Protective Effects of Different Molecular Weights of Purslane (Portulaca oleracea L.) Aqueous Extract on DSS-Induced Ulcerative Colitis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Purslane Extraction
2.3. Analysis of Components and Antioxidant Capacity in Purslane Extracts
2.4. Liquid Chromatography–Mass Spectrometry (LC/MS) Analysis of the Components of Purslane Extracts
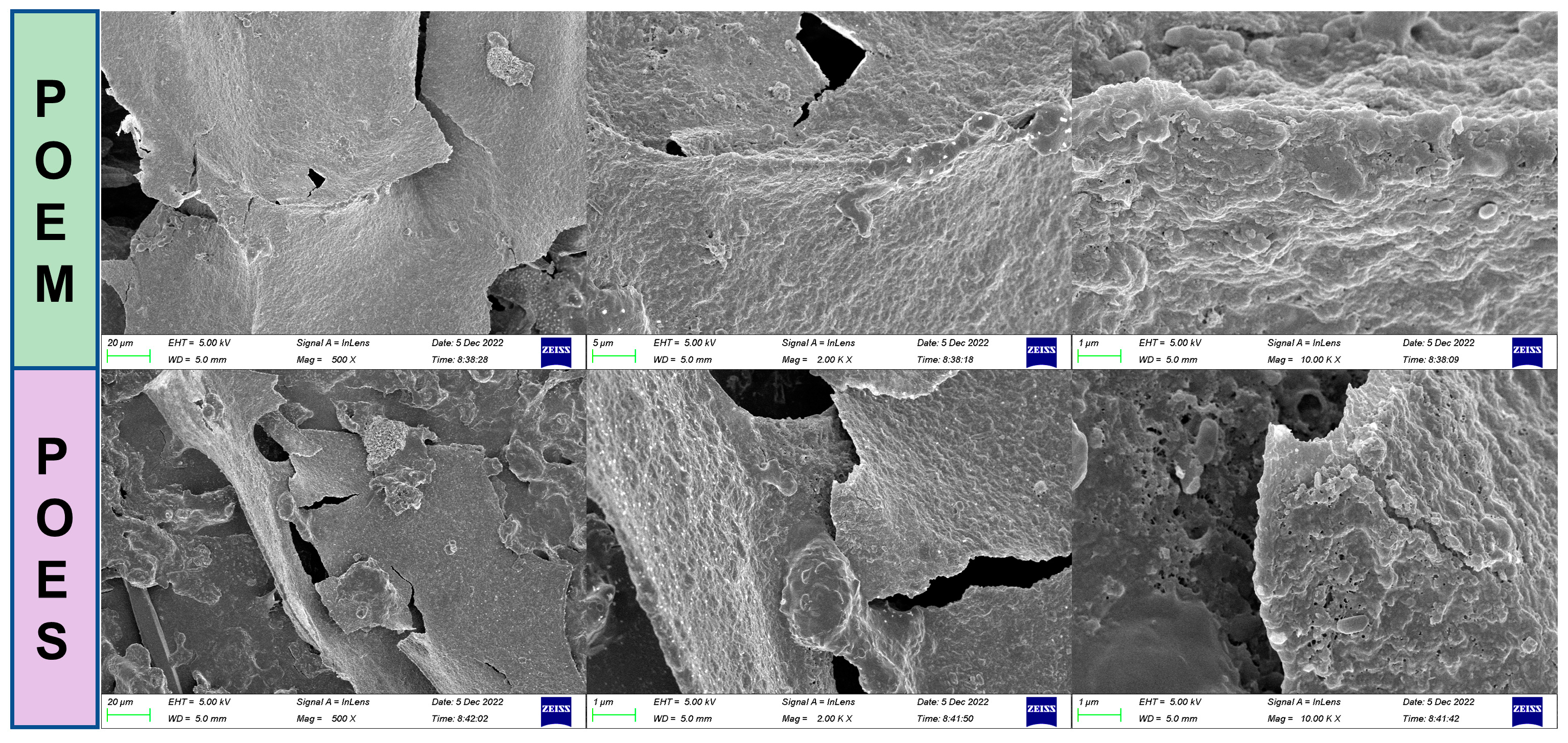
2.5. Scanning Electron Microscope Observation
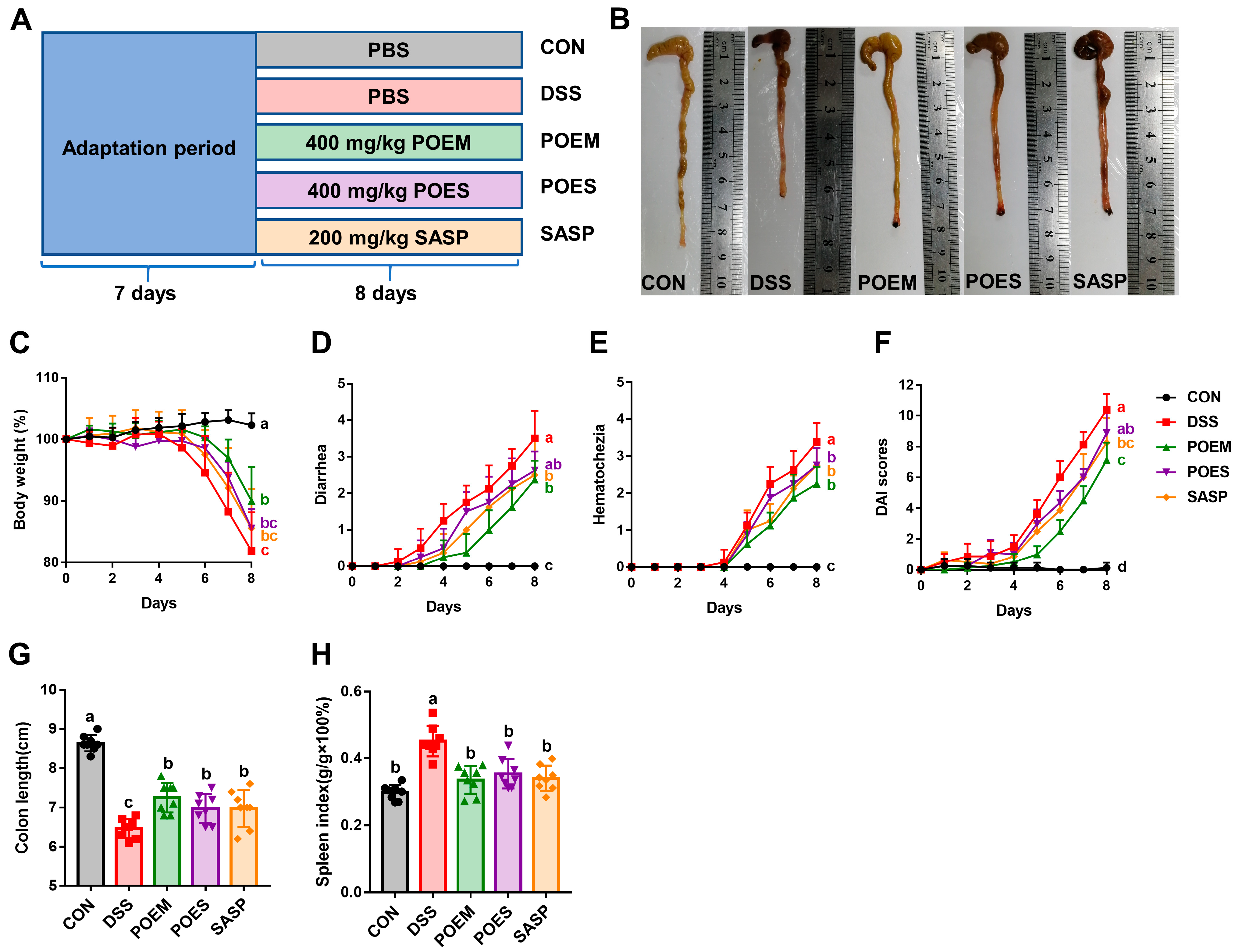
2.6. Animals and the Experimental Design
- (1)
- CON group: Drinking water was given to the mice for Days 1–8 of intervention. Then, the same volume of phosphate-buffered saline (PBS) was administered by gavage during the experiment.
- (2)
- DSS group: Drinking water containing 4% DSS was given to the mice for Days 1–8 of intervention. Then, the same volume of PBS was administered by gavage during the experiment.
- (3)
- POEM group: Drinking water containing 4% DSS was given to the mice for Days 1–8 of intervention. Then, 400 mg/kg/day POEM was administered by gavage during the experiment.
- (4)
- POES group: Drinking water containing 4% DSS was given to the mice for Days 1–8 of intervention. Then, 400 mg/kg/day POES was administered by gavage during the experiment.
- (5)
- SASP group: Drinking water containing 4% DSS was given to the mice for Days 1–8 of intervention. Then, 200 mg/kg/day SASP was administered by gavage during the experiment.
2.7. Assessment of Disease Activity Index (DAI)
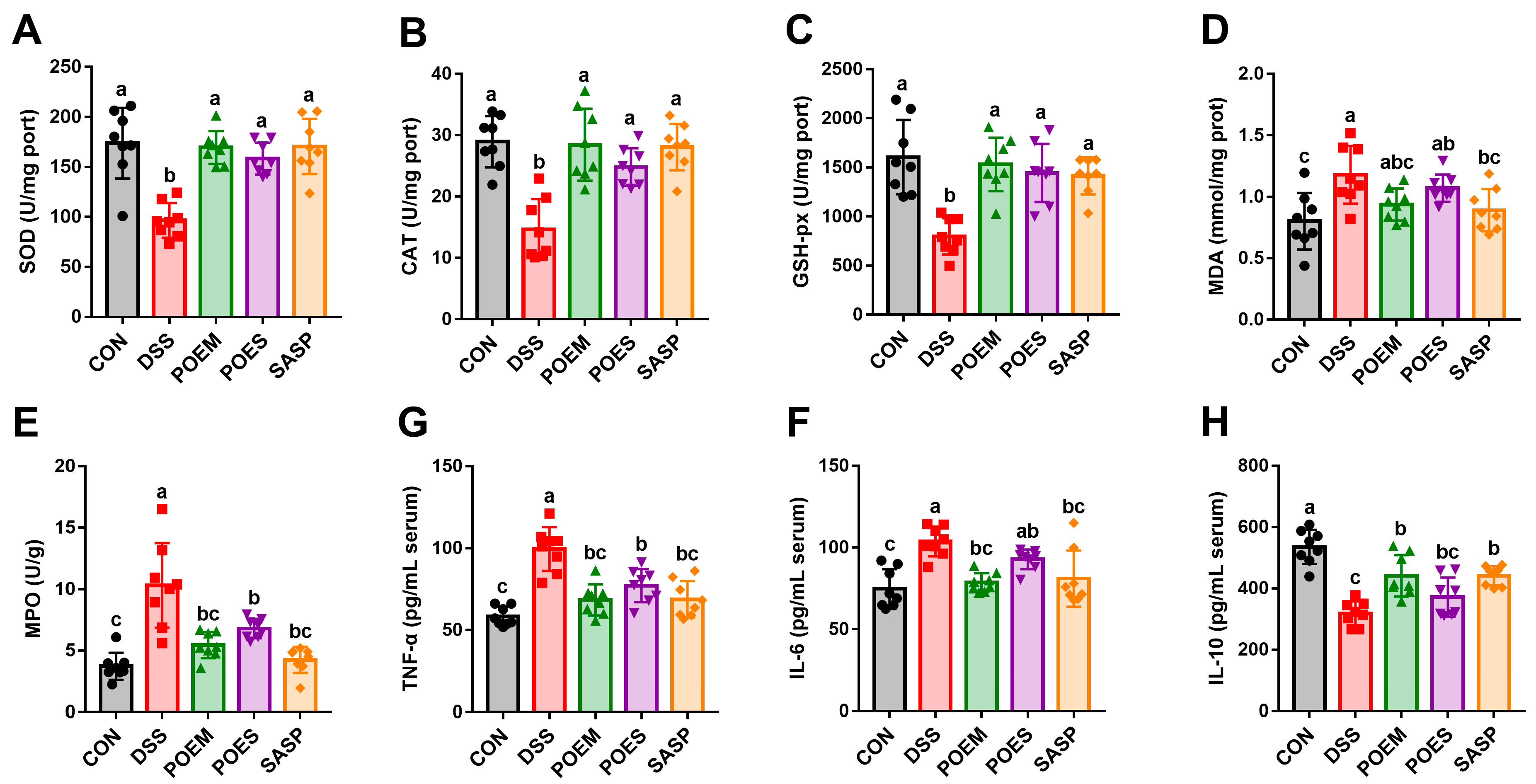
2.8. Assessment of Oxidative Stress Markers and Inflammatory Cytokines
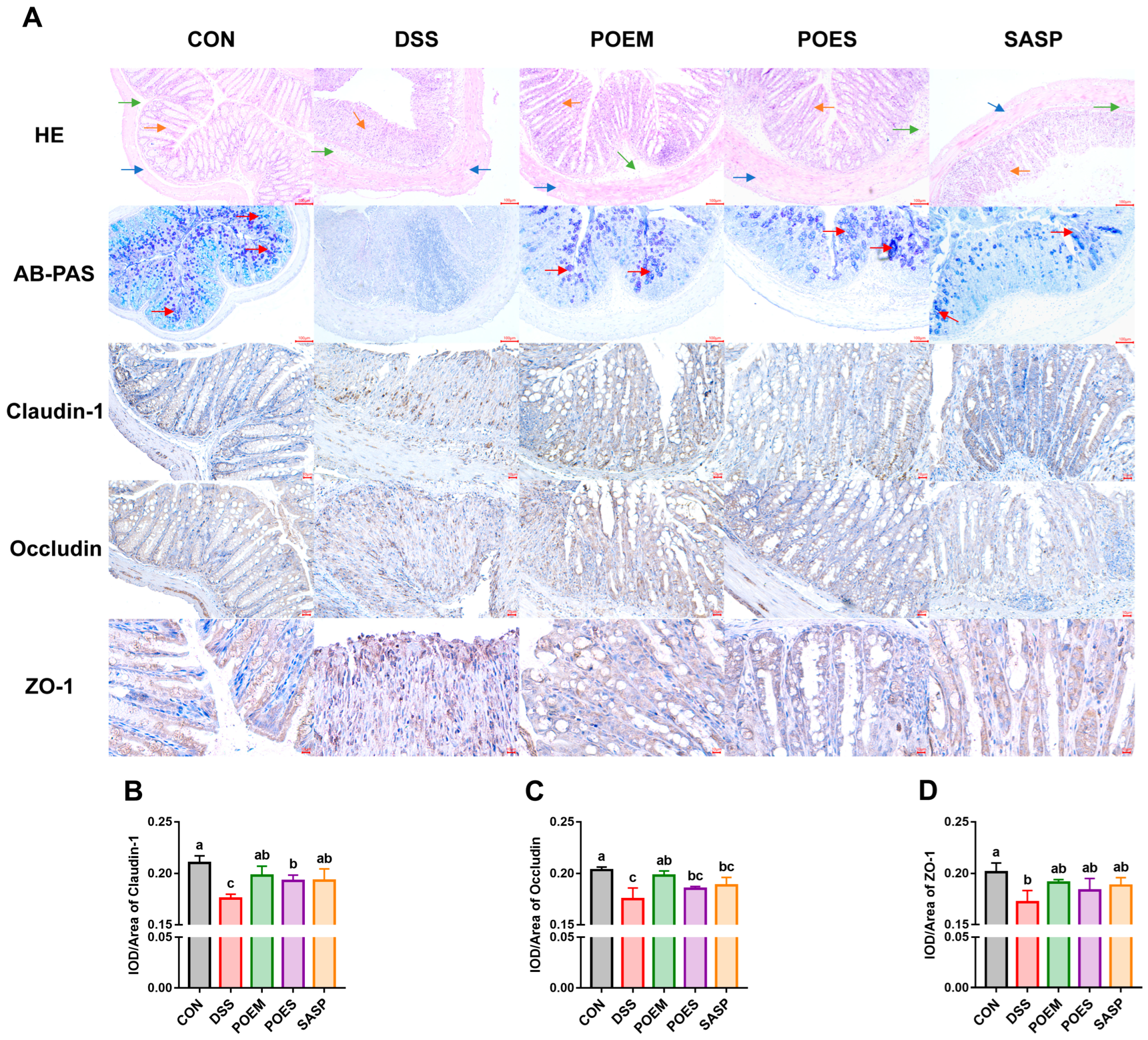
2.9. Histological and Immunohistochemical Analyses
2.10. Analysis of Gut Microbiota
2.11. Determination of SCFAs Using High-Performance Liquid Chromatography (HPLC)
2.12. Statistical Analysis
3. Results
3.1. Preparation, Composition Analysis, and Antioxidant Capacity Determination of Aqueous Extracts from Purslane with Different Molecular Weight Ranges
3.2. POEM and POES Alleviated the Pathological Features of DSS-Induced UC in Mice
