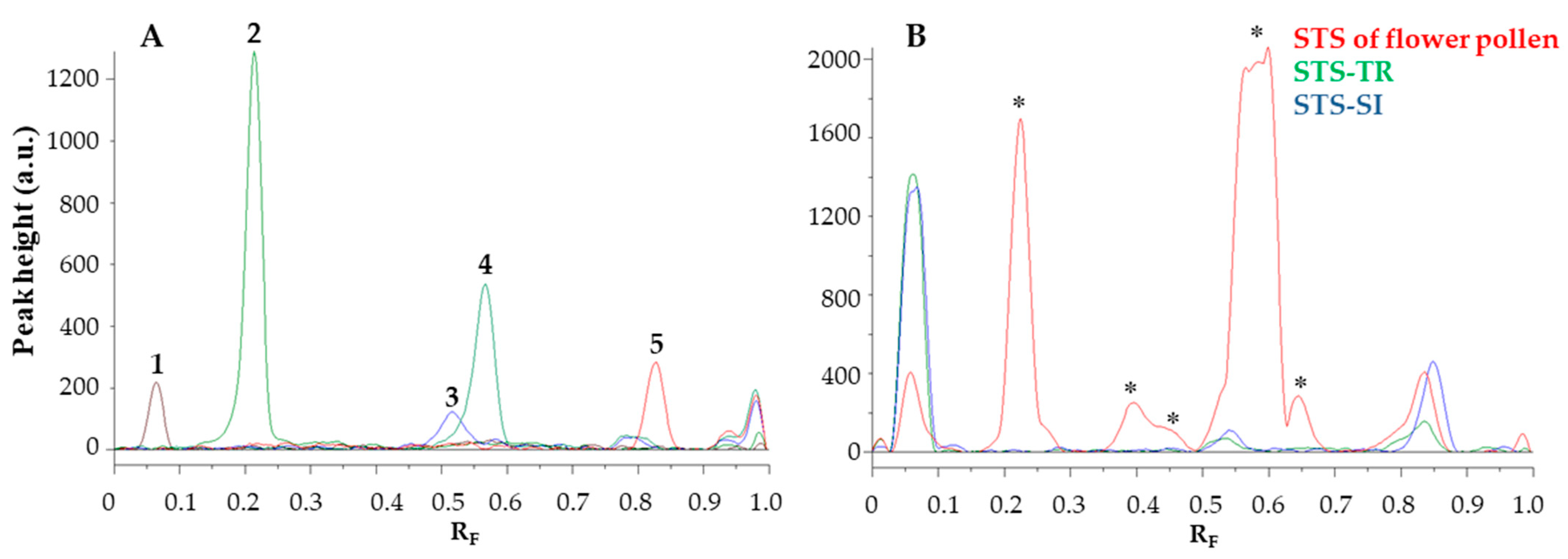
| Compounds | MS (m/z) [Relative Intensity] | MSn (m/z) [Relative Intensity] |
|---|---|---|
| Quercetin-3-O-β- glucopyrnosyl-(1→2)- β-galactopyranoside | 625 [100], 626 [41], 627 [10], 723 [6], 661 [3], 1251 [3] | MS2 [625]: 300 [100], 301 [73], 445 [33], 505 [17], 271 [16], 463 [11], 355 [9], 255 [8], 343 [8], 299 [6], 325 [5], 273 [4], 409 [4], 427 [4], 367 [3] |
| MS3 [625 → 300]: 271 [100], 255 [61], 272 [12], 254 [7], 243 [3] | ||
| MS2 [723]: 625 [100], 399 [34], 437 [21], 687 [21], 685 [21]. 437 [21] MS3 [723 → 625]: 300 [100], 301 [76], 445 [40], 271 [17], 505 [17], 463 [13] MS2 [661]: 625 [100] MS3 [661 → 625]: 300 [100] | ||
| Platanoside | 723 [100], 724 [49], 725 [13], 759 [13], 739 [9], 1447 [5] | MS2 [723]: 437 [100], 285 [36], 577 [4] MS3 [723 → 437]: 145 [100], 291 [72], 163 [71], 273 [63], 187 [23], 211 [16], 229 [14], 419 [14], 201 [10], 375 [7] MS2 [759]: 723 [100] MS3 [759 → 723]: 437 [100], 285 [31], 577 [4] MS4 [759 → 723 → 437]: 145 [100], 291 [70], 163 [68], 273 [54], 187 [21], 211 [16], 229 [15], 419 [14], 201 [8] MS2 [739]: 453 [100], 593 [24], 285 [19], 301 [14], 307 [4], 437 [3], 300 [2] MS3 [739 → 453]: 307 [100], 161 [45], 179 [34], 289 [22], 163 [13], 291 [12], 1445 [10], 217 [9], 263 [7], 135 [6], 227 [6] MS4 [739 → 453 → 307]: 161 [100], 135 [39], 179 [34] |
| Afzelin | 431 [100], 432 [27], 863 [19], 467 [15], 630 [9], 285 [3] | MS2 [431]: 285 [100], 284 [44], 327 [5] MS3 [431→285]: 257 [100], 229 [46], 267 [40], 241 [30], 213 [20], 285 [18], 197 [18], 239 [15], 163 [15] MS4 [431→285 → 257]: 229 [100], 163 [67], 239 [38], 213 [22], 185 [11], 187 [9], 189 [9], 257 [7] MS2 [863]: 701 [100], 717 [97], 727 [64], 697 [32], 831 [24], 571 [17], 561 [17] 285 [6], 431 [2] MS2 [467]: 431 MS3 [467 → 431]: 285 [100], 327 [8], 255 [6] |
| Standards | Linearity Range (µg/mL) | r2 | S * | Intercept | SD ** | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|---|---|
| Chlorogenic acid | 0.5–50 | 0.9995 | 20.590 | 2.950 | 0.186 | 0.027 | 0.090 |
| Quercetin-3-O-β-glucopyranosyl- (1→2)-β-galactopyranoside | 0.5–50 | 0.9998 | 7.899 | 2.244 | 0.058 | 0.022 | 0.073 |
| 3,5-Dicaffeoylquinic acid | 1–100 | 0.9994 | 31.248 | 9.637 | 0.790 | 0.076 | 0.253 |
| Afzelin | 1–50 | 0.9974 | 23.451 | 30.016 | 0.266 | 0.041 | 0.136 |
| Platanoside | 0.5–50 | 0.9998 | 38.132 | 0.982 | 0.271 | 0.021 | 0.071 |
| Intraday Precision | Interday Precision | ||||