3.3. POEM and POES Regulate Antioxidant Enzymes Levels and Reduce Inflammation
3.4. POEM and POES Protect Colon Integrity and Increase the Tight Junction
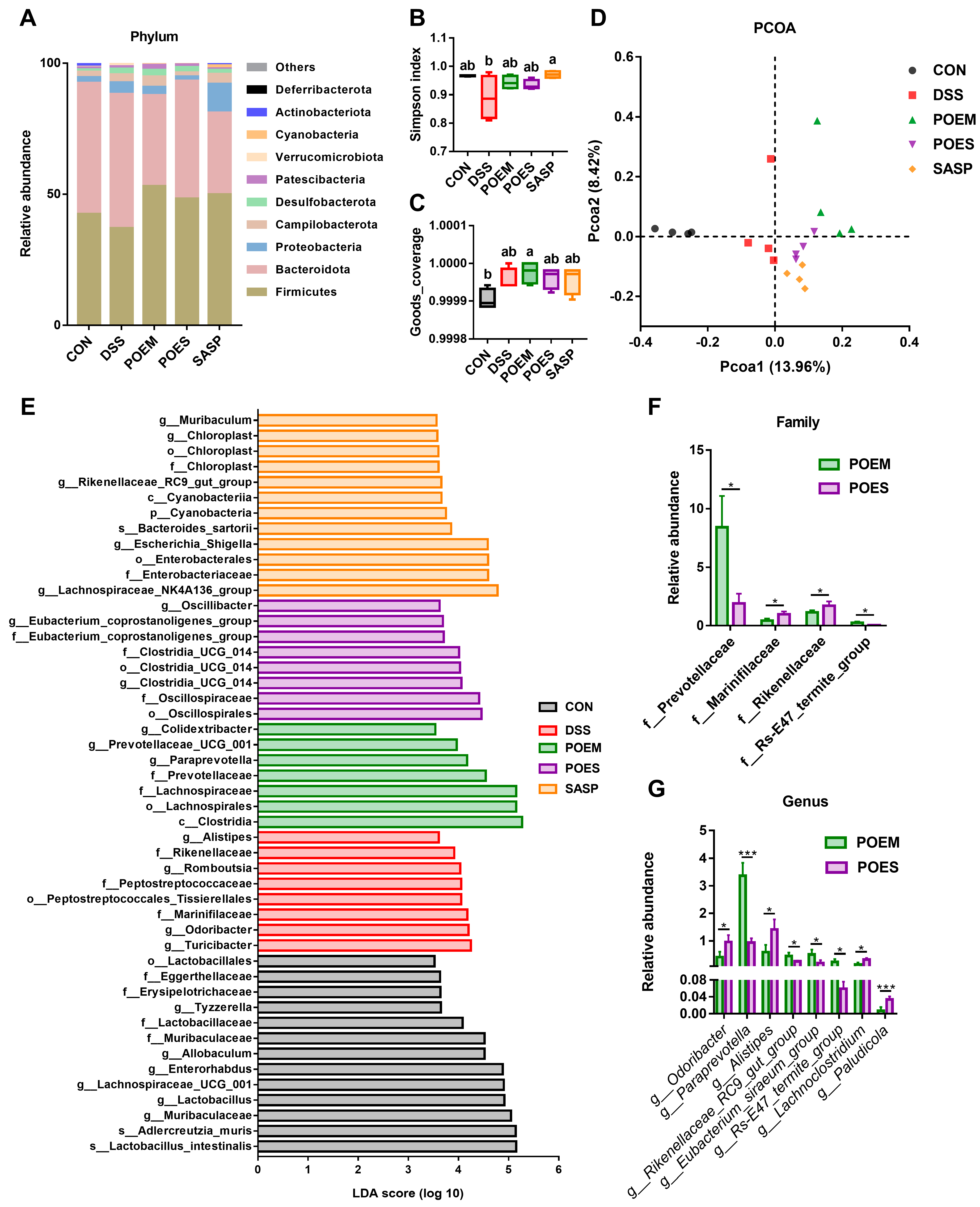
3.5. POEM and POES Regulated the Intestinal Microbiota Composition
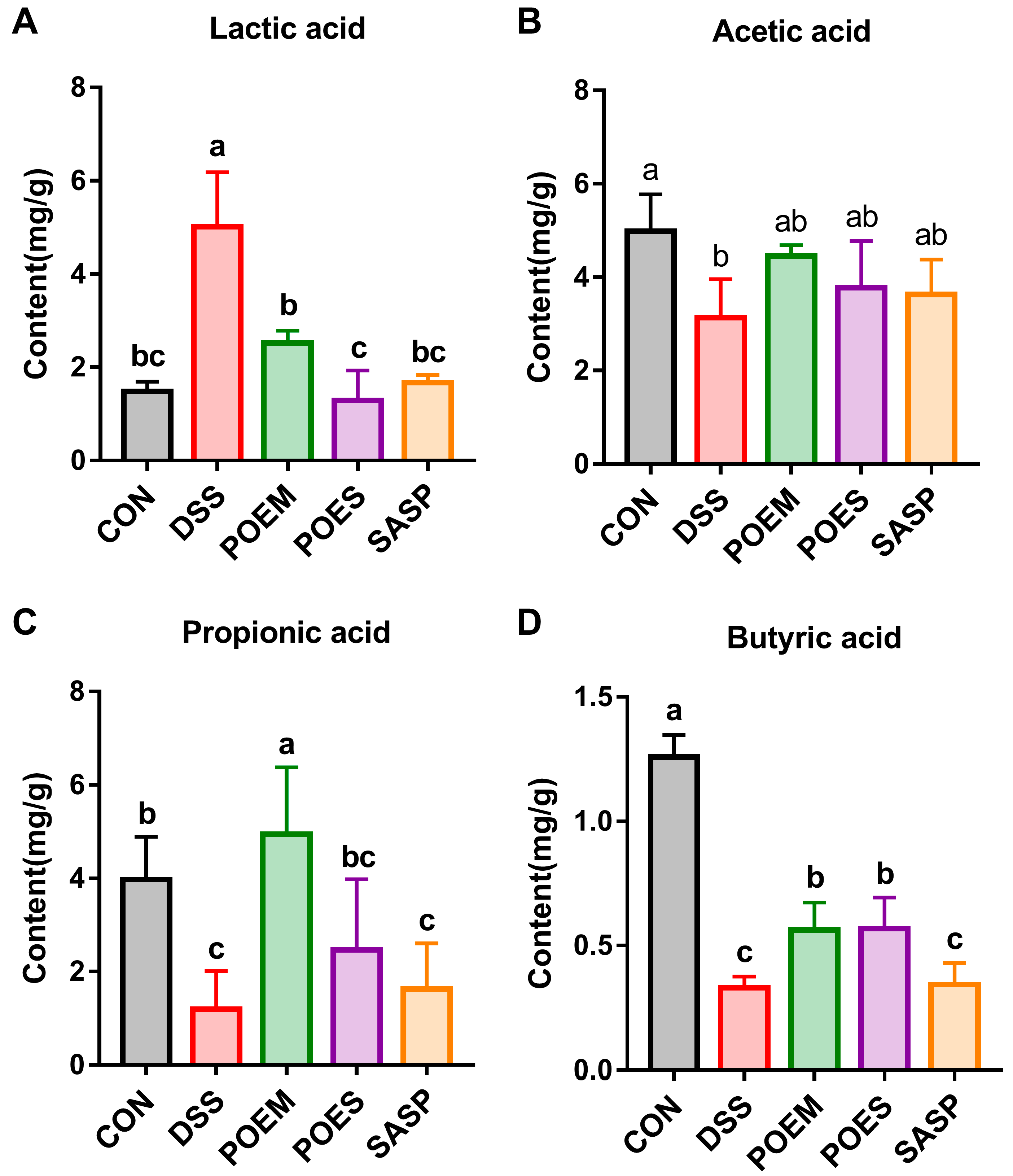
3.6. POEM and POES Affected SCFA Contents in the Intestinal Contents of Mice
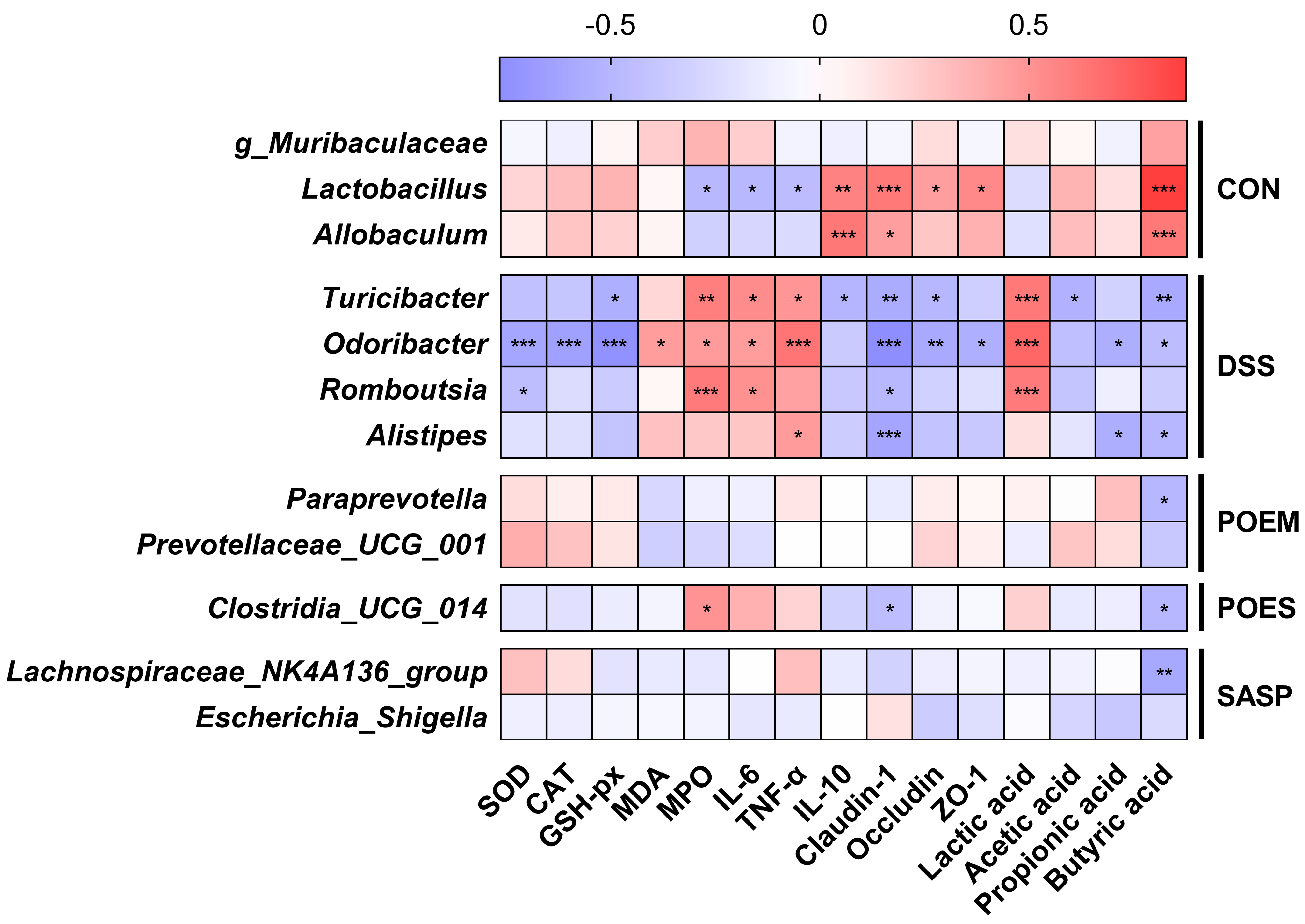
3.7. Correlation between the Main Factors of UC Development and the Gut Microflora
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, B.G.; Su, Y.H.; Zhao, R.X.; Song, P.; Li, H.; Cui, X.H.; Gao, H.M.; Zhai, R.X.; Fu, X.J.; et al. Potential activity of Traditional Chinese Medicine against Ulcerative colitis: A review. J. Ethnopharmacol. 2022, 289, 115084. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Moss, A.C.; Farraye, F.A. Ulcerative Colitis. Mayo. Clin. Proc. 2019, 94, 1357–1373. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, R.P.D.; Machado, A.; Galvez, J.; Cazarin, C.B.B.; Marostica, M.R., Jr. Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. 2020, 258, 118129. [Google Scholar] [CrossRef]
- Ji, K.L.; Wu, M.Z.; Huang, C.Y.; GongPan, P.C.; Sun, P.; Sun, Y.L.; Li, J.; Xiao, C.F.; Xu, Y.K.; Fan, Q.F.; et al. Alpinia hainanensis Rhizome Extract Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis: Active Ingredient Investigation and Evaluation. J. Agric. Food Chem. 2022, 70, 3989–3999. [Google Scholar] [CrossRef]
- Chen, J.F.; Luo, D.D.; Lin, Y.S.; Liu, Y.H.; Wu, J.Z.; Yi, X.Q.; Wu, Y.; Zhang, Q.; Gao, C.J.; Cai, J.; et al. Aqueous extract of Bruguiera gymnorrhiza leaves protects against dextran sulfate sodium induced ulcerative colitis in mice via suppressing NF-kappaB activation and modulating intestinal microbiota. J. Ethnopharmacol. 2020, 251, 112554. [Google Scholar] [CrossRef]
- Subpiramaniyam, S. Portulaca oleracea L. for phytoremediation and biomonitoring in metal-contaminated environments. Chemosphere 2021, 280, 130784. [Google Scholar] [CrossRef]
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.P.; Mahyari, S.; Hassani, F.V.; Karimi, G. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiao, D.; Zhang, Y.; Chen, Q.; Chen, Y.; Tang, Y.; Que, R.; Chen, Y.; Zheng, L.; Dai, Y.; et al. Portulaca oleracea L. Extract Ameliorates Intestinal Inflammation by Regulating Endoplasmic Reticulum Stress and Autophagy. Mol. Nutr. Food Res. 2022, 66, e2100791. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, Y.; Zhang, D.; Wu, X.; Yu, J.; Zhang, Z.; Li, Y.; Sun, C.; Tang, Z.; Liu, L. Protective Effects of Polysaccharide Extracted from Portulacae oleracea L. on Colitis Induced by Dextran Sulfate Sodium. J. Med. Food 2020, 23, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Luo, H.; Wang, N.; Li, J.; Xu, S.; Chen, K.; Feng, J.; Wu, L.; Li, S.; Liu, T.; et al. Portulaca Extract Attenuates Development of Dextran Sulfate Sodium Induced Colitis in Mice through Activation of PPARgamma. PPAR Res. 2018, 2018, 6079101. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef] [PubMed]
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Guo, H.; Xie, Y.; Chen, Y.; Lu, C.; Yang, Z.; Zhu, Y.; Ouyang, Y.; Zhang, Y.; Wang, X. Mo3Se4 nanoparticle with ROS scavenging and multi-enzyme activity for the treatment of DSS-induced colitis in mice. Redox Biol. 2022, 56, 102441. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Su, Y.; Sun, J.; Yang, Y. Chicken embryo extracts enhance spleen lymphocyte and peritoneal macrophages function. J. Ethnopharmacol. 2012, 144, 255–260. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Z.; Guo, Y.; Lu, S.; Du, H.; Cao, Y. Antioxidant capacity of flavonoids from Folium Artemisiae Argyi and the molecular mechanism in Caenorhabditis elegans. J. Ethnopharmacol. 2021, 279, 114398. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yuan, Z.W.; Qu, C.; Yu, X.T.; Huang, T.; Chen, P.V.; Su, Z.R.; Dou, Y.X.; Wu, J.Z.; Zeng, H.F.; et al. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota. Pharmacol. Res. 2018, 137, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, H.; Li, W.; He, Q.; Liang, J.; Yan, X.; Yuan, Y.; Yue, T. Selenium-containing tea polysaccharides ameliorate DSS-induced ulcerative colitis via enhancing the intestinal barrier and regulating the gut microbiota. Int. J. Biol. Macromol. 2022, 209, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shao, D.; Zhou, J.; Gu, J.; Qin, J.; Chen, W.; Wei, W. Signatures within esophageal microbiota with progression of esophageal squamous cell carcinoma. Chin. J. Cancer Res. 2020, 32, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Q.; Zhang, Y.; Ma, W.; Ning, K.; Xiang, J.Y.; Cui, J.; Xiang, H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020, 104, 335–349. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef]
- LeBlanc, J.F.; Segal, J.P.; de Campos Braz, L.M.; Hart, A.L. The Microbiome as a Therapy in Pouchitis and Ulcerative Colitis. Nutrients 2021, 13, 1780. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef]
- Xu, A.; Zhao, Y.; Shi, Y.; Zuo, X.; Yang, Y.; Wang, Y.; Xu, P. Effects of oxidation-based tea processing on the characteristics of the derived polysaccharide conjugates and their regulation of intestinal homeostasis in DSS-induced colitis mice. Int. J. Biol. Macromol. 2022, 214, 402–413. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Shi, S.; Jiang, L. Evaluation of antioxidant and immuno-enhancing activities of Purslane polysaccharides in gastric cancer rats. Int. J. Biol. Macromol. 2014, 68, 113–116. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Singh, S.K.; Chaubey, S.; Bansal, A.; Kaur, G.; Malik, D.S. Cosmeceutical Aptitudes of Azelaic Acid. Curr. Drug Res. Rev. 2021, 13, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Jeengar, M.K.; Thummuri, D.; Magnusson, M.; Naidu, V.G.M.; Uppugunduri, S. Uridine Ameliorates Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Sci. Rep. 2017, 7, 3924. [Google Scholar] [CrossRef] [PubMed]
- Fikry, A.M.; Attia, A.I.; Ismail, I.E.; Alagawany, M.; Reda, F.M. Dietary citric acid enhances growth performance, nutrient digestibility, intestinal microbiota, antioxidant status, and immunity of Japanese quails. Poult. Sci. 2021, 100, 101326. [Google Scholar] [CrossRef]