|---|---|---|---|---|---|
| Standards | Concentration (µg/mL) | Average Concentration (µg/mL ± SD) (n = 3) | RSD (%) (n = 3) | Average Concentration (µg/mL ± SD) (n = 3) | RSD (%) (n = 3) |
| Chlorogenic acid | 5 | 5.283 ± 0.028 | 0.539 | 5.265 ± 0.014 | 0.266 |
| 5.272 ± 0.008 | 0.160 | 5.269 ± 0.006 | 0.106 | ||
| 5.259 ± 0.003 | 0.053 | 5.338 ± 0.023 | 0.430 | ||
| Quercetin-3-O-β-glucopyraosyl- (1→2)-β-galactopyranoside | 5 | 5.155 ± 0.032 | 0.618 | 5.172 ± 0.013 | 0.245 |
| 5.142 ± 0.039 | 0.752 | 5.218 ± 0.015 | 0.280 | ||
| 5.146 ± 0.013 | 0.246 | 5.311 ± 0.022 | 0.413 | ||
| Platanoside | 5 | 5.134 ± 0.007 | 0.135 | 5.165 ± 0.007 | 0.128 |
| 5.141 ± 0.004 | 0.078 | 5.201 ± 0.008 | 0.162 | ||
| 5.142 ± 0.007 | 0.135 | 5.263 ± 0.003 | 0.050 | ||
| 3,5-Dicaffeoylquinic acid | 10 | 9.936 ± 0.033 | 0.335 | 9.921 ± 0.032 | 0.318 |
| 9.907 ± 0.004 | 0.037 | 9.951 ± 0.020 | 0.201 | ||
| 9.904 ± 0.002 | 0.019 | 10.052 ± 0.043 | 0.424 | ||
| Afzelin | 10 | 9.492 ± 0.020 | 0.206 | 9.526 ± 0.004 | 0.045 |
| 9.489 ± 0.002 | 0.026 | 9.590 ± 0.011 | 0.118 | ||
| 9.501 ± 0.004 | 0.045 | 9.704 ± 0.009 | 0.091 | ||
| Added Concentration (µg/mL) | Recovery (%) (n = 3) | Added Concentration (µg/mL) | Recovery (%) (n = 3) | ||||
|---|---|---|---|---|---|---|---|
| Samples | Chlorogenic Acid | Quercetin-3-O-β- glucopyranosyl-(1→2)-β-galactopyranoside | Platanoside | 3,5-Dicaffeoyl- quinic Acid | Afzelin | ||
| BP-TR | 12.5 | 103.9 | 102.9 | 104.9 | 25 | 108.4 | 95.8 |
| 6.25 | 97.3 | 104.4 | 94.6 | 12.5 | 90.9 | 82.6 | |
| 3.125 | 94.0 | 102.6 | 105.9 | 6.25 | 72.3 | 85.0 | |
| BP-SI | 12.5 | 100.8 | 98.6 | 105.0 | 25 | 107.2 | 95.2 |
| 6.25 | 103.6 | 102.5 | 107.4 | 12.5 | 91.8 | 87.1 | |
| 3.125 | 103.8 | 106.1 | 105.9 | 6.25 | 72.2 | 92.4 | |
| FP | 12.5 | 100.6 | 96.3 | 93.1 | 25 | 83.8 | 78.4 |
| 6.25 | 97.3 | 93.7 | 104.4 | 12.5 | 99.6 | 86.3 | |
| 3.125 | 90.3 | 80.0 | 96.6 | 6.25 | 76.9 | 87.7 | |
| Samples | Chlorogenic Acid | Quercetin-3-O-β- glucopyranosyl-(1→2)-β- galactopyranoside | Platanoside | 3,5-Dicaffeoylquinic Acid | Afzelin |
|---|---|---|---|---|---|
| mg/g ± SD (n = 3) | |||||
| BP-TR | N.d. | 29.041 ± 0.088 a | 2.030 ± 0.009 c | N.d. | 2.052 ± 0.002 c |
| BP-SI | N.d. | 24.400 ± 0.211 b | 7.283 ± 0.107 a | N.d. | 2.419 ± 0.007 b |
| FP | 3.480 ± 0.010 | 3.959 ± 0.082 c | 6.242 ± 0.039 b | 22.372 ± 0.032 | 6.598 ± 0.010 a |
| Compounds and Samples | DPPH | FRAP | ABTS | CUPRAC |