- Sanchez, A.G.; Ibargoyen, M.N.; Mastrogiovanni, M.; Radi, R.; Keszenman, D.J.; Peluffo, R.D. Fast and biphasic 8-nitroguanine production from guanine and peroxynitrite. Free Radic. Biol. Med. 2022, 193, 474–484. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef]
- Zhang, B.H.; Chen, W.; Li, H.Q.; Zhou, E.M.; Hu, W.Y.; Duan, Y.Q.; Mohamad, O.A.; Gao, R.; Li, W.J. An antialgal compound produced by Streptomyces jiujiangensis JXJ 0074(T). Appl. Microbiol. Biotechnol. 2015, 99, 7673–7683. [Google Scholar] [CrossRef]
- Livingston, M.; Heaney, L.G.; Ennis, M. Adenosine, inflammation and asthma—A review. Inflamm. Res. 2004, 53, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.Y.; Tan, L.; Han, C.; Zheng, Z.Y.; Wu, G.S.; Luo, H.R.; Li, S.L. Trigonelline Extends the Lifespan of C. Elegans and Delays the Progression of Age-Related Diseases by Activating AMPK, DAF-16, and HSF-1. Oxid. Med. Cell Longev. 2021, 2021, 7656834. [Google Scholar] [CrossRef]
- Kwiecien, I.; Miceli, N.; D’Arrigo, M.; Marino, A.; Ekiert, H. Antioxidant Potential and Enhancement of Bioactive Metabolite Production in In Vitro Cultures of Scutellaria lateriflora L. by Biotechnological Methods. Molecules 2022, 27, 1140. [Google Scholar] [CrossRef]
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Rodriguez, A.B.; Barriga, C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age 2013, 35, 1277–1285. [Google Scholar] [CrossRef] [Green Version]
- Niu, W.; Chen, X.; Xu, R.; Dong, H.; Yang, F.; Wang, Y.; Zhang, Z.; Ju, J. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: A review. Carbohydr. Polym. 2021, 254, 117189. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Cao, Q.; Ye, L.; Wang, J.; Guo, M. The Function of Natural Polysaccharides in the Treatment of Ulcerative Colitis. Front. Pharmacol. 2022, 13, 927855. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Backhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Giudici, F.; Fiorindi, C.; Ficari, F.; Scaringi, S.; Amedei, A. Immunomodulating Activity and Therapeutic Effects of Short Chain Fatty Acids and Tryptophan Post-biotics in Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 2754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminsky, L.W.; Al-Sadi, R.; Ma, T.Y. IL-1beta and the Intestinal Epithelial Tight Junction Barrier. Front. Immunol. 2021, 12, 767456. [Google Scholar] [CrossRef]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, S.; Cao, H.; Shen, P.; Liu, J.; Fu, Y.; Cao, Y.; Zhang, N. The protective role of phloretin against dextran sulfate sodium-induced ulcerative colitis in mice. Food Funct. 2019, 10, 422–431. [Google Scholar] [CrossRef]
- Mills, R.H.; Dulai, P.S.; Vazquez-Baeza, Y.; Sauceda, C.; Daniel, N.; Gerner, R.R.; Batachari, L.E.; Malfavon, M.; Zhu, Q.; Weldon, K.; et al. Multi-omics analyses of the ulcerative colitis gut microbiome link Bacteroides vulgatus proteases with disease severity. Nat. Microbiol. 2022, 7, 262–276. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Z.; Wu, G.; Zhang, R.; Dong, L.; Huang, F.; Zhang, M.; Su, D. Lychee (Litchi chinensis Sonn.) Pulp Phenolics Activate the Short-Chain Fatty Acid-Free Fatty Acid Receptor Anti-inflammatory Pathway by Regulating Microbiota and Mitigate Intestinal Barrier Damage in Dextran Sulfate Sodium-Induced Colitis in Mice. J. Agric. Food Chem. 2021, 69, 3326–3339. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Teofani, A.; Marafini, I.; Laudisi, F.; Pietrucci, D.; Salvatori, S.; Unida, V.; Biocca, S.; Monteleone, G.; Desideri, A. Intestinal Taxa Abundance and Diversity in Inflammatory Bowel Disease Patients: An Analysis including Covariates and Confounders. Nutrients 2022, 14, 260. [Google Scholar] [CrossRef]
- Jalanka, J.; Cheng, J.; Hiippala, K.; Ritari, J.; Salojarvi, J.; Ruuska, T.; Kalliomaki, M.; Satokari, R. Colonic Mucosal Microbiota and Association of Bacterial Taxa with the Expression of Host Antimicrobial Peptides in Pediatric Ulcerative Colitis. Int. J. Mol. Sci. 2020, 21, 6044. [Google Scholar] [CrossRef] [PubMed]