|---|---|---|---|---|
| mg TE/g (n = 3) | ||||
| Quercetin-3-O-β-glucopyranosyl- (1→2)-β-galactopyranoside | 400.21 ± 11.78 c | 227.30 ± 2.65 c | 242.13 ± 5.88 c | 560.53 ± 14.74 c |
| Chlorogenic acid | 812.72 ± 4.99 a | 676.51 ± 2.34 a | 803.52 ± 1.12 a | 950.43 ± 7.64 a |
| Afzelin | 149.35 ± 4.28 d | 58.54 ± 2.37 d | 104.71 ± 0.25 e | 171.40 ± 8.24 e |
| 3,5-Dicaffeoylquinic acid | 665.53 ± 1.96 b | 542.36 ± 15.41 b | 701.64 ± 13.63 b | 760.83 ± 12.84 b |
| Platanoside | 143.11 ± 1.31 d | 49.06 ± 0.91 de | 173.15 ± 0.86 d | 213.68 ± 8.41 d |
| FP | 42.28 ± 0.68 e | 34.49 ± 0.05 e | 41.82 ± 2.87 f | 98.96 ± 4.22 f |
| BP-TR | 11.00 ± 0.04 f | 6.99 ± 0.12 f | 6.24 ± 0.14 g | 22.20 ± 1.31 g |
| BP-SI | 14.61 ± 0.75 f | 7.61 ± 0.27 f | 9.35 ± 0.15 g | 30.35 ± 1.56 g |
| Compounds and DSTSs | XO Inhibitory Activity | SOD Activity |
|---|---|---|
| IC50 (µg/mL ± SD) (n = 3) | ||
| Quercetin-3-O-β-glucopyranosyl-(1→2)-β-galactopyranoside | 73.01 ± 3.42 d | 5.09 ± 0.36 a |
| Chlorogenic acid | 12.68 ± 1.12 b | 1.58 ± 0.09 a |
| Afzelin | 14.46 ± 1.47 bc | 20.61 ± 1.30 b |
| 3,5-Dicaffeoylquinic acid | 17.47 ± 2.23 c | 5.87 ± 0.32 a |
| Platanoside | N.d. | N.d. |
| FP | N.d. | 36.92 ± 3.49 c |
| BP-TR | N.d. | 63.08 ± 4.00 e |
| BP-SI | N.d. | 48.14 ± 2.42 d |
| Allopurinol | 2.25 ± 0.04 a | 3.03 ± 0.10 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, N.B.; Guzelmeric, E.; Vovk, I.; Glavnik, V.; Kırmızıbekmez, H.; Yesilada, E. Phytochemical and Bioactivity Studies on Hedera helix L. (Ivy) Flower Pollen and Ivy Bee Pollen. Antioxidants 2023, 12, 1394. https://doi.org/10.3390/antiox12071394
Sen NB, Guzelmeric E, Vovk I, Glavnik V, Kırmızıbekmez H, Yesilada E. Phytochemical and Bioactivity Studies on Hedera helix L. (Ivy) Flower Pollen and Ivy Bee Pollen. Antioxidants. 2023; 12(7):1394. https://doi.org/10.3390/antiox12071394
Chicago/Turabian StyleSen, Nisa Beril, Etil Guzelmeric, Irena Vovk, Vesna Glavnik, Hasan Kırmızıbekmez, and Erdem Yesilada. 2023. "Phytochemical and Bioactivity Studies on Hedera helix L. (Ivy) Flower Pollen and Ivy Bee Pollen" Antioxidants 12, no. 7: 1394. https://doi.org/10.3390/antiox12071394
APA StyleSen, N. B., Guzelmeric, E., Vovk, I., Glavnik, V., Kırmızıbekmez, H., & Yesilada, E. (2023). Phytochemical and Bioactivity Studies on Hedera helix L. (Ivy) Flower Pollen and Ivy Bee Pollen. Antioxidants, 12(7), 1394. https://doi.org/10.3390/antiox12071394