- Ran, B.; Guo, C.E.; Zhang, Y.; Han, C.; Cao, T.; Huang, H.; Geng, Z.; Li, W. Preventive effect of Chinese dwarf cherry [Cerasus humilis (Bge.) Sok.] fermentation juice on dextran sulfate sodium-induced ulcerative colitis rats through the regulation of IgA and the intestinal immune barrier. Food Funct. 2022, 13, 5766–5781. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.; Dai, L.; Chen, H.; Yi, B.; Niu, J.; Wang, L.; Zhang, F.; Luo, J.; Wang, K.; et al. Ecological and network analyses identify four microbial species with potential significance for the diagnosis/treatment of ulcerative colitis (UC). BMC Microbiol. 2021, 21, 138. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Li, W.B.; Wang, H.Y.; Ma, Y.M.; Zhao, X.H.; Yang, H.; Qian, J.M.; Li, J.N. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar] [CrossRef]
- Yang, C.; Du, Y.; Ren, D.; Yang, X.; Zhao, Y. Gut microbiota-dependent catabolites of tryptophan play a predominant role in the protective effects of turmeric polysaccharides against DSS-induced ulcerative colitis. Food Funct. 2021, 12, 9793–9807. [Google Scholar] [CrossRef]
- Li, Y.; Watanabe, E.; Kawashima, Y.; Plichta, D.R.; Wang, Z.; Ujike, M.; Ang, Q.Y.; Wu, R.; Furuichi, M.; Takeshita, K.; et al. Identification of trypsin-degrading commensals in the large intestine. Nature 2022, 609, 582–589. [Google Scholar] [CrossRef]
- Gong, X.; Liu, L.; Li, X.; Xiong, J.; Xu, J.; Mao, D.; Liu, L. Neuroprotection of cannabidiol in epileptic rats: Gut microbiome and metabolome sequencing. Front Nutr. 2022, 9, 1028459. [Google Scholar] [CrossRef]
- Yang, L.; Wan, Y.; Li, W.; Liu, C.; Li, H.F.; Dong, Z.; Zhu, K.; Jiang, S.; Shang, E.; Qian, D.; et al. Targeting intestinal flora and its metabolism to explore the laxative effects of rhubarb. Appl. Microbiol. Biotechnol. 2022, 106, 1615–1631. [Google Scholar] [CrossRef]

| No. | Sample | Weight (g/kg) | Polysaccharide (mg/g) | Protein (mg/g) |
|---|---|---|---|---|
| 1 | Aqueous extract 1 | 27.85 | 117.15 ± 14.30 | 183.38 ± 21.60 |
| 2 | >10 kDa (POEM) | 9.70 | 159.87 ± 8.83 | 269.13 ± 11.50 |
| 3 | 3–10 kDa | 0.04 | 75.49 ± 10.52 | 173.70 ± 7.27 |
| 4 | 1–3 kDa | 0.02 | 61.89 ± 2.87 | 136.76 ± 18.53 |
| 5 | <1 kDa (POES) | 12.39 | 56.94 ± 3.47 | 120.49 ± 6.64 |
| No. | Classification | <1 kDa (POES) | 1–3 kDa | 3–10 kDa | >10 kDa (POEM) |
|---|---|---|---|---|---|
| 1 | Organic acids | Citric acid | Citric acid | - | Citric acid |
| 2 | Ferulic acid | Ferulic acid | Ferulic acid | - | |
| 3 | Azelaic acid | Azelaic acid | Azelaic acid | - | |
| 4 | - | Pantothenic acid | - | - | |
| 5 | - | - | Isocitric acid | - | |
| 6 | - | - | D-(-)-Quinic acid | - | |
| 7 | Nucleotide related substances | Guanine | Guanine | Guanine | Guanine |
| 8 | Adenosine | Adenosine | Adenosine | Adenosine | |
| 9 | Uridine | Uridine | Uridine | - | |
| 10 | 2′-Deoxyadenosine | 2′-Deoxyadenosine | 2′-Deoxyadenosine | 2′-Deoxyadenosine | |
| 11 | Amino acids | L-Phenylalanine | L-Phenylalanine | L-Phenylalanine | L-Phenylalanine |
| 12 | L-Tryptophan | - | - | - | |
| 13 | - | D-(+)-Tryptophan | - | - | |
| 14 | - | - | DL-Tryptophan | DL-Tryptophan | |
| 15 | Alkaloids | Trigonelline | Trigonelline | Trigonelline | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, K.; Duan, Y.; Tong, W.; Chen, Y.; Zhang, Q.; Xie, Q.; Xiang, H. Protective Effects of Different Molecular Weights of Purslane (Portulaca oleracea L.) Aqueous Extract on DSS-Induced Ulcerative Colitis in Mice. Antioxidants 2023, 12, 1400. https://doi.org/10.3390/antiox12071400
Ning K, Duan Y, Tong W, Chen Y, Zhang Q, Xie Q, Xiang H. Protective Effects of Different Molecular Weights of Purslane (Portulaca oleracea L.) Aqueous Extract on DSS-Induced Ulcerative Colitis in Mice. Antioxidants. 2023; 12(7):1400. https://doi.org/10.3390/antiox12071400
Chicago/Turabian StyleNing, Ke, Yameng Duan, Weiwei Tong, Yue Chen, Qinghui Zhang, Qiuhong Xie, and Hongyu Xiang. 2023. "Protective Effects of Different Molecular Weights of Purslane (Portulaca oleracea L.) Aqueous Extract on DSS-Induced Ulcerative Colitis in Mice" Antioxidants 12, no. 7: 1400. https://doi.org/10.3390/antiox12071400





